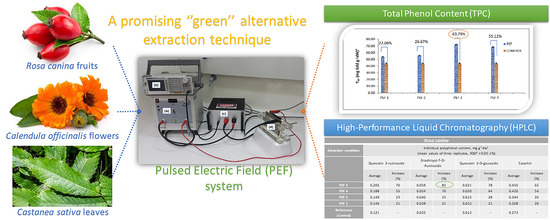
Enhancement of Polyphenols Recovery from Rosa canina, Calendula officinalis and Castanea sativa Using Pulsed Electric Field
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. PEF Apparatus
2.4. PEF Extraction
2.5. Total Polyphenol Content of Extracts
2.6. High-Performance Liquid Chromatography (HPLC)
2.7. Statistical Analysis
3. Results and Discussion
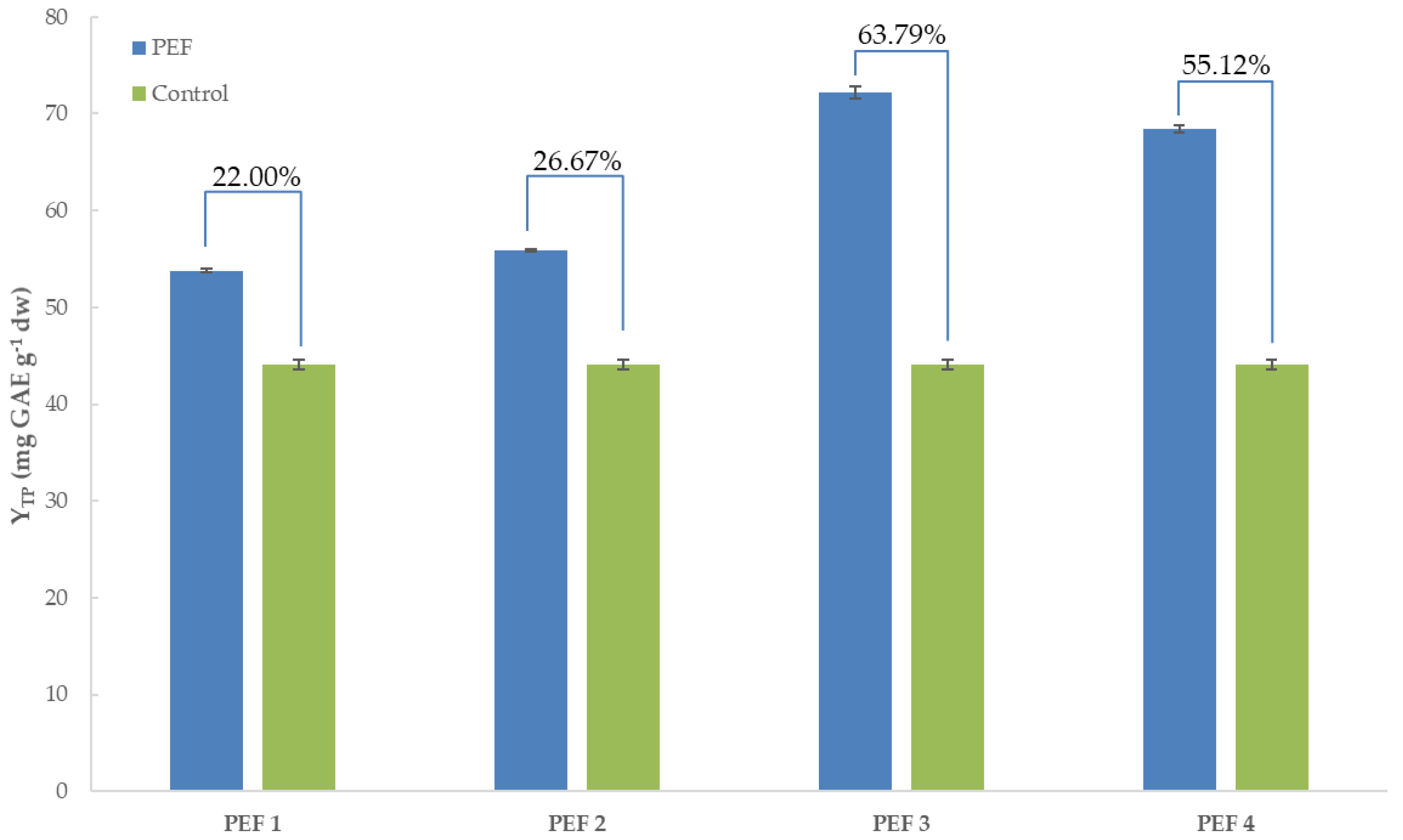
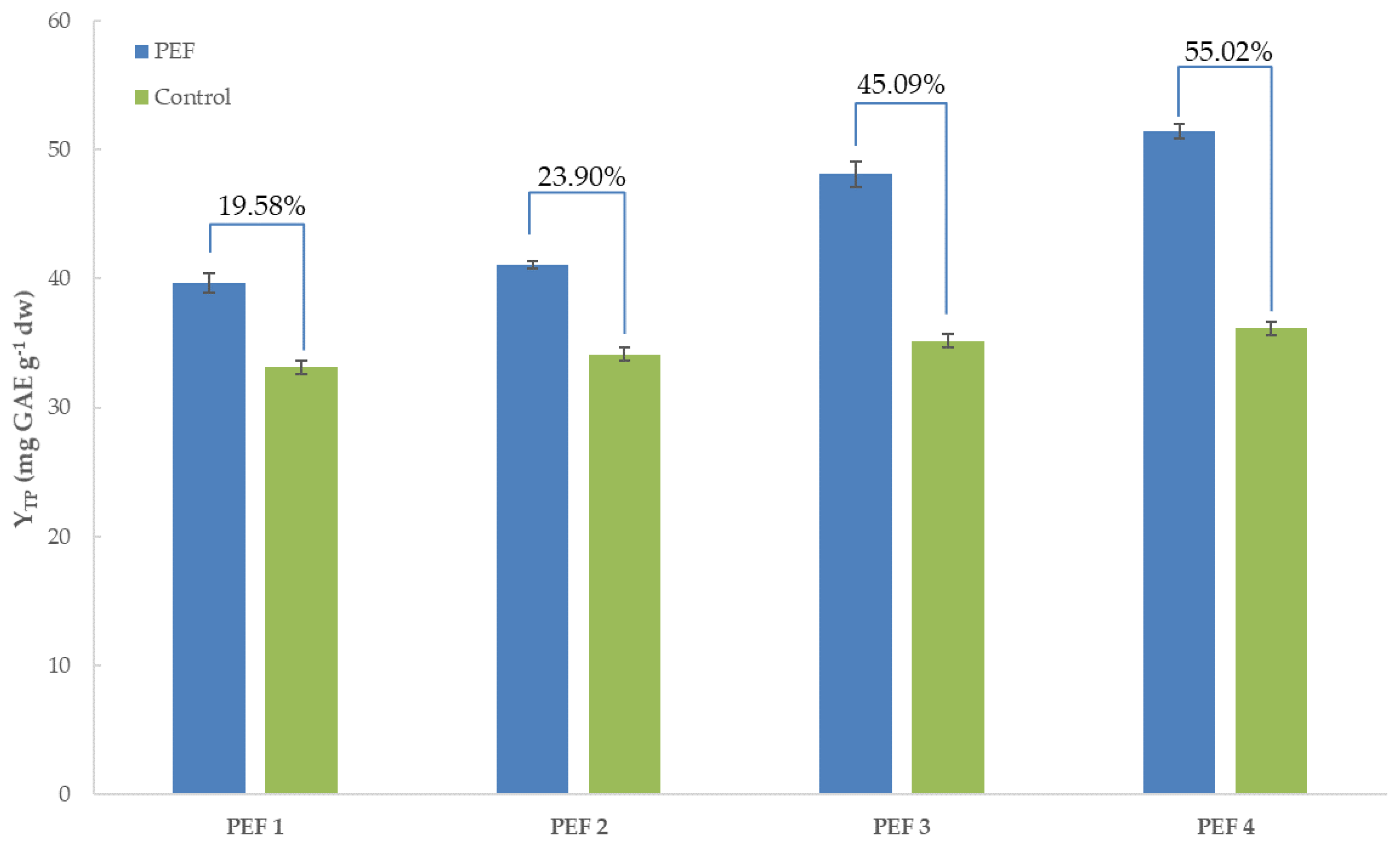
3.1. Total Polyphenol Content of Extracts
3.2. Composition of Polyphenolic Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad Khan, M.S.; Ahmad, I. Chapter 1—Herbal Medicine: Current Trends and Future Prospects. In New Look to Phytomedicine: Advancements in Herbal Products as Novel Drug Leads, 1st ed.; Ahmad Khan, M.S., Ahmad, I., Chattopadhyay, D., Eds.; Academic Press: London, UK, 2019; pp. 3–13. [Google Scholar] [CrossRef]
- Atmakuri, L.R.; Dathi, S. Current Trends in Herbal Medicines. J. Pharm. Res. 2010, 3, 109–113. [Google Scholar]
- Fatima, N.; Nayeem, N. Toxic effects as a result of herbal medicine intake. In Toxicology-New Aspects to This Scientific Conundrum; Larramendy, M., Soloneski, S., Eds.; InTech Open: London, UK, 2016; pp. 193–207. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, M.R.; Strobel, B.W.; Hama, J.R.; Cedergreen, N. Toxicity and risk of plant-produced alkaloids to Daphnia magna. Environ. Sci. Eur. 2021, 33, 1–12. [Google Scholar] [CrossRef]
- Hajšlová, J.; Schulzová, V.; Botek, P.; Lojza, J. Natural toxins in food crops and their changes during processing. Czech. J. Food Sci. 2018, 22, 29–34. [Google Scholar] [CrossRef]
- Kregiel, D.; Berlowska, J.; Witonska, I.; Antolak, H.; Proestos, C.; Babic, M.; Babic, L.; Zhang, B. Saponin-based, biological-active surfactants from plants. In Application and Characterization of Surfactants; Najjar, R., Ed.; InTech Open: London, UK, 2017; pp. 183–205. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Wang, B.; Jiang, J.; Fitzgerald, M.; Huang, Q.; Yu, Z.; Li, H.; Zhang, J.; Wei, J.; Yang, C.; et al. Heavy metal contaminations in herbal medicines: Determination, comprehensive risk assessments, and solutions. Front. Pharmacol. 2021, 11, 2016. [Google Scholar] [CrossRef]
- Abuajah, C.I. Functional Components and Medicinal Properties of Food. In Bioactive Molecules in Food, Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–34. [Google Scholar] [CrossRef]
