1. Introduction
Fruits spirits are consumed in several countries around the world. The pre-tax turnover for the distillation industry in the EU is estimated to be 26.5 billion euros [
1]. In Germany, there are 14,671 small- and medium-sized (SME) distilleries that in the year 2018 produced 3.8 million L ethanol [
2]. Both distillers and consumers would like to achieve products with consistently high quality in a reproducible manner. The quality of distillates depends on several aspects such as fruit quality, mashing, fermentation, distillation parameters and aging. The Federation of German Food and Drink Industries considers food and drink quality to be the most important factor for consumer decisions [
3]. They also state that through innovations in quality improvement, food manufacturers have been able to increase product sales by 2.2 percent annually over the past 12 years. Investments to increase the product quality in the European spirits industries, therefore, have the potential to increase sales volumes by 583 million euros annually. Overall, the most important quality marker for distilled beverages is the aroma, which is defined by the volatile compound composition of the product [
4]. We know that the distillation process itself has a major impact on the volatile compound composition of the product, and therefore, its quality [
5,
6].
In general, fruit spirit distillation processes are based on physical principles of heat and mass transfers, which are decisive for the separation of desired and undesired aroma compounds [
7,
8]. The process principles are similar for each batch distillation process and can be categorized into consecutive process sequences. Initially, the process starts with heating a fermented fruit mash in the reboiler of the still. Due to the constant thermal energy input, a multicomponent vapor, which contains ethanol, water and various other volatile compounds (congeners) evaporates to the top of the column [
9]. The evaporation rate can be controlled by the thermal energy input [
10,
11]. These vapors condense when they come in contact with cooler surface areas, which causes a liquid reflux that flows downward again [
12]. This evaporation/condensation process is crucial for the separation of substances with different volatilities [
7,
8,
9,
12,
13]. Substances with low boiling points become enriched in the vapor phase while compounds with lower boiling points concentrate in the liquid phase. A recent study investigating the best distillation technique for improving the quality of apricot brandies also concluded that the right balance between heating parameters, which determine evaporation rates, and reflux conditions plays a decisive role in improving the product quality [
14]. This indicates that it is important to control and monitor both process parameters in fruit spirit distillation processes.
During the distillation run, ethanol and water are the two main components that support the carryover of other volatile compounds. This carryover is influenced by the relative volatility of each compound in respect to the present ethanol and water concentrations [
7,
9,
15]. The concentration of ethanol in ethanol-water mixtures can be determined by measuring the temperature under boiling conditions [
16]. The monitoring of temperatures in the distillation device is, therefore, additionally important to be able to estimate and control the carryover of volatile compounds in regard to the apparent ethanol concentration.
In chemical industries, distillation accounts for 90–95% of all separations processes [
17]. The separation of multicomponent mixtures is known to be challenging but includes some of the world’s largest and most profitable separations, such as crude oil fractionation, hydrocarbon separation from steam cracking, and natural gas-liquid separation. To separate a multicomponent mixture that contains
n components into
n product streams, each enriched in one of the components, a sequence of distillation columns known as a distillation configuration is required [
18]. Such distillation setups are equipped with extensive process control and monitoring that enable the regulation of evaporation rates and reflux streams [
12,
18]. Similar control strategies could be transferred to fruit spirit distillation processes to be able to investigate distillation separation processes in more detail. Bastidas et al. [
19] transferred thermodynamic models and unit operation data from a fuel production plant to evaluate optimized wine distillation processes by simulation models. They stated that deep knowledge and understanding of the fuel ethanol process allows for identifying the main operating conditions of the process, to keep the product flowrate and quality within the desired values.
Industrial fruit spirit production is mainly performed with two different distillation systems, the traditional Charentais alembic stills and modern batch distillation columns [
9]. In alembics, internal reflux is caused by condensation in the head and swan neck, which mainly depends on the external temperature. This reflux can only be modified by regulation of the thermal energy input to the boiler, and therefore, represents a very limited system in terms of control and modification during the distillation process. On the other hand, modern batch distillation columns are equipped with an additional internal partial condenser, which can be controlled and operated independently. This allows for rather simple control of the internal reflux by adjusting the cooling water flow rate of the partial condenser [
20,
21]. The condensate can be collected on the trays and the vapor from the lower tray must pass through the holes, and therefore, through the reflux condensate, causing rectification and thus more efficient separation of the different volatile components [
13].
The dephlegmator works as a thermal barrier that provides resistance to the distillate vapors, enabling them to pass on toward the product cooler. The mass transfer of vapors exiting the distillation column is mainly influenced by the cooling power of the dephlegmator and the thermal energy input to the distillation column [
14,
22]. This indicates the importance of regulating and monitoring both parameters for controlling mass transfer rates.
Earlier studies focused on product differences between both distillation systems [
20,
21,
23]. It was shown that the internal reflux rates and the thermal energy input are two important parameters that control the volatile concentrations, and in turn, aroma attributes in the final product [
5,
23,
24,
25,
26]. Despite their importance, very limited information on both parameters is available in scientific publications dealing with fruit spirit distillation [
27]. García-Llobodanin et al. [
20] emphasized the importance of both parameters and considered that “they are severely affected by heat loss, ambient temperature, cooling water flow rates and temperatures.” They concluded that there is a prominent lack of control of these operation variables that leads to a lack of reproducibility of fruit spirit distillation processes, which then significantly affects the volatile composition of the produced spirits.
We upgraded a 120-L batch distillation column with digitized instrumentation and a control technique to gain control of the crucial process parameters of the thermal energy input and reflux rates and to monitor six distillation parameters. We hypothesized that reproducible fruit spirit distillation processes and products can largely be achieved when the two crucial process parameters are controlled and monitored. This study aimed to identify the magnitude of reproducibility errors for (i) six distillation process parameters and (ii) 13 volatile compound compositions in the product in repeatedly performed distillations, when two process parameters were controlled during the distillation process.
