Anethole Stability in Aniseed Spirits: Storage Condition Repercussions on Commercial Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Solutions
2.2. High-Pressure Liquid Chromatography (HPLC) and Gas Chromatography (GC) Methods
2.3. Ultraviolet (UV) and Visible (VIS) Lights Exposure Assay
2.4. High-Temperature Assay
2.5. Low-Temperature Assay
2.6. Statistical Analyses
3. Results and Discussion
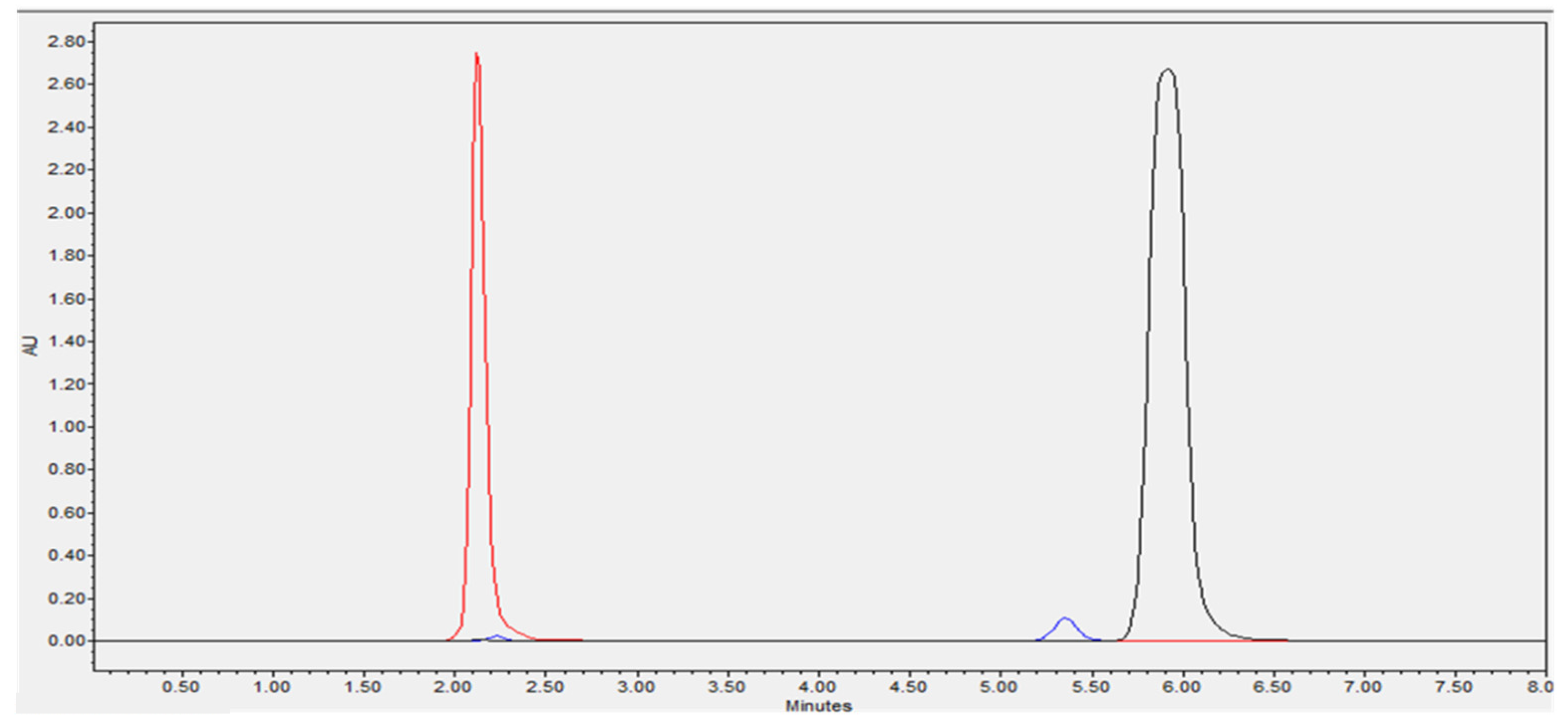
3.1. HPLC Method Validation
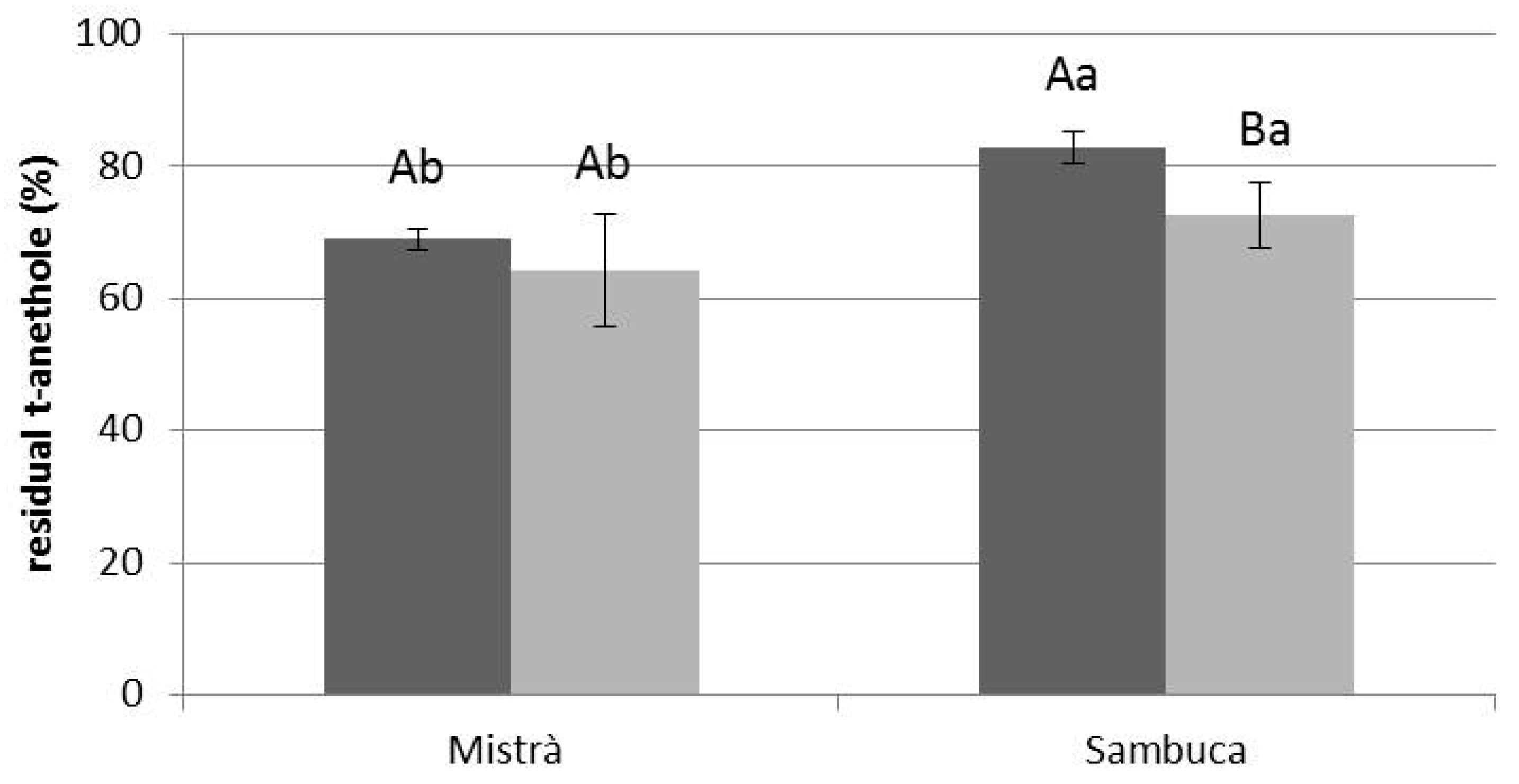
3.2. Effect of UV Exposure
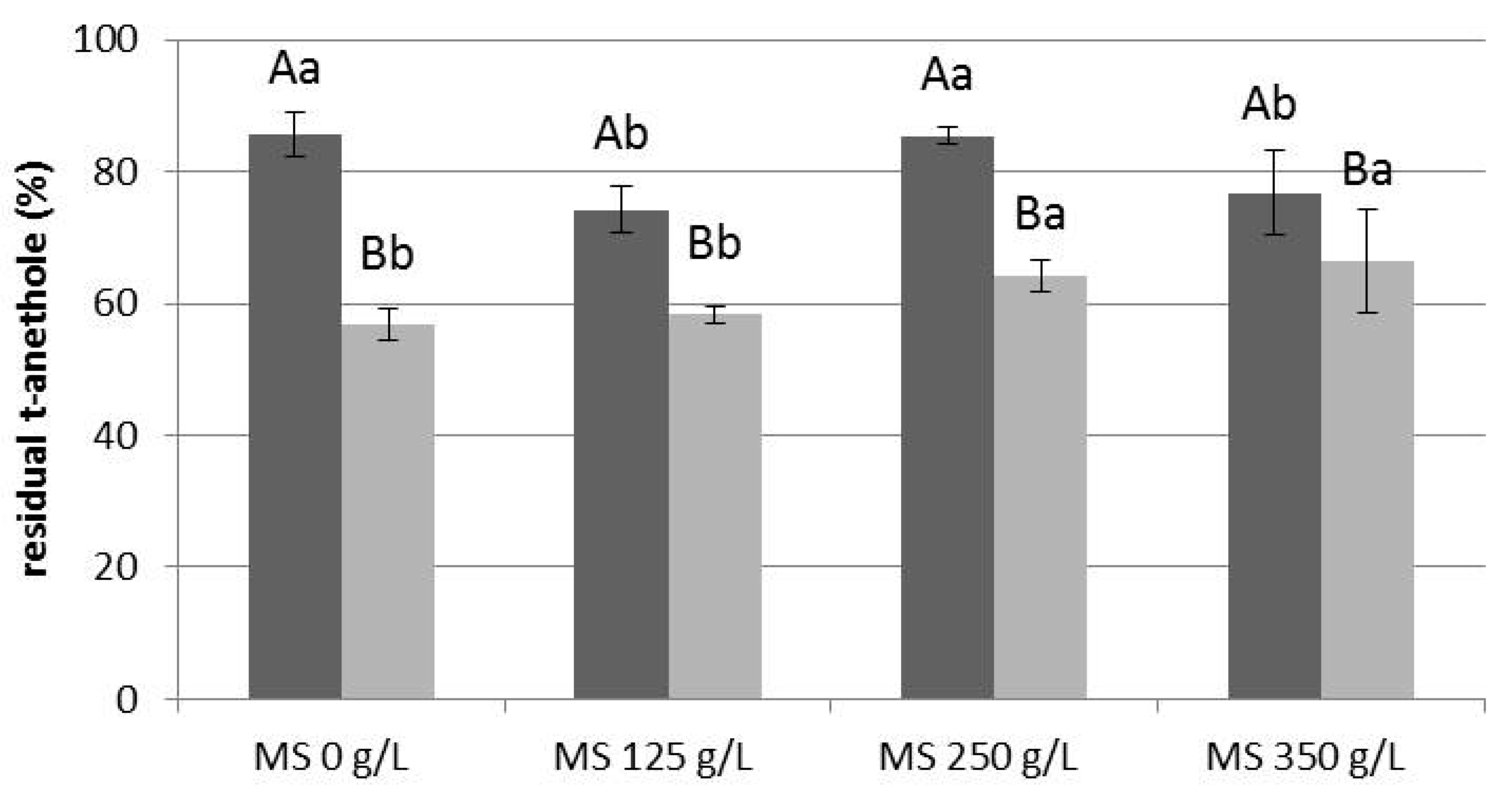
3.3. Effect of UV Exposure on Model Solutions
3.4. Effect of VIS Light Exposure
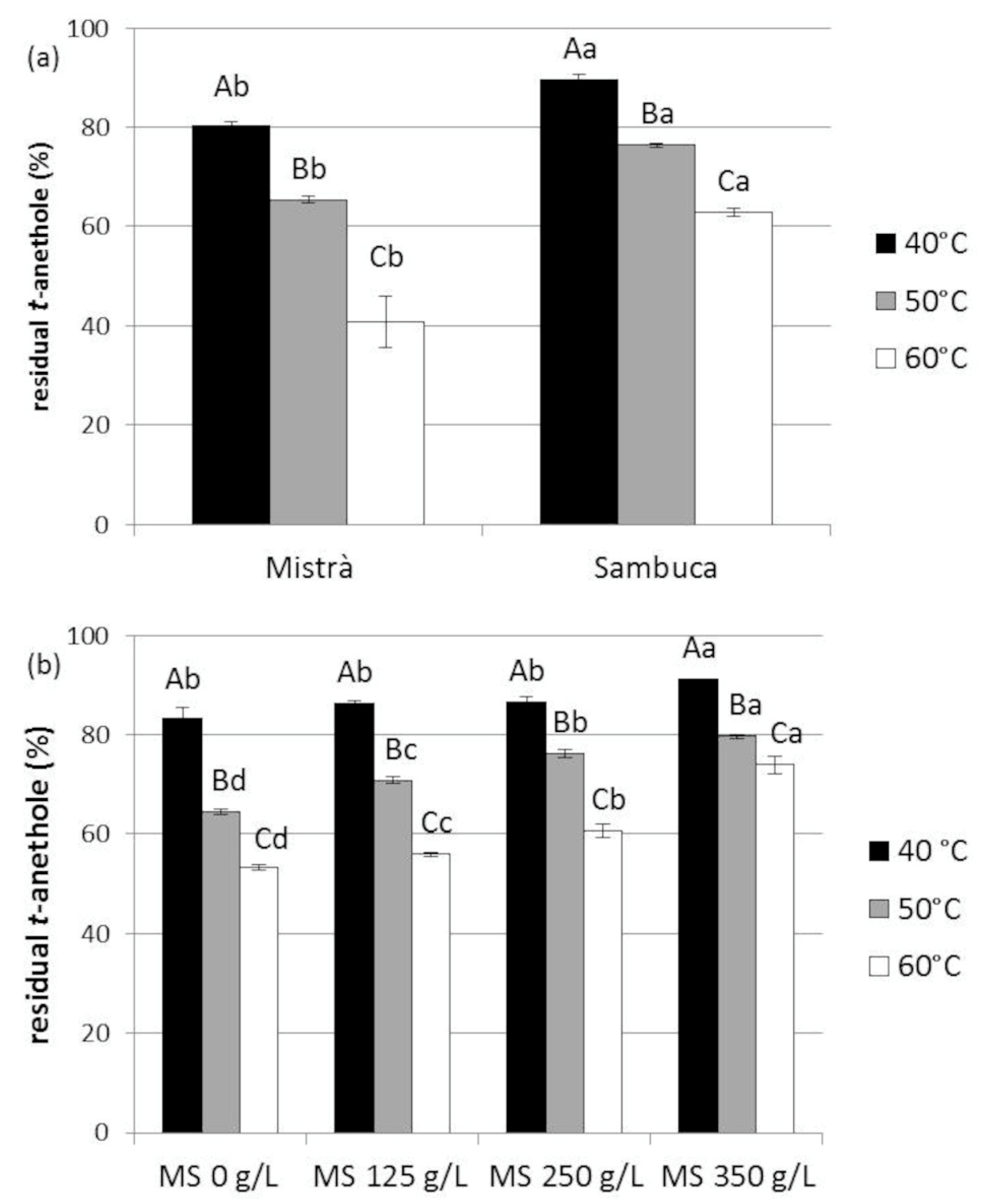
3.5. High-Temperature Stability of Trans-Anethole
3.6. Low-Temperature Stability of Trans-Anethole
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carteau, D.; Brunerie, P.; Guillemat, B.; Bassani, D.M. Photochemistry in everyday life: The effect of spontaneous emulsification on the photochemistry of Trans-anethole. Photochem. Photobiol. Sci. 2007, 6, 423–430. [Google Scholar] [CrossRef]
