Classification of Pummelo (Citrus grandis) Extracts through UV-VIS-Based Chemical Fingerprint
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction Procedure
2.3. UV–VIS Acquisition Spectra
2.4. NMR Measurements
2.5. Volatile Organic Compounds Determination
2.6. Multivariate Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. 2020. Available online: http://www.fao.org/economic/est/est-commodities/citrus-fruit/en/ (accessed on 23 April 2022).
- Igual, M.; García-Martínez, E.; Camacho, M.M.; Martínez-Navarrete, N. Stability of Micronutrients and Phytochemicals of Grapefruit Jam as Affected by the Obtention Process. Food Sci. Technol. Int. Cienc. Tecnol. Los Aliment. Int. 2016, 22, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Restani, P. Food Supplements Containing Botanicals: Benefits, Side Effects and Regulatory Aspects; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Schipilliti, L.; Bonaccorsi, I.; Cotroneo, A.; Dugo, P.; Mondello, L. Evaluation of Gas Chromatography−Combustion−Isotope Ratio Mass Spectrometry (GC-C-IRMS) for the Quality Assessment of Citrus Liqueurs. J. Agric. Food Chem. 2013, 61, 1661–1670. [Google Scholar] [CrossRef]
- Magbalot-Fernandez, A.; de Guzman, C.C. Phenology of ‘Magallanes’ Pummelo (Citrus maxima) Trees and Its Growth and Development as Influenced by Potassium Nutrition. Asian J. Res. Agric. For. 2019, 3, 1–18. [Google Scholar] [CrossRef]
- Tsai, H.I.; Chang, S.C.K.; Chang, S.J. Antioxidant content and free radical scavenging ability of fresh red pummel [Citrus grandis (L.) Osbeck] Juice and Freeze-Dried Products. J. Agric. Food Chem. 2007, 55, 2867–2872. [Google Scholar] [CrossRef]
- Gonzalez-Mas, M.C.; Rambla, J.L.; Lopez-Gresa, M.P.; Amparo Blazquez, M.; Granell, A. Volatile compounds in citrus essential oils: A comprehensive review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.-G.; Liu, Y.