2. Materials and Methods
2.1. Batch Distillation System
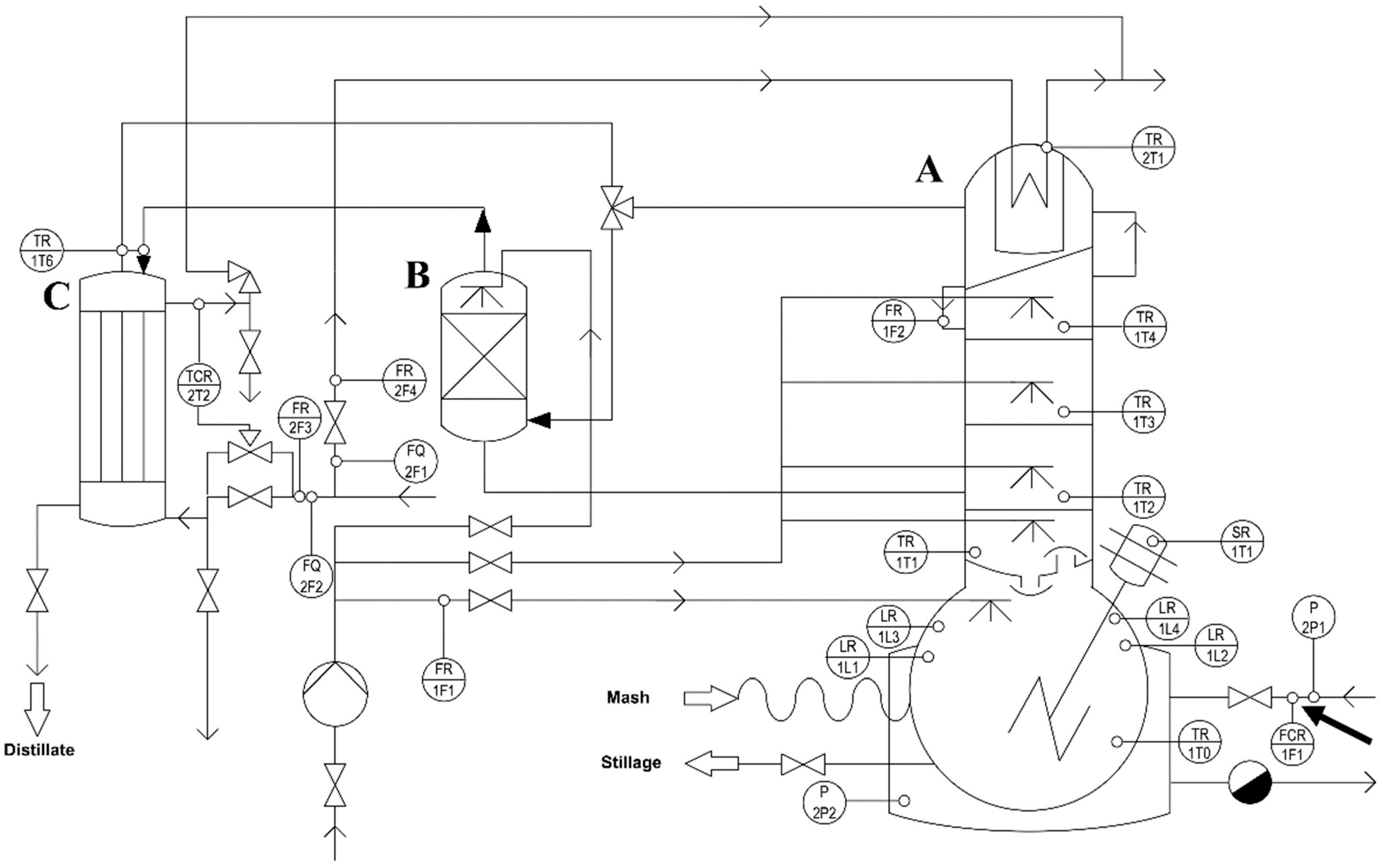
This study was performed with a steam-heated copper batch still (Carl GmbH, Eislingen, Germany) equipped with a 120-L reboiler, three sieve trays, rectification column with a partial condenser, a separate reactive copper packing and a product cooler (
Figure 1). The system was upgraded with digitized technical sensors to control and monitor the thermal energy input, temperatures at four different positions, internal reflux induced by the partial condenser and product volume flow rate. All controllers and sensors were calibrated by the batch still producer (R
2 ≥ 0.95). The crucial process parameter, the thermal energy input, was controlled by an electro-pneumatic valve positioner (SP400, Spirax-Sarco Engineering plc, Cheltenham, UK). The crucial process parameter, the internal reflux rate, was monitored via a Coriolis flowmeter (Emerson micro motion H series, St. Louis, MO, USA) and indirectly controlled (SM6120 flowmeter, lfm electronic GmbH, Essen, Germany) by adjusting the cooling water flow rate to the partial condenser. For this investigation, the cooling water flow rate to the partial condenser was kept constant at 2 L/min. Additional process monitoring was performed by four PT100 temperature sensors and an additional Coriolis flowmeter (Emerson micro motion H series, St. Louis, MO, USA) to determine the product volume flow rates. The cooling water flow rate for the product cooler was constantly set to 7 L/min. All control and monitor data were logged every 10 sec and stored via a manufactured automation system (DPC500, Carl GmbH, Eislingen, Germany). After each distillation run, data were transferred to Excel (Microsoft Office 2010, Microsoft Corporation, Redmond, DC, USA). To avoid presenting illegible diagrams on distillation process parameters, we averaged the mean values and standard deviations of six consecutive logging points and present these as one data point (
Figure 2,
Figure 3 and
Figure 4). The distillation system contained additional technical sensors that were not considered in this study. More specifics of the digitized distillation system are given in Heller et al. [
11].
2.2. Experimental Setup
Eight different distillation profiles (A–H) were generated in duplicate (
n = 16) while the thermal energy input was adjusted (
Table 1). Every distillation run of the duplicates was performed with 100 L fermented fruit mash of the same batch. The distillation profiles started with an initial thermal energy input of 450 W/L. When the mash temperature reached 90 °C, the thermal energy input was reduced to a defined value (Q1). When the third tray of the rectification column reached a temperature of 75 °C, the thermal energy input was adapted again to a different value (Q2). From this point on, the thermal energy input constantly increased by a defined value (Q3) until the product flow began to run. Subsequently, the DPC500 automation system regulated the thermal energy input to hold a constant product flow rate of 10 ± 0.5 L/h. These different distillation profiles were generated to simulate different distillation conditions, such as fast distillation or different internal reflux intensities, induced by variations in the thermal energy input. Means and relative standard deviations (RSDs) were calculated from two distillation replicates.
2.3. Mash Preparation and Fermentation
Red wine was ordered from research facility Plant Product Quality (University of Hohenheim, Stuttgart, Germany). Williams pears (
Pyrus communis L.) were purchased from Kaiser Destillerie-Obstweinkellerei (Salach, Germany). Plums (
Prunus domestica subsp.
domestica L.) originated from agricultural farm Heidfeldhof (University of Hohenheim, Stuttgart, Germany). Our investigation into the substrate characteristics (
Table 2) included the determination of total solids and ash, as described in VDLUFA [
28] and ICC [
29], respectively. Total carbohydrates were quantified via the phenol-sulfuric acid method [
30]. Total protein concentrations were analyzed using the method by Bradford [
31]. Total phenol determination was performed as described in Lim et al. [
32]. pH and conductivity were measured via a multimeter (HQ40D, Hach, Loveland, CO, USA).
For mash preparation, Williams pears were water cleaned, fruit mill shredded (Helmut Rink GmbH, Amtzell, Germany) and transferred to a 1000-L stainless steel tank. The pH was lowered to 3.1 by phosphoric and lactic acid addition (product no. 5862, Schliessmann, Schwäbisch Hall, Germany). Additionally, 10 mL/hL pectin lyase (IUB 4.2.2.10, Schliessmann, Schwäbisch Hall, Germany) was applied to the mash to ensure sufficient substrate liquefaction. Then, 20 g/hL selected yeast (Saccharomyces cerevisiae) strains (Aroma plus, Schliessmann, Schwäbisch Hall, Germany) were added to start mash fermentation.