- Lalas, S.; Dourtoglou, V. Use of rosemary extract in preventing oxidation during deep-fat frying of potato chips. J. Am. Oil Chem. Soc. 2003, 80, 579–583. [Google Scholar] [CrossRef]
- Hanlidou, E.; Karousou, R.; Kleftoyanni, V.; Kokkini, S. The herbal market of Thessaloniki (N. Greece) and its relation to the ethnobotanical tradition. J. Ethnopharmacol. 2004, 91, 281–299. [Google Scholar] [CrossRef]
- Petrakou, K.; Iatrou, G.; Lamari, F.N. Ethnopharmacological survey of medicinal plants traded in herbal markets in the Peloponnisos, Greece. J. Herb. Med. 2020, 19, 100305. [Google Scholar] [CrossRef]
- Arora, D.; Rani, A.; Sharma, A. A review on phytochemistry and ethnopharmacological aspects of genus Calendula. Pharmacogn. Rev. 2013, 7, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Braga, N.; Rodrigues, F.; Beatriz, M.; Oliveira, P.P. Castanea sativa by-products: A review on added value and sustainable application. Nat. Prod. Res. 2015, 29, 1–18. [Google Scholar] [CrossRef]
- Chrubasik, C.; Roufogalis, B.D.; Müller-Ladner, U.; Chrubasik, S. A systematic review on the Rosa canina effect and efficacy profiles. Phytother. Res. 2008, 22, 725–733. [Google Scholar] [CrossRef]
- Almeida, I.F.; Valentão, P.; Andrade, P.B.; Seabra, R.M.; Pereira, T.M.; Amaral, M.H.; Costa, P.C.; Bahia, M.F. In vivo skin irritation potential of a Castanea sativa (Chestnut) leaf extract, a putative natural antioxidant for topical application. Basic Clin. Pharmacol. Toxicol. 2008, 103, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Akobirshoeva, A.; Zilfikarov, I.N.; Vennos, C. Isorhamnetin and Quercetin Derivatives as Anti-Acetylcholinesterase Principles of Marigold (Calendula officinalis) Flowers and Preparations. Int. J. Mol. Sci. 2017, 18, 1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Oliveira, P.; Fraga-Corral, M.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Scientific basis for the industrialization of traditionally used plants of the Rosaceae family. Food Chem. 2020, 330, 127197. [Google Scholar] [CrossRef] [PubMed]