-J. Chemical Composition and Antimicrobial Activity of the Essential Oil from the Peel of Shatian Pummelo (Citrus grandis Osbeck). Int. J. Food Prop. 2012, 15, 709–716. [Google Scholar] [CrossRef]
- MetaboAnalyst 5.0. Available online: https://www.metaboanalyst.ca/ (accessed on 23 April 2022).
- Wehrens, R.; Mevik, B.H. The pls Package: Principal Component and Partial Least Squares Regression in R. J. Stat. Softw. 2007, 18, 1–23. [Google Scholar]
- Sun, H.; Ni, H.; Yang, Y.; Chen, F.; Cai, H.; Xiao, A. Sensory evaluation and gas chromatography-mass spectrometry (GC-MS) analysis of the volatile extracts of pummelo (Citrus maxima) peel. Flavour Fragr. J. 2014, 29, 305–312. [Google Scholar] [CrossRef]
- Intekhab, J.; Aslam, M. Isolation of flavonoid from the roots of citrus sinensis. Malays. J. Pharm. Sci. 2009, 7, 1–8. [Google Scholar]
- Cordenonsi, L.M.; Sponchiado, R.M.; Campanharo, S.C.; Garcia, C.V.; Raffin, R.P.; Schapoval, E.E.S. Study of flavo-noids present in Pomelo (Citrus máxima) by DSC, UV-VIS, IR, 1H and 13C NMR and MS. Drug Anal. Res. 2017, 1, 31–37. [Google Scholar] [CrossRef]
- Sudto, K.; Pornpakakul, S.; Wanichwecharungruang, S. An efficient method for the large scale isolation of naringin from pomelo (Citrus grandis) peel. Food Sci. Technol. 2009, 9, 1737–1742. [Google Scholar] [CrossRef]
- Kirtil, E.; Cikrikci, S.; McCarthy, M.J.; Oztop, M.H. Recent advances in time domain NMR & MRI sensors and their food applications. Curr. Opin. Food Sci. 2017, 17, 9–15. [Google Scholar]
- Hatzakis, E. Nuclear Magnetic Resonance (NMR) Spectroscopy in Food Science: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 18, 189–220. [Google Scholar] [CrossRef] [Green Version]
- Mannu, A.; Poddighe, M.; Garroni, S.; Malfatti, L. Application of IR and UV–VIS spectroscopies and multivariate analysis for the classification of waste vegetable oils. Resour. Conserv. Recycl. 2021, 178, 106088. [Google Scholar] [CrossRef]