For plum mash preparation, all plums were thoroughly mixed with a mixing drill (product no. 6681, Schliessmann, Schwäbisch Hall, Germany). The mixing drill was used to disintegrate the fruits and prevent excessive seed abrasion or destruction. The disintegrated fruits were subsequently pumped into a 1000-L stainless steel tank, with the pH adjusted to 3 (product no. 5862, Schliessmann, Schwäbisch Hall, Germany), and inoculated with 0.15 g/L selected yeast strains (Aroma Plus, product no. 5828, Schliessmann, Schwäbisch Hall, Germany). To this, 5 mL/hL pectin lyase enzyme (IUB 4.2.2.10, Schliessmann, Schwäbisch Hall, Germany) was also added to enhance liquefaction of the mash. All mashes were thoroughly mixed and the pH readjusted after 24 h. Similar mash preparation procedures were described by Liebminger et al. [
22]. Mash fermentations were performed for 21 days at 20 °C room temperature.
2.4. Product Analysis
The produced distillates were collected in 20 defined fractions for subsequent analysis. Sampling was performed every 100 mL for the first ten fractions, while fractions 11–15 were sampled every 200 mL and fractions 16–20 were sampled every 1000 mL. Additional distillate was collected as an undefined final fraction and not considered in this study. Williams pear mashes resulted in reduced product yields and were only sampled until fraction 19.
A headspace gas chromatograph (GC-2010 Plus, Shimadzu Scientific Instruments, Kyoto, Japan) equipped with a flame ionization detector (FID) and a Rtx-Volatiles column (Restek Corp., Bellefonte, PA, USA) was used for volatile compound analysis in distillate fractions. All samples were adjusted to 40% (
v/
v) ethanol before volatile compound quantification. The product fractions were quantified for typical fruit spirit volatile compounds [
6,
23,
33,
34,
35] ethanol, methanol, acetaldehyde, 1-propanol, 2-butanol, ethyl acetate, isobutanol, isoamyl alcohol, 2-methyl-1-butanol, ethyl 2-methylbutanoate, 1-hexanol, trans-2-hexen-1-ol and hexyl acetate. As GC-FID is not a technique for absolute quantification [
36], it is mandatory to perform calibration runs with standard solutions of fixed concentrations. For the calibration runs, all analyzed substances were ordered from Merck KGaA (Darmstadt, Germany) in standard analytical grades with a purity of ≥99.9% for ethanol, methanol and 1-propanol, ≥99.5% for acetaldehyde, 2-butanol and ethyl acetate, ≥99% for isobutanol, 2-methyl-1-butanol, ethyl 2-methylbutanoate, 1-hexanol and hexyl acetate, ≥98% for isoamyl alcohol and ≥97% for trans-2-hexen-1-ol. Each standard substance was diluted with distilled water to gain five defined concentrations. The five standard concentrations were prepared in a range that covered the concentrations at which the analytes typically appear in fruit spirits. The peak area of all five standard concentrations was determined in chromatograms, which can be used to establish a linear equation between two parameters. The fit of both parameters to the linear equation significantly correlated with an accuracy of R
2 ≥ 0.99 for each analyzed volatile compound. This linear equation, gained by five-point calibration, was thus used to quantify the concentration of an analyte in the fruit spirit sample by determining its peak area in the chromatogram.
2.5. Data Analysis
To identify whether fruit spirit distillations can be controlled by two variables, we evaluated the accuracy of replicated distillations by determining deviations between two replicated distillation runs in terms of distillation process parameters and the volatile compound composition of the product. The basis of data analysis in this study was the evaluation of deviations between duplicate data points. Median RSD analysis is often used to investigate the precision of analytical replicate data or process variability [
37,
38,
39]. The calculation of median RSD values reflects the magnitude of reproducibility errors. To evaluate process replicability, the data of six process parameters (mash temperature, temperatures at three trays, internal reflux rate, product flow rate), which were logged every 10 s, were compared between the replicate distillation runs. A maximum of 36 data points was thus comparable for every minute of a distillation run. Data on the internal reflux and product flow were only considered after they started to run (flow rates > 0 L/h). To evaluate the product replicability in terms of their volatile compound composition, equally sampled product fractions were analyzed for 13 volatile concentrations. One distillation run with 20 sampled fractions thus allowed a comparison of a maximum of 260 data points.
Initially, the relative standard deviation (RSD) was calculated for every set of two duplicate data points. In a second step, we calculated the median RSD, which considered all RSD data evaluated within a single parameter, for instance, one distillation parameter (e.g., mash temperature) or the concentration of one volatile compound (e.g., 1-propanol).
3. Results & Discussion
3.1. Distillation Process Parameters
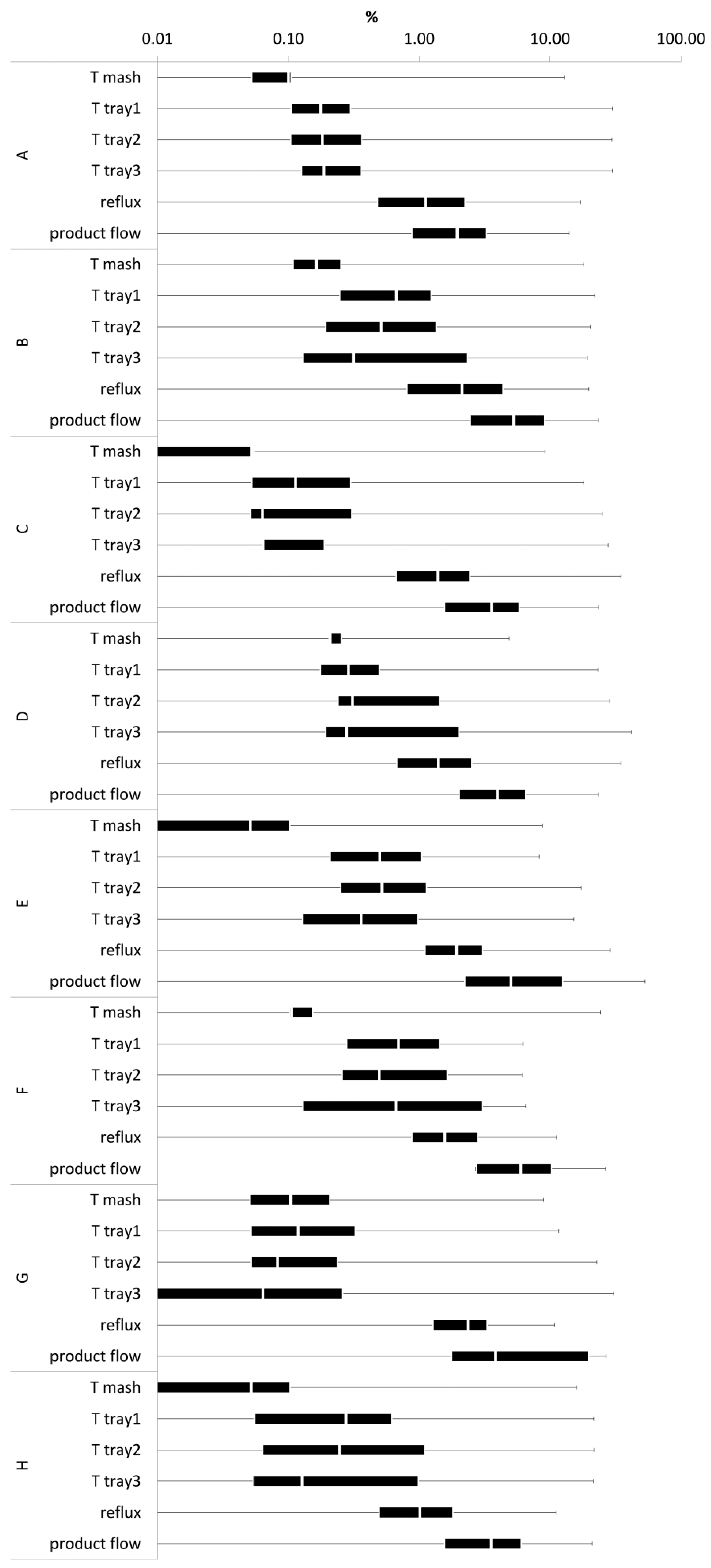
The control of thermal energy input and cooling water flow rate to the partial condenser resulted in variations of the six monitored distillation parameters. Since the cooling water flow rate was set to a constant rate, the main impact on process variations was induced by altering the thermal energy input. All distillation processes showed that thermal energy regulation had a decisive influence on the development of process temperatures.