- Elmastaş, M.; Demir, A.; Genç, N.; Dölek, Ü.; Güneş, M. Changes in flavonoid and phenolic acid contents in some Rosa species during ripening. Food Chem. 2017, 235, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Queiroz, M.J.; Santos-Buelga, C.; Ferreira, I.C. Characterisation of phenolic compounds in wild fruits from Northeastern Portugal. Food Chem. 2013, 141, 3721–3730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Lalas, S.; Athanasiadis, V.; Karageorgou, I.; Batra, G.; Nanos, G.D.; Makris, D.P. Nutritional characterization of leaves and herbal tea of Moringa oleifera cultivated in Greece. J. Herbs Spices Med. Plants 2017, 23, 320–333. [Google Scholar] [CrossRef]
- Nowosad, K.; Sujka, M.; Pankiewicz, U.; Kowalski, R. The application of PEF technology in food processing and human nutrition. J. Food Sci. Technol. 2021, 58, 397–411. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Munir, A.; Buntat, Z.; Ahmad, M.H.; Jusoh, Y.M.M.; Bekhit, A.E.; Roobab, U.; Muhammad, F.M.; Aadil, R.M. Electrical systems for pulsed electric field applications in the food industry: An engineering perspective. Trends Food Sci. Technol. 2020, 104, 1–13. [Google Scholar] [CrossRef]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Wouters, P.C.; Alvarez, I.; Raso, J. Critical factors determining inactivation kinetics by pulsed electric field food processing. Trends Food Sci. Technol. 2001, 12, 112–121. [Google Scholar] [CrossRef]
- Bozinou, E.; Karageorgou, I.; Batra, G.; Dourtoglou, V.; Lalas, S. Pulsed Electric Field Extraction and Antioxidant Activity Determination of Moringa oleifera Dry Leaves: A Comparative Study with Other Extraction Techniques. Beverages 2019, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Vorobiev, E.; Lebovka, N. Electrotechnologies for Extraction from Food Plants and Biomaterial, 1st ed.; Springer: New York, NY, USA, 2008. [Google Scholar] [CrossRef] [Green Version]
- Brodelius, P.E.; Funk, C.; Shillito, R.D. Permeabilization of cultivated plant cells by electroporation for release of intracellularly stored secondary products. Plant. Cell Rep. 1988, 7, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Bobinaitė, R.; Pataro, G.; Lamanauskas, N.; Šatkauskas, S.; Viškelis, P.; Ferrari, G. Application of pulsed electric field in the production of juice and extraction of bioactive compounds from blueberry fruits and their by-products. J. Food Sci. Technol. 2015, 52, 5898–5905. [Google Scholar] [CrossRef] [PubMed]