- Mannu, A.; Karabagias, I.K.; Di Pietro, M.E.; Baldino, S.; Karabagias, V.K.; Badeka, A.V. 13C NMR-Based Chemical Fingerprint for the Varietal and Geographical Discrimination of Wines. Foods 2020, 9, 1040. [Google Scholar] [CrossRef]
- Brereton, R.G.; Lloyd, G.R. Partial least squares discriminant analysis: Taking the magic away. J. Chemom. 2014, 28, 213–225. [Google Scholar] [CrossRef]
- Ramadan, Z.; Jacobs, D.; Grigorov, M.; Kochhar, S. Metabolic profiling using principal component analysis, discriminant partial least squares, and genetic algorithms. Talanta 2006, 68, 1683–1691. [Google Scholar] [CrossRef]
- Westerhuis, J.A.; Hoefsloot, H.C.J.; Smith, S.; Vis, D.J.; Smilde, A.K.; van Velzen, E.J.J.; van Duijnhoven, J.P.M.; van Dorsten, F.A. Assessment of PLSDA cross validation. Metabolomics 2008, 4, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Kim, L.C.; Debra, R.; Christèle, R.G.; Philippe, B. A sparse PLS for variable selection when integrating omics data Statistical applications in genetics and molecular biology. Sat. Appli. Genet. Mol. 2008, 7, 1–32. [Google Scholar]
- Camacho, J.; Saccenti, E. Group-wise partial least square regression. J. Chemom. 2018, 32, e2964. [Google Scholar] [CrossRef] [Green Version]
- Colombani, C.; Croiseau, P.; Fritz, S.; Guilleaume, F.; Legarra, A.; Ducroq, V.; Robert-Granié, C. A comprasion of partial least squares (PLS) and sparse PLS regression in genomic selection in French dairy cattle. J. Dairy Sci. 2012, 95, 2120–2131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erik, A. Sparse models by iteratively reweighted feature scaling: A framework for wavelength and sample selection. J. Chemom. 2013, 27, 50–62. [Google Scholar]