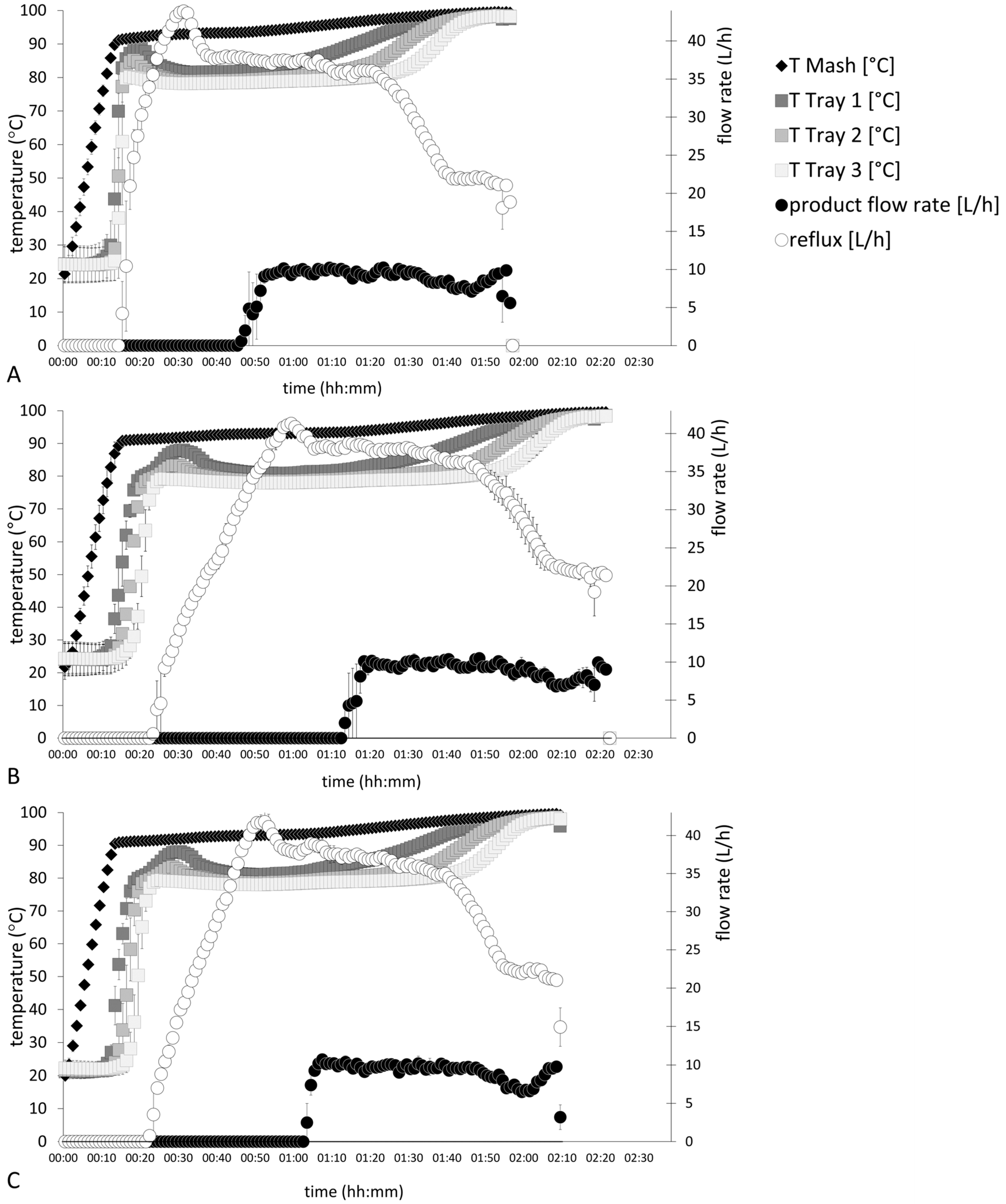
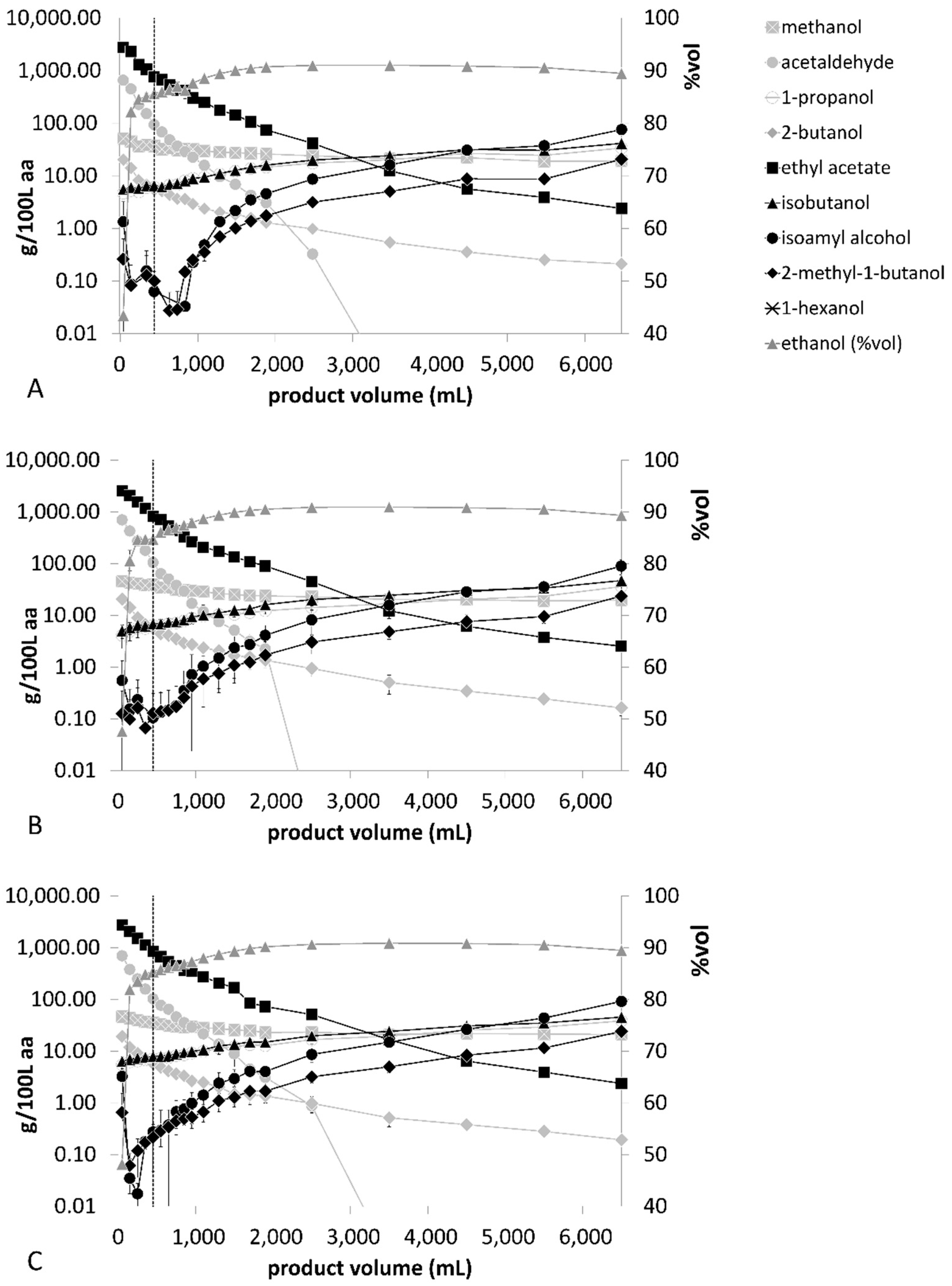
Figure 2 shows distillation parameters of processes A–C of the fermented wine mashes. Different heating profiles clearly influenced the total distillation time, which was 116 ± 2 min in distillation process A. Due to their lower thermal energy inputs, distillation profiles B and C were finalized after 141 ± 3 min and 128 ± 1 min, respectively. Processes B and C showed a slower increase of internal reflux volumes but reached a maximum value of 42 ± 1 L/h within all distillation profiles.