- Boussetta, N.; Vorobiev, E.; Le, L.H.; Cordin-Falcimaigne, A.; Lanoisellé, J.L. Application of electrical treatments in alcoholic solvent for polyphenols extraction from grape seeds. LWT Food Sci. Technol. 2012, 46, 127–134. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 79–84. [Google Scholar] [CrossRef]
- Martín, B.; Tylewicz, U.; Verardo, V.; Pasini, F.; Caravaca, A.M.G.; Caboni, M.; Dalla Rosa, M. Pulsed electric field (PEF) as pre-treatment to improve the phenolic compounds recovery from brewers’ spent grains. Innov. Food Sci. Emerg. Technol. 2020, 64, 102402. [Google Scholar] [CrossRef]
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369. [Google Scholar] [CrossRef]
- Sukardi, S.; Purwaningsih, I.; Sita, P. Extraction of phenolic compounds from basil (Ocimum americanum L.) leaves with pretreatment using pulsed electric field (PEF). IOP Conf. Ser. Earth Environ. Sci. 2020, 475, 012056. [Google Scholar] [CrossRef]
- Turk, M.F.; Baron, A.; Vorobiev, E. Effect of Pulsed Electric Fields Treatment and Mash Size on Extraction and Composition of Apple Juices. J. Agric. Food Chem. 2010, 58, 9611–9616. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Bals, O.; Grimi, N.; Vorobiev, E. A new way for the oil plant biomass valorization: Polyphenols and proteins extraction from rapeseed stems and leaves assisted by pulsed electric fields. Ind. Crop. Prod. 2015, 74, 309–318. [Google Scholar] [CrossRef]
- Yu, X.; Gouyo, T.; Grimi, N.; Bals, O.; Vorobiev, E. Pulsed electric field pretreatment of rapeseed green biomass (stems) to enhance pressing and extractives recovery. Bioresour. Technol. 2016, 199, 194–201. [Google Scholar] [CrossRef]
- Lakka, A.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Evaluation of Pulsed Electric Field Polyphenol Extraction from Vitis vinifera, Sideritis scardica and Crocus sativus. ChemEngineering 2021, 5, 25. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Barba, F.J.; Parniakov, O.; Galanakis, C.M.; Lebovka, N.; Grimi, N.; Vorobiev, E. High Voltage Electrical Discharges, Pulsed Electric Field, and Ultrasound Assisted Extraction of Protein and Phenolic Compounds from Olive Kernel. Food Bioproc. Tech. 2015, 8, 885–894. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Kaltsa, O.; Bozinou, E.; Lalas, S.; Makris, D.P. Saffron Processing Wastes as a Bioresource of High-Value Added Compounds: Development of a Green Extraction Process for Polyphenol Recovery Using a Natural Deep Eutectic Solvent. Antioxidants 2019, 8, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakashov, B.