- Castro, H.T.; Martínez, J.R.; Stashenko, E. Anethole isomerization and dimerization induced by acid sites or UV irradiation. Molecules 2010, 15, 5012–5030. [Google Scholar] [CrossRef] [Green Version]
- Zabetakis, I. Anise spirits: Types, sensory properties and sensory analysis. In Alcoholic Beverages: Sensory Evaluation and Consumer Research; Piggott, J., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2012; pp. 229–241. [Google Scholar]
- Sitnikova, N.L.; Sprik, R.; Wegdam, G.; Eiser, E. Spontaneously formed trans-anethol/water/alcohol emulsions: Mechanism of formation and stability. Langmuir 2005, 21, 7083–7089. [Google Scholar] [CrossRef] [PubMed]
- Grillo, I. Small-angle neutron scattering study of a world-wide known emulsion: Le pastis. Colloids Surf. A Physicochem. Eng. Asp. 2003, 225, 153–160. [Google Scholar] [CrossRef]
- Dawidar, A.M.; Abou-Elzahab, M.M.; Abdel-Mogib, M.; Hussien, K.; Mostafa, M.E.-H. Photo-oxygenation of trans anethole. Int. J. Sci. Eng. Appl. 2015, 4, 294–298. [Google Scholar] [CrossRef]
- Lewis, F.D.; Kojima, M. Electron transfer induced photoisomerization, dimerization, and oxygenation of trans- and cis-anethole. The role of monomer and dimer cation radicals. J. Am. Chem. Soc. 1988, 110, 8664–8670. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Costache, I.I.; Miron, A. Anethole and its role in chronic diseases. In Drug Discovery from Mother Nature. Advances in Experimental Medicine and Biology; Gupta, S.C., Prasad, S., Aggarwal, B.B., Eds.; Springer International Publishing: Basel, Switzerland, 2016; pp. 247–267. ISBN 1420026399. [Google Scholar]
- Akçan, R.; Lale, A.; Tümer, A.R. Trans-anethole: A key compound in Bogma Raki. Acta Med. 2018, 49, 26–31. [Google Scholar]