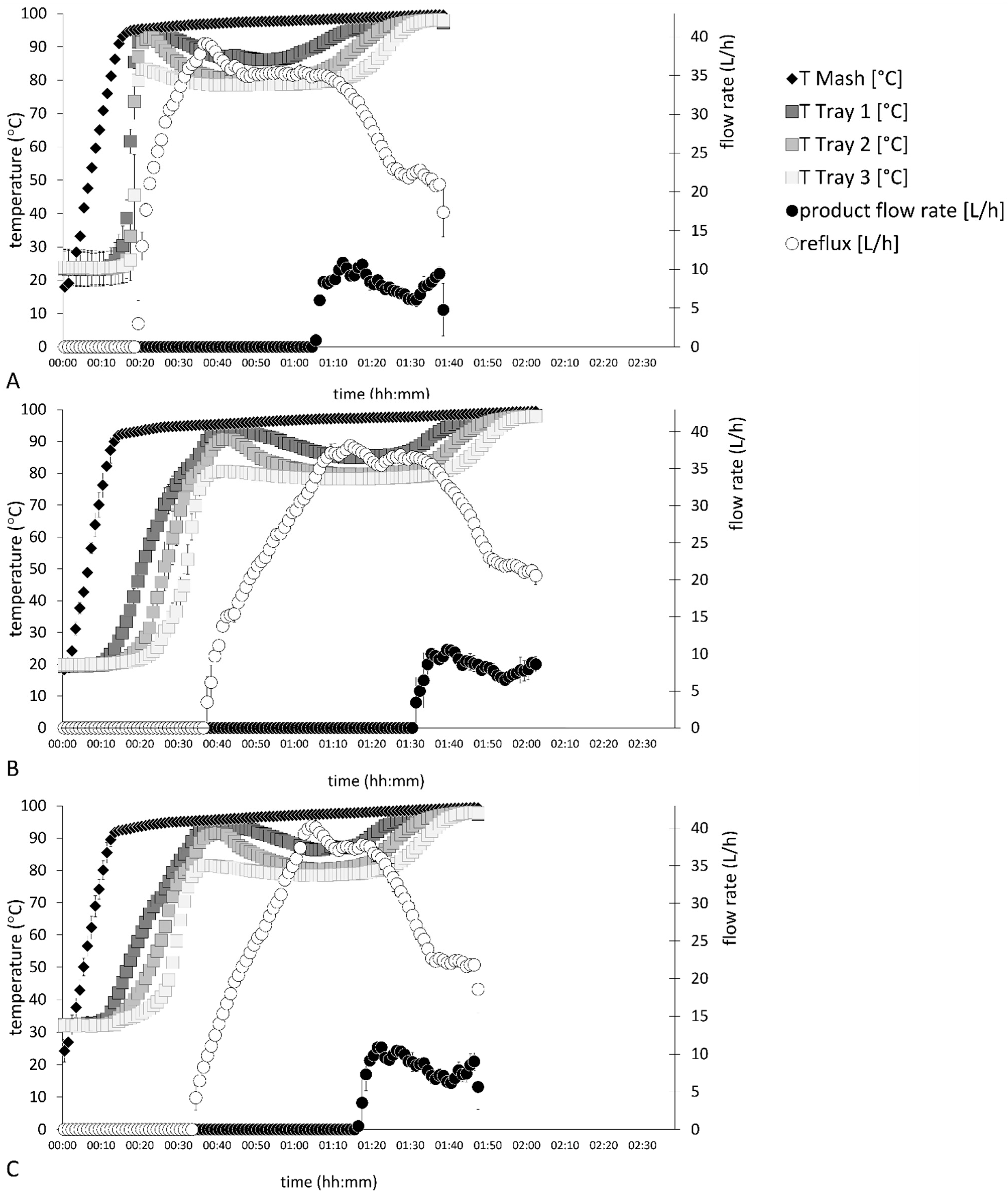
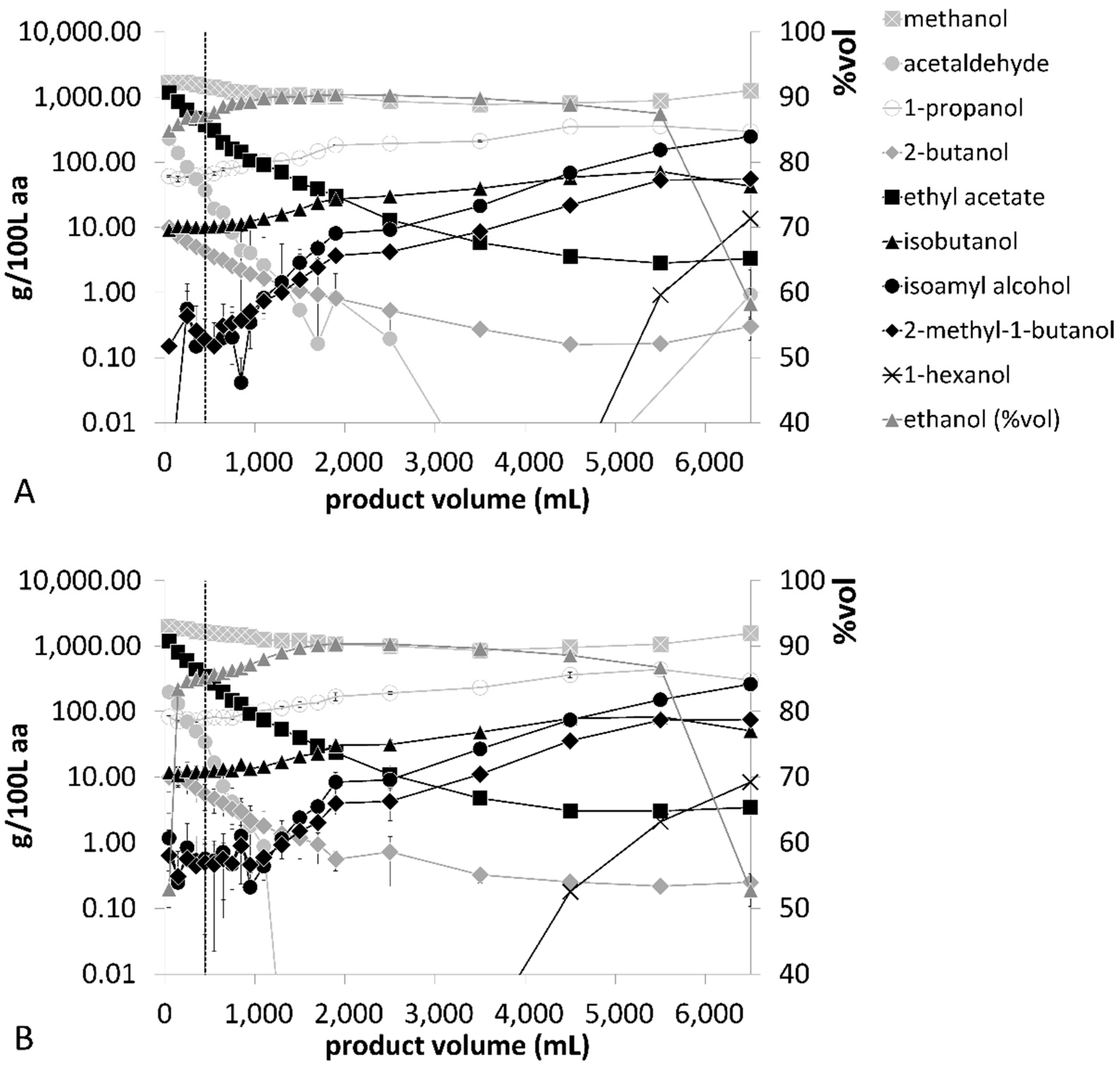
Distillation processes D–F in
Figure 3 show the process data of fermented Williams pear mashes. The fastest distillation run was finalized after 98 ± 1 min in distillation profile D, which also had the highest initial thermal energy input. While product flow started after 65 ± 0 min in distillation profile D, this was shifted to 91 ± 1 min and 76 ± 0 min in distillation profiles E and F, respectively. Different thermal energy inputs during the distillation profiles also affected the internal reflux rates of the distillation processes. The highest reflux rates were 41 ± 0 L/h after 64 min in distillation profile F, while distillation profiles D and E showed a maximum value of 39 ± 0 L/h after 37 ± 1 min and 74 ± 1 