- Jiménez-Carvelo, A.M.; Martín-Torres, S.; Ortega-Gavilán, F.; Camacho, J. PLS-DA vs. sparse PLS-DA in food tracea-bility. A case study: Authentication of avocado samples. Talanta 2021, 224, 121904. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, T.; Liland, K.H.; Snipen, L.; Saebo, S. A review of variable selection methods in partial least squares regression. Chemometr. Intell. Lab. Syst. 2012, 118, 62–69. [Google Scholar] [CrossRef]
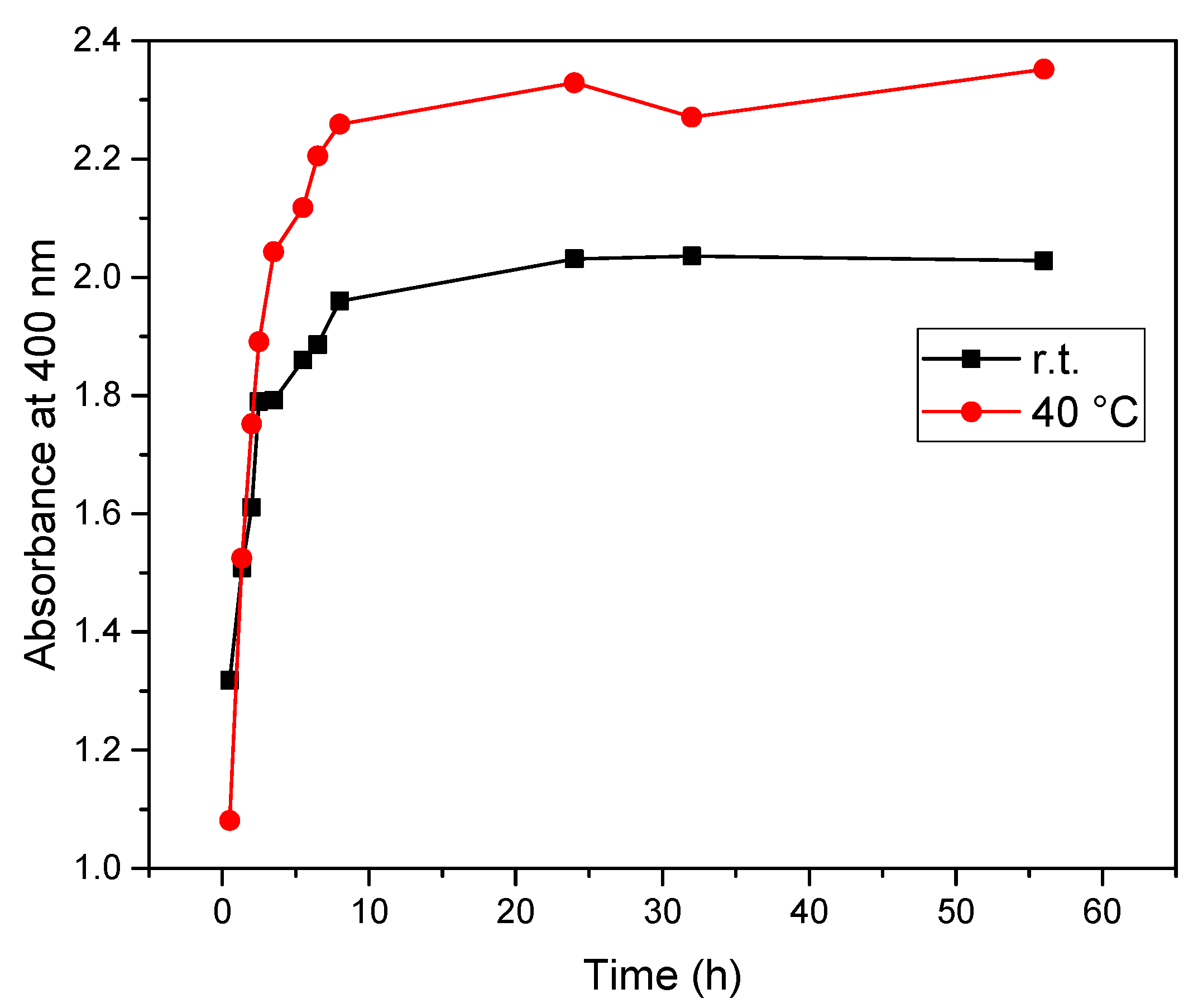
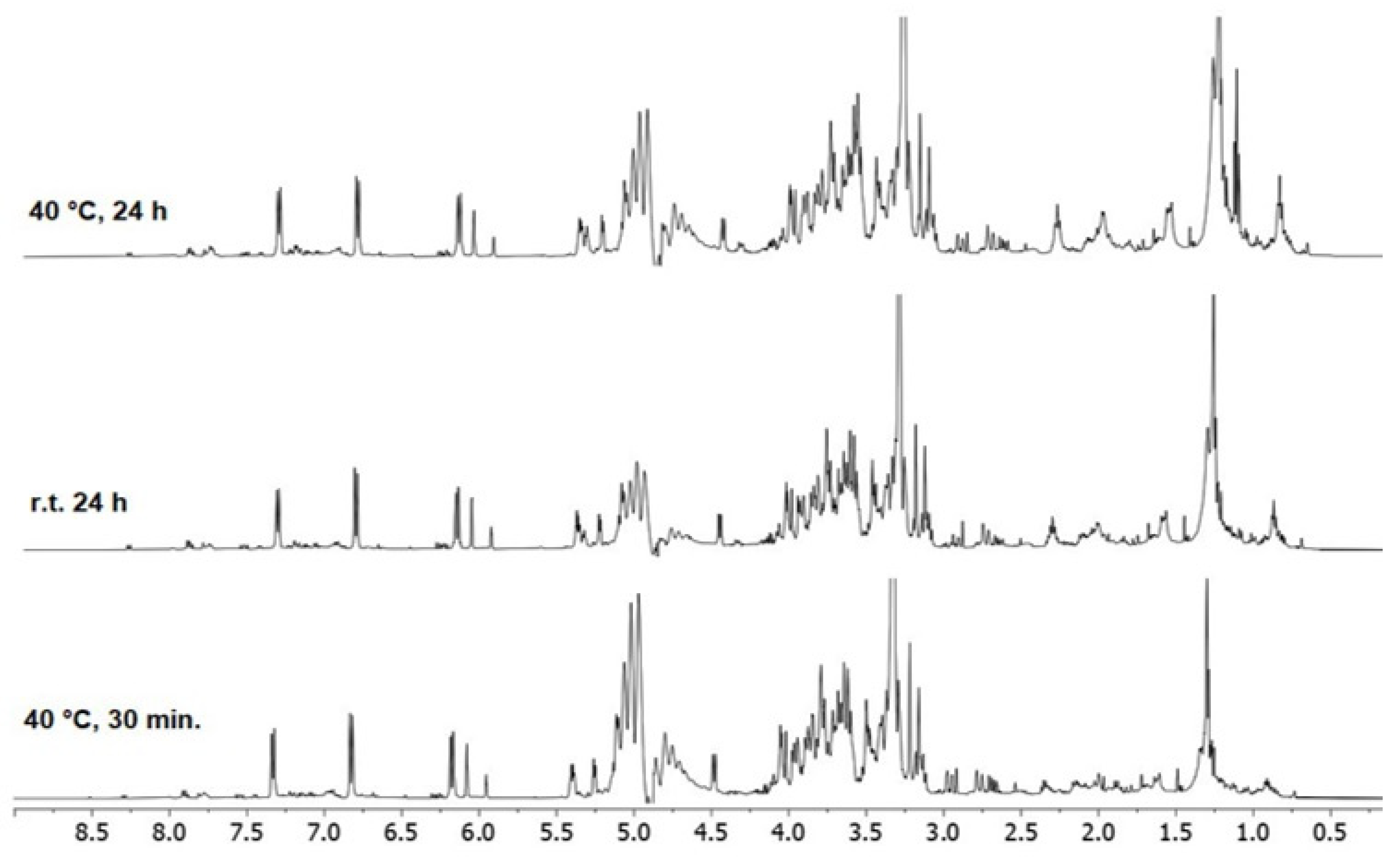
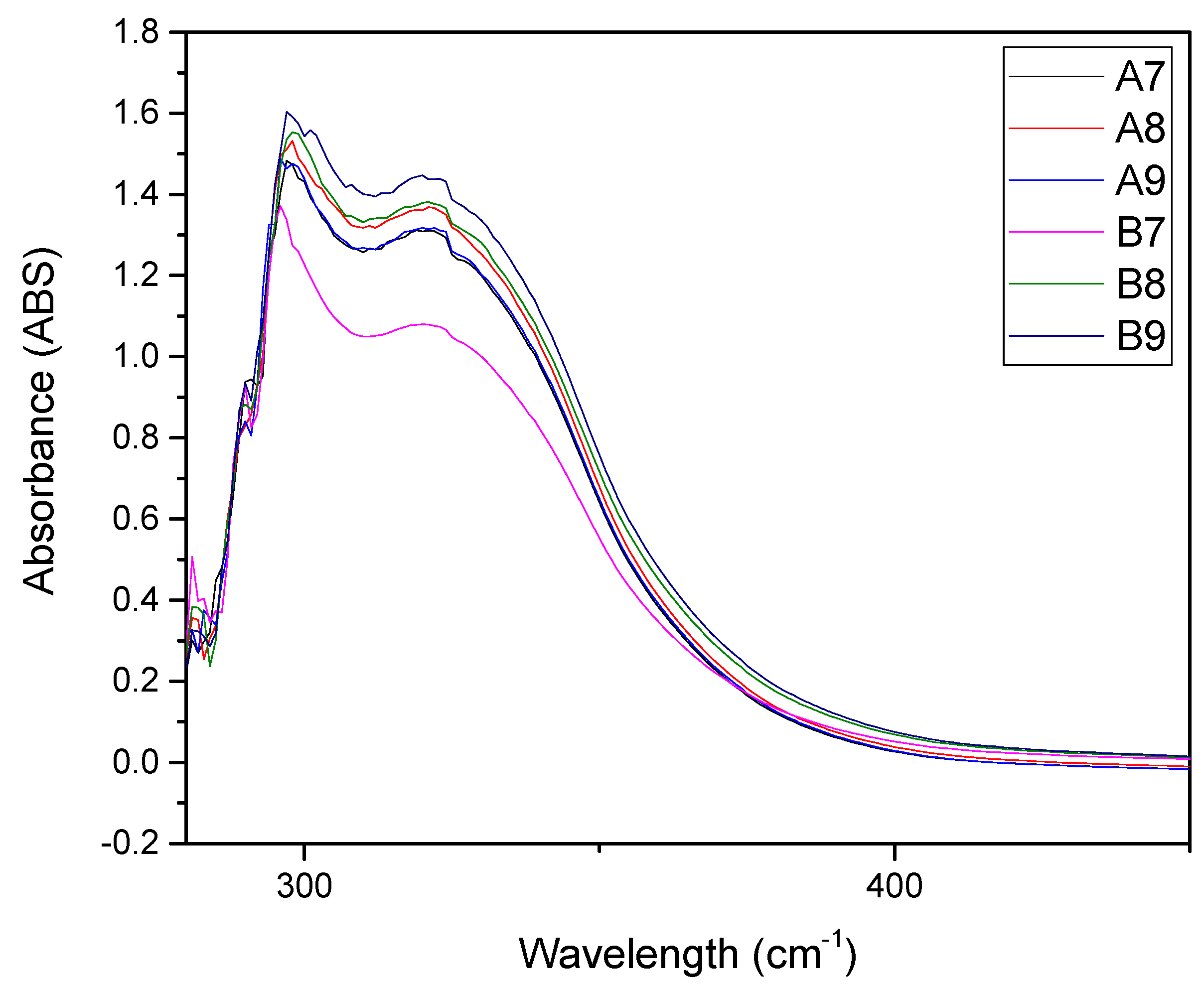
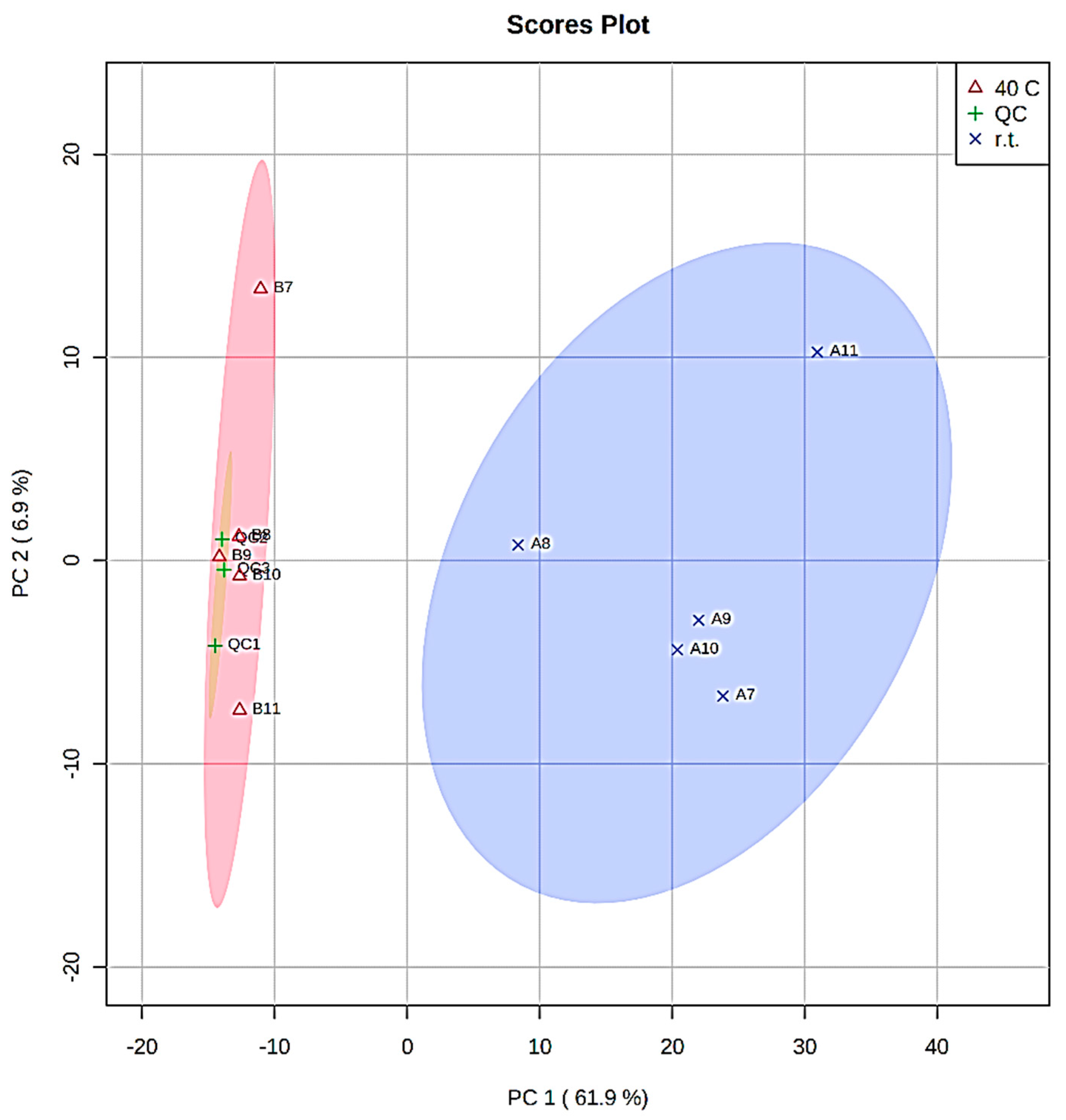

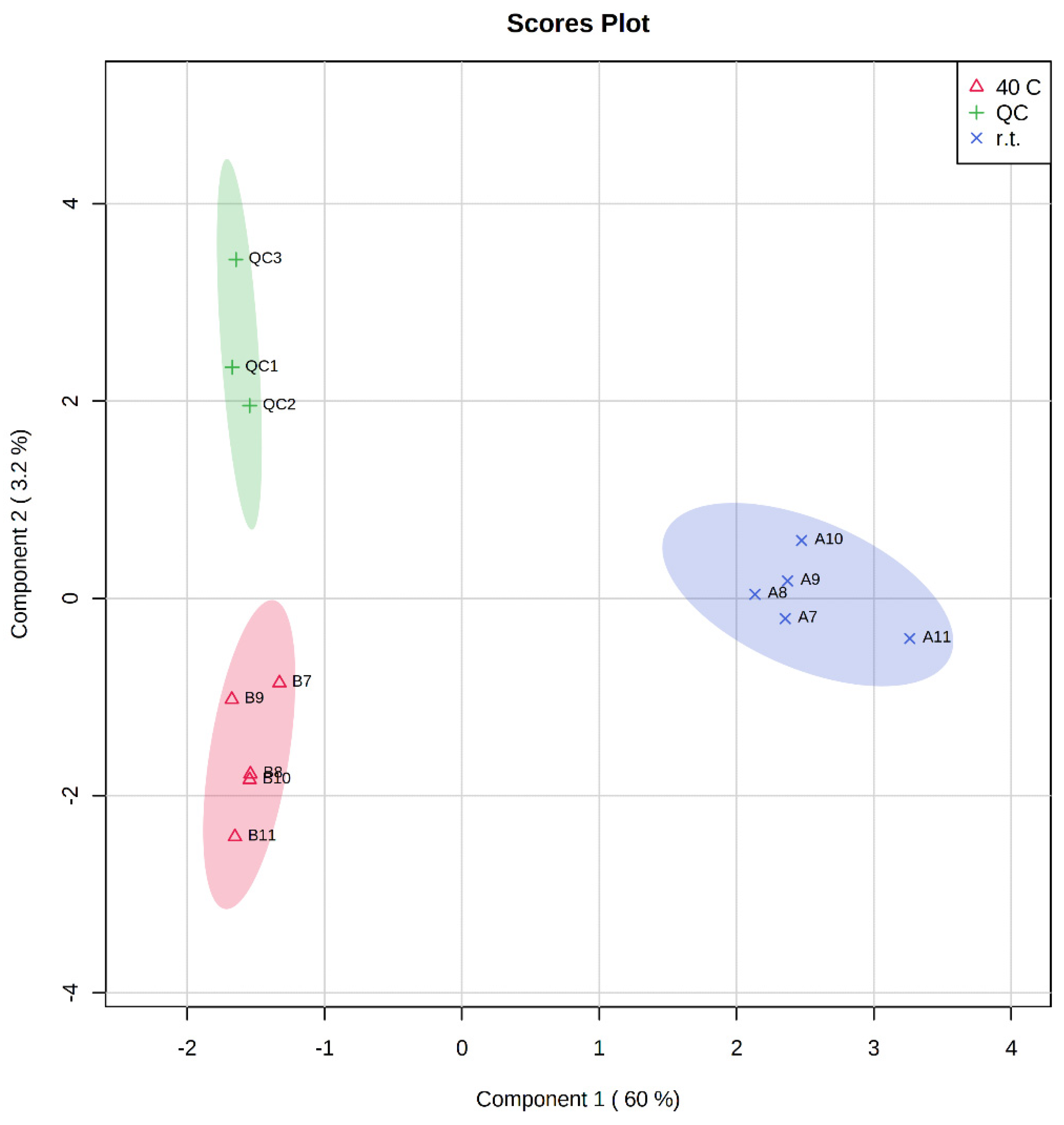

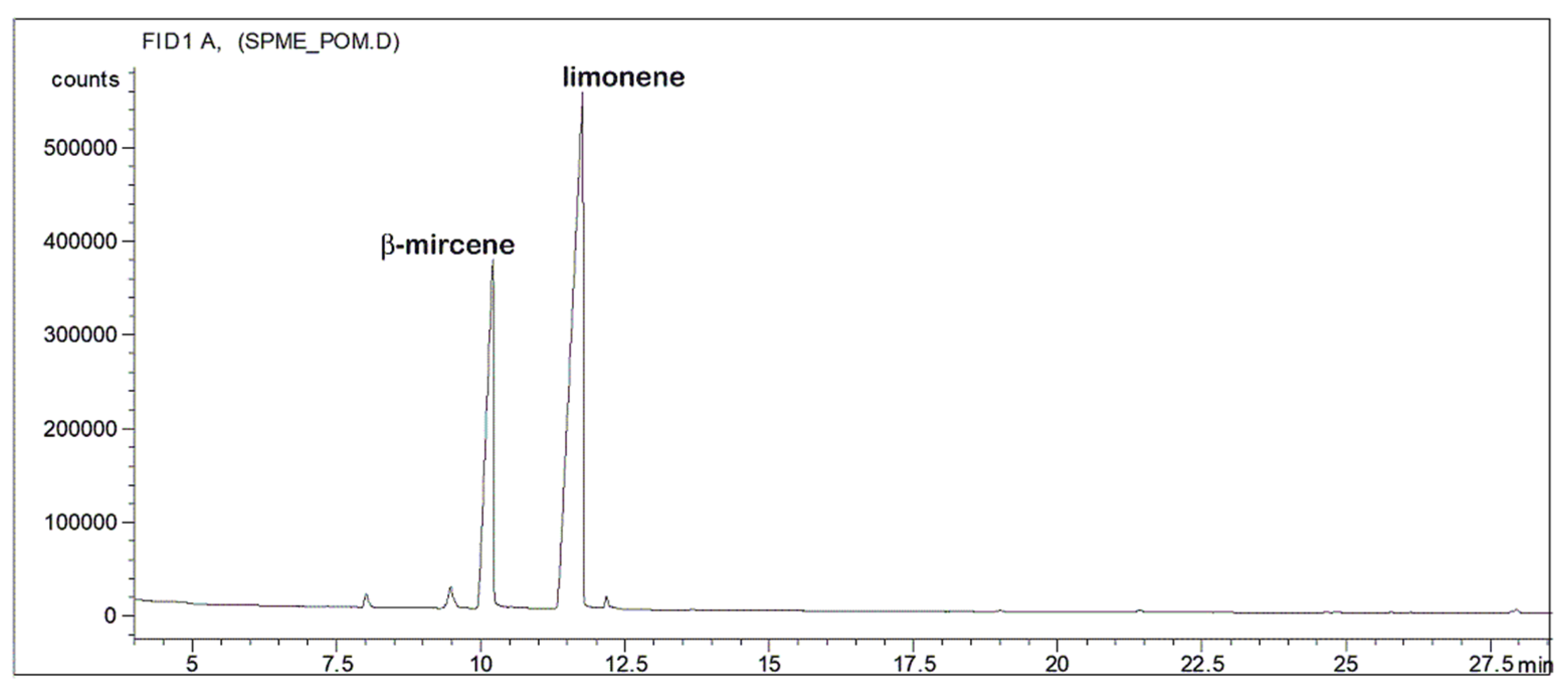
| Extract A (r.t.) | Extract B (40 °C) | Time (h) |
|---|---|---|
| A1 | B1 | 0.5 |
| A2 | B2 | 1.3 |
| A3 | B3 | 2.0 |
| A4 | B4 | 2.5 |
| A5 | B5 | 3.5 |
| A6 | B6 | 5.5 |
| A7 | B7 | 6.5 |
| A8 | B8 | 8 |
| A9 | B9 | 24 |
| A10 | B10 | 32 |