- Mohan, R.S.; Whalen, D.L. Acid-catalyzed hydrolysis of cis- and trans-anethole oxides: Discrete carbocation intermediates and syn/anti hydration ratios. J. Org. Chem. 1993, 58, 2663–2669. [Google Scholar] [CrossRef]
- O’Brien, P.; Siraki, A.; Shangari, N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 2005, 35, 609–662. [Google Scholar] [CrossRef]
- Özgüven, M. Aniseed. In Handbook of Herbs and Spices; Peter, K.V., Ed.; Taylor & Francis: London, UK, 2012; pp. 138–150. ISBN 9780857095688. [Google Scholar]
- Zafeiropoulou, T.; Evageliou, V.; Gardeli, C.; Yanniotis, S.; Komaitis, M. Retention of trans-anethole by gelatine and starch matrices. Food Chem. 2010, 123, 364–368. [Google Scholar] [CrossRef]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Ruellan, S.; Fourmentin, S. Cyclodextrin, an efficient tool for trans-anethole encapsulation: Chromatographic, spectroscopic, thermal and structural studies. Food Chem. 2014, 164, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.D.; Elmore, J.S.; Langley, K.R.; Bakker, J. Effects of sucrose, guar gum, and carboxymethylcellulose on the release of volatile flavor compounds under dynamic conditions. J. Agric. Food Chem. 1996, 44, 1321–1326. [Google Scholar] [CrossRef]
- Misharina, T.A.; Polshkov, A.N. Antioxidant properties of essential oils: Autoxidation of essential oils from laurel and fennel and effects of mixing with essential oil from coriander. Appl. Biochem. Microbiol. 2005, 41, 610–618. [Google Scholar] [CrossRef]
- Miething, H.; Seger, V.; Hänsel, R. Determination of photoanethole from a stored essential oil of anise fruits as 4,4′-dimethoxystilbene by high performance liquid chromatography–ultraviolet coupling. Phyther. Res. 1990, 4, 121–123. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. C. R. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Btaun, M.; Franz, G. Quality criteria of bitter fennel oil in the german pharmacopoeia. Pharm. Pharmacol. Lett. 1999, 9, 48–51. [Google Scholar]
- Regulation (EU) 2019/787. Off. J. Eur. Union 2019, L 130, 1–54.