min, respectively. Replicates of each distillation profile showed the highest standard deviations during the heating phase until the third tray reached thermal equilibrium.
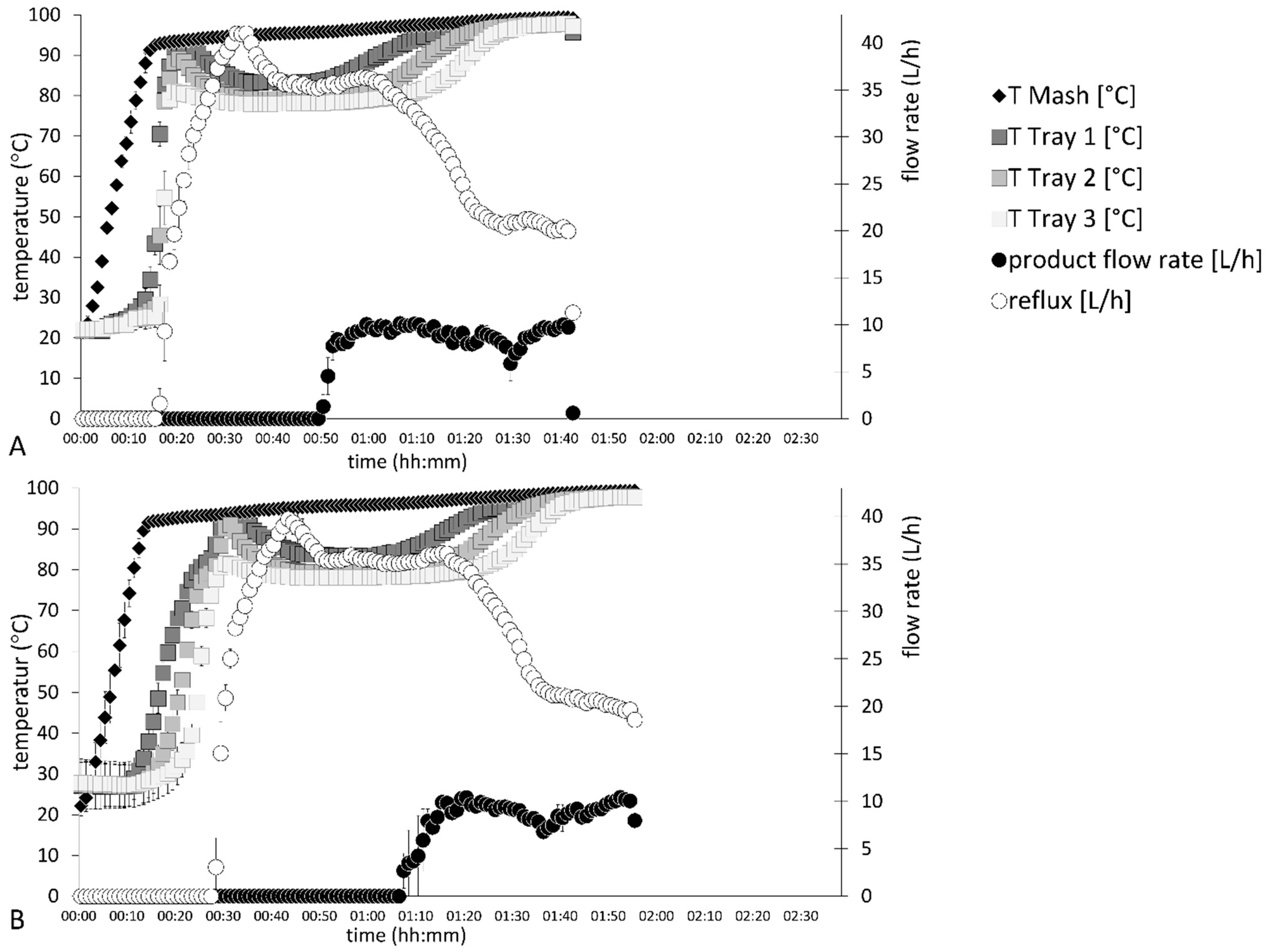
Distillation processes G–H in
Figure 4 show the process data of fermented plum mashes. Different thermal energy inputs resulted in a 13 ± 0 min longer distillation run of distillation profile H. A timely shift could also be determined during the distillation run. For instance, the maximum temperature of 92 ± 0 °C on tray one was reached 10 ± 0 min later in distillation process H. This also induced a 13-min delay until the initial reflux flow rates were detected. Replicates of each distillation profile showed the highest standard deviations during the heating phase and at the beginning of product volume flow.