| A11 | B11 | 56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petretto, G.L.; Di Pietro, M.E.; Piroddi, M.; Pintore, G.; Mannu, A. Classification of Pummelo (Citrus grandis) Extracts through UV-VIS-Based Chemical Fingerprint. Beverages 2022, 8, 34. https://doi.org/10.3390/beverages8020034
Petretto GL, Di Pietro ME, Piroddi M, Pintore G, Mannu A. Classification of Pummelo (Citrus grandis) Extracts through UV-VIS-Based Chemical Fingerprint. Beverages. 2022; 8(2):34. https://doi.org/10.3390/beverages8020034
Chicago/Turabian StylePetretto, Giacomo Luigi, Maria Enrica Di Pietro, Marzia Piroddi, Giorgio Pintore, and Alberto Mannu. 2022. "Classification of Pummelo (Citrus grandis) Extracts through UV-VIS-Based Chemical Fingerprint" Beverages 8, no. 2: 34. https://doi.org/10.3390/beverages8020034
APA StylePetretto, G. L., Di Pietro, M. E., Piroddi, M., Pintore, G., & Mannu, A. (2022). Classification of Pummelo (Citrus grandis) Extracts through UV-VIS-Based Chemical Fingerprint. Beverages, 8(2), 34. https://doi.org/10.3390/beverages8020034