- Jurado, J.M.; Alcázar, A.; Pablos, F.; Martín, M.J. LC Determination of anethole in aniseed drinks. Chromatographia 2006, 64, 223–226. [Google Scholar] [CrossRef]
- Regulation (EC) No 2091/2002. Off. J. Eur. Communities 2002, L 322, 11–27.
- Moretón-Lamas, E.; Lago-Crespo, M.; Lage-Yusty, M.A.; López-Hernández, J. Comparison of methods for analysis of resveratrol in dietary vegetable supplements. Food Chem. 2017, 224, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, B.; Örnemark, U. (Eds.) Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; EURACHEM Working Group, LGC: Middlesex, UK, 2014; ISBN 0-94948926-12-0. [Google Scholar]
- Maury, C.; Clark, A.C.; Scollary, G.R. Determination of the impact of bottle colour and phenolic concentration on pigment development in white wine stored under external conditions. Anal. Chim. Acta 2010, 660, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Lewis, F.D.; Kojimat, M. Photodimerization of singlet trans- and cis-anethole. Concerted or stepwise? J. Am. Chem. Soc. 1988, 110, 8660–8664. [Google Scholar] [CrossRef]
- Lukes, P.; Clupek, M.; Babicky, V.; Sunka, P. Ultraviolet radiation from the pulsed corona discharge in water. Plasma Sources Sci. Technol. 2008, 17, 024012. [Google Scholar] [CrossRef]
- Hennemeyer, M.; Burghardt, S.; Stark, R.W. Cantilever micro-rheometer for the characterization of sugar solutions. Sensors 2008, 8, 10–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, H.M.; Barbosa, D.P.; Fricks, A.T.; Aranda, D.A.G.; Valdés, R.H.; Antunes, O.A.C. Production of piperonal, vanillin, and p-anisaldehyde via solventless supported iodobenzene diacetate oxidation of isosafrol, isoeugenol, and anethol under microwave irradiation. Org. Process. Res. Dev. 2006, 10, 941–943. [Google Scholar] [CrossRef]

| Sambuca | Mistrà | Model Solution | |||
|---|---|---|---|---|---|
| GC | HPLC | GC | HPLC | GC | HPLC |
| 1.11 ± 0.001 * | 1.18 ± 0.071 * | 0.66 ± 0.078 * | 0.63 ± 0.042 * | 0.87 ± 0.036 ** | 0.90 ± 0.031 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vendramin, V.; Pesce, A.; Vincenzi, S. Anethole Stability in Aniseed Spirits: Storage Condition Repercussions on Commercial Products. Beverages 2021, 7, 73. https://doi.org/10.3390/beverages7040073
Vendramin V, Pesce A, Vincenzi S. Anethole Stability in Aniseed Spirits: Storage Condition Repercussions on Commercial Products. Beverages. 2021; 7(4):73. https://doi.org/10.3390/beverages7040073
Chicago/Turabian StyleVendramin, Veronica, Antonio Pesce, and Simone Vincenzi. 2021. "Anethole Stability in Aniseed Spirits: Storage Condition Repercussions on Commercial Products" Beverages 7, no. 4: 73. https://doi.org/10.3390/beverages7040073
APA StyleVendramin, V., Pesce, A., & Vincenzi, S. (2021). Anethole Stability in Aniseed Spirits: Storage Condition Repercussions on Commercial Products. Beverages, 7(4), 73. https://doi.org/10.3390/beverages7040073