All distillation processes commonly showed that with the start of product flow streams, the internal reflux flow rates reduced over time. This is since initially, all the vapor mass condensed on the cool surface of the partial condenser. The partial condenser is heated up during the process, until a stream of vapor passes the partial condenser, which is condensed in the product cooler and the product flow starts. As far as we know, no other study on fruit spirit distillation processes has directly evaluated reflux flow rates. García-Llobodanin et al. [
20] showed the energy demand for cooling the partial condenser without providing information on reflux flow rate quantities. Balcerek et al. [
24] evaluated the effects of double- and single-stage fruit mash distillations without defining the magnitude of energy input or reflux ratio. They also stated they varied the reflux rate by adjusting the cooling water flow rate without giving more specifics. Spaho et al. [
23] compared alembic and column distillation techniques without providing any specifics on the thermal energy input or reflux rates for pot or column still distillations. They mentioned, however, distillate flow rates of 5.3 L/h, which provides information on the mass stream that exited the distillation column. The product flow rates of our study showed average values of 8.5 ± 1.8 L/h in distillation profiles A–H, which indicated that the product flow rate differs by simply adjusting the thermal energy input, without changing the cool water flow of the partial condenser. As no more specifics were given in the study of Spaho et al. [
23], it is not clear whether reduced energy inputs or increased reflux rates led to lower distillate flow rates compared to our study.
Liebminger et al. [
22] evaluated column distillation processes for three fruit mashes. This study is interesting as it provided insights into the effects of limited process control. The data indicated strong variations in the temperature profiles between the three distillation processes. Despite using a similar distillation setup, the temperature sensor measured values ranging from 79 °C to 92 °C 20 min after beginning distillate collection in the three distillations. Based on the vapor phase diagram of ethanol-water mixtures [
7], this indicated deviating ethanol concentrations of 53% to 86% (
w/
w) in the vapor stream despite utilizing fruit mashes (plum, pear) with similar ethanol concentrations. This shows that the distillation process was not able to perform mass and heat transfer with similar efficiency, which affected the physical separation of ethanol and water. In addition, the collected tails fraction was increased by 82%, which suggests that the separation of volatile compounds was also affected by the different relative volatility of the apparent ethanol or water concentration [
7,
9,
15]. Since the thermal energy input was not monitored, the authors assumed that the energy input of the oil-heating system varied, which possibly led to uncontrolled heating patterns. The temperature sensor was placed at the exit pipe close to the product cooler, and additional temperature control was not mentioned in this study. This indicated that the energy input could not be controlled or monitored by temperature sensors positioned within the distillation column. Different dephlegmator cooling setups were also chosen for the three distillation runs. The study did not describe the amount of cooling energy or the quantities of internal reflux rates. Since both parameters, thermal energy input and reflux rates, could not be controlled or monitored, this resulted in largely deviating product flow rates, ranging from 12.1 L/h to 23.3 L/h. The lack of process regulation and monitoring technique led an uncontrolled vapor mass transfer to exit the distillation column.
Several other studies also investigated the volatile compound composition of the distilled product without providing specifics for the crucial distillation process parameters that define the physical principles of heat and mass transfer [
40,
41,
42,
43]. None of these studies had the potential to provide data that allow the evaluation of the physical separation principles on behalf of mass or heat transfer rates during the distillation process. This makes it difficult to evaluate beneficial or disadvantageous distillation process conditions.
Our study showed additional process data to be able to evaluate separation processes on behalf of physical principles. In terms of the dependency of distillation time, the distillation profiles A–H provided a minimum of 2927 comparable data points for distillation profile G and a maximum of 4358 for profile B. In total, the acquisition of process data from six process parameters within eight duplicate distillation profiles enabled the comparison of 28,600 duplicate data points. The median RSD (
Figure 5) was determined at a range of <0.1–7%. This indicated that enabling control over two distillation parameters allows largely reproducible distillation processes with a replication error ≤7%.
3.2. Volatile Compound Composition of Product Fractions
The volatile compound compositions of wine, pear and plum distillate fractions are presented in
Figure 6,
Figure 7 and
Figure 8. The figures also indicate the cut of the heads-to-hearts fraction, which was consistently assessed at 450 mL. All distillation profiles commonly showed that the concentrations of acetaldehyde, ethyl acetate and 2-butanol significantly decreased during the shift from heads to hearts fractions. Acetaldehyde and ethyl acetate are known to implement strong pungent characteristics and negatively influence product quality [
44]. Due to their low boiling point, both substances are typically enriched in the hearts fraction of fruit spirit distillation processes [
40,
45]. In addition, this study indicated that 2-butanol is also enriched in the hearts fraction. Similar behavior of 2-butanol was described by Spaho et al. [
6]. This might be beneficial for the final product quality as high 2-butanol concentrations (>50 mg/100 mL aa) are considered a marker for spoilage of the raw material or negative microbiological influences of the fermentation process [
33]. The averaged 2-butanol concentrations of each distillation run ranged from 2.7 ± 0.7 mg/100 mL to 4.3 ± 0.3 mg/100 mL ethanol and were thus below the considered perceivable odor threshold of 5 mg/100 mL ethanol [
46].
Higher alcohols 1-propanol, isobutanol, isoamyl alcohol and 2-methyl-1-butanol steadily increased during the distillation run. They are important congeners of the yielded product as optimal levels of higher alcohols impart fruity characters on the distillate. However, excessive concentrations of higher alcohols can result in a strong pungent and fusel-like smell and taste [
47,
48,
49,
50]. As the concentration of higher alcohols increases toward the end of the distillation run, a final tails cut of the distillate is separated from the value product.
The methanol concentrations indicated a typical behavior for fruit spirit distillation processes [
45,
51]. They showed slightly higher concentrations during the beginning and the end of the distillation run. However, the presented data state once more that an efficient separation of methanol from ethanol-rich solutions is not possible with a simple fruit spirit distillation technique. Based on the European legal limits [
52] of 200 mg/100 mL ethanol for wine spirits, 1200 mg/00 mL ethanol for plum spirits and 1350 mg/100 mL ethanol for Williams pear spirit, the data on wine spirit production never exceeded the limit. In contrast, all distillation runs with Williams pears exceeded the legal methanol limit until 1100 mL of distillate was produced, independently of the distillation profile. For plum spirit production, distillation profile G resulted in exceeding the legal methanol limit until 750 mL of distillate was produced. Distillation profile H exceeded the legal limit until a product yield of 1700 mL. This indicated that the degree of thermal energy input had an impact on the separation efficiency of methanol from ethanol-water solutions. Overall, it is essential to mix all hearts fractions of one distillation process together, to ensure that the final product contained a sufficiently low methanol concentration.
Wine mash distillation profiles A–C showed relatively similar ethanol concentrations. The averaged ethanol concentrations were 85.8 ± 0.5% (
v/
v), 85.9 ± 0.8% (
v/
v) and 85.7 ± 0.3% (
v/
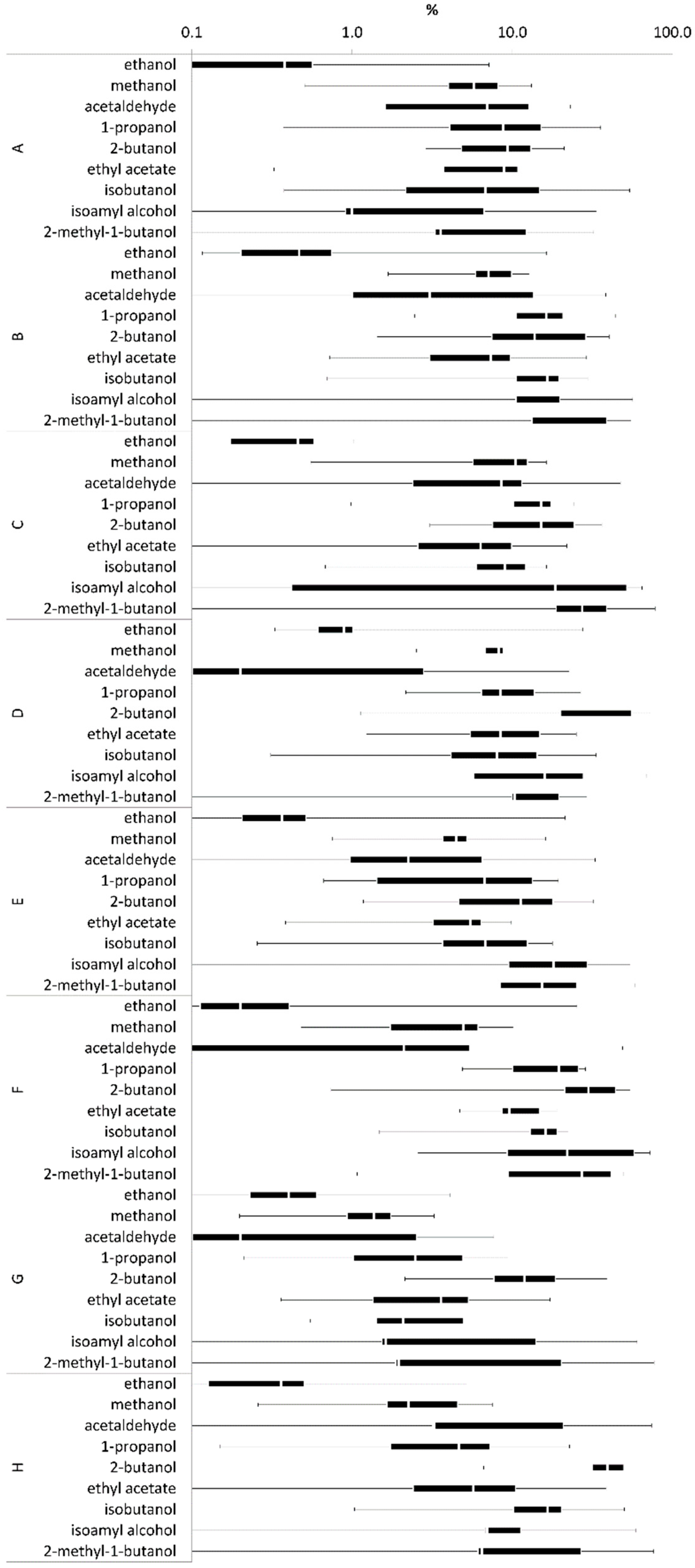
v), respectively. This indicated that different distillation profiles did not affect the separation efficiency of ethanol in the rectification column. The replicates of wine distillations showed the largest median RSD for isoamyl alcohol and 2-methyl-1-butanol in distillation profile C. Here, the median RSD values were 27% and 19%, respectively (
Figure 9).
It should be considered that the RSD values for volatiles such as isoamyl alcohol and 2-methyl-1-butanol might be affected by methodical and analytical limits when volatile concentrations reach a limit of <1 mg/100 mL. Small differences at low concentration values result in relatively large standard deviations. In fact, when the mean value is close to zero, the coefficient of variation will approach infinity and is, therefore, sensitive to small changes. For instance, the isoamyl alcohol concentration in sampled fraction 11 of distillation profile A showed a value of 0.37 mg/100 mL ethanol in the first distillate run, while the duplicate sample showed a value of 0.6 mg/100 mL ethanol, resulting in a relatively large RSD of 34%. Both concentration values can be considered low while the RSD determination becomes less resilient to deviations. This might impair the evaluated RSD values of volatiles with especially low concentrations, without having a prominent effect on the sensory quality of the yielded product. All other volatiles of distillation profiles A–C showed a median RSD ≤16%, indicating a high repetitiveness of the duplicate distillation runs.
The Williams pear distillation runs D–F indicated that the averaged ethanol concentrations decreased from 84 ± 1.7% (
v/
v) and 82.4 ± 1.1% (
v/
v) in distillation profiles D and E to 77.7 ± 1% (
v/
v) in distillation profile F. This is possibly due to the different thermal energy input of profile F and a slower increase of the ethanol concentration during the distillation run. The largest median RSD of the replicates was apparent for 2-butanol with 30% in distillation profile F. This volatile substance again showed relatively low concentrations from 0.3 mg/100 mL ethanol to 12.7 mg/100 mL ethanol, which affects the methodical and analytical limits of volatile quantification. In addition, low concentrated volatiles isoamyl alcohol and 2-methyl-1-butanol again showed maximum median RSDs of 22% and 27%, respectively. Beyond this, 1-propanol also indicated a larger median RSD of 19% in distillation profile F. The largest discrepancy between 1-propanol concentrations of the duplicates of distillation profile F was 81 mg/100 mL ethanol within the sampled fraction at 3500 mL. Since the odor threshold of 1-propanol is estimated at 83 mg/100 mL ethanol, this difference might not be detectible for the consumer [
33]. All other volatiles showed median RSD values ≤16%.
For plum spirit distillations, the averaged ethanol concentrations decreased from 87.1 ± 0.5% (v/v) in distillation profile G to 83.9 ± 0.5% (v/v) in distillation profile H. A slower increase of the ethanol concentration during the process might again be the result of different thermal energy inputs. The highest median RSD value was found for 2-butanol with 39% in distillation profile H. We found 2-butanol was again present in relatively low concentrations. All other volatiles showed a median RSD value ≤17%.
Volatiles ethyl-2-methylbutanoate, trans-2-hexen-1-ol and hexyl acetate were not detected in any of the distillate fractions. The evaluation of 10 volatile compound concentrations in eight duplicate distillation runs enabled the comparison of a total of 1540 duplicate data points, which resulted in a median RSD range of 0.2% to 39% (average 9 ± 8%). This indicated that enabling control over two distillation parameters allows largely reproducible fruit spirit products with similar volatile compound concentrations, considering an average median RSD deviation of about 9 ± 8%. When we excluded volatile concentrations ≤1 mg/100 mL ethanol from this evaluation, the median RSD range changed to values from 0.2% to 23% (average 7 ± 6%). This indicated that low-concentrated volatiles have a major impact on median RSD determination, and thus should be evaluated carefully, since they will not affect the sensory profile of the product in a similar manner.