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimisation of polyphenol extraction from Hypericum perforatum (St. John’s Wort) using aqueous glycerol and response surface methodology. J. Appl. Res. Med. Aromat. Plants 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A Green Extraction Process for Polyphenols from Elderberry (Sambucus nigra) Flowers Using Deep Eutectic Solvent and Ultrasound-Assisted Pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef] [Green Version]
- Ricci, A.; Parpinello, G.P.; Versari, A. Recent advances and applications of pulsed electric fields (PEF) to improve polyphenol extraction and color release during red winemaking. Beverages 2018, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.G.; He, L.; Xi, J. High intensity pulsed electric field as an innovative technique for extraction of bioactive compounds—A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2877–2888. [Google Scholar] [CrossRef]
- Gabrić, D.; Barba, F.; Roohinejad, S.; Gharibzahedi, S.M.T.; Radojčin, M.; Putnik, P.; Bursać Kovačević, D. Pulsed electric fields as an alternative to thermal processing for preservation of nutritive and physicochemical properties of beverages: A review. J. Food Process. Eng. 2018, 41, 12638. [Google Scholar] [CrossRef]
- Heinz, V.; Toepfl, S.; Knorr, D. Impact of temperature on lethality and energy efficiency of apple juice pasteurization by pulsed electric fields treatment. Innov. Food Sci. Emerg. Technol. 2003, 4, 167–175. [Google Scholar] [CrossRef]
- Niu, D.; Zeng, X.-A.; Ren, E.-F.; Xu, F.-Y.; Li, J.; Wang, M.-S.; Wang, R. Review of the application of pulsed electric fields (PEF) technology for food processing in China. Food Res. Int. 2020, 137, 109715. [Google Scholar] [CrossRef] [PubMed]
- Ntourtoglou, G.; Tsapou, E.A.; Drosou, F.; Bozinou, E.; Lalas, S.; Tataridis, P.; Dourtoglou, V. Pulsed Electric Field Extraction of α and β-Acids From Pellets of Humulus lupulus (Hop). Front. Bioeng. Biotechnol. 2020, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Tsapou, E.A.; Ntourtoglou, G.; Drosou, F.; Tataridis, P.; Dourtoglou, T.; Lalas, S.; Dourtoglou, V. In situ Creation of the Natural Phenolic Aromas of Beer: A Pulsed Electric Field Applied to Wort-Enriched Flax Seeds. Front. Bioeng. Biotechnol. 2020, 8, 1219. [Google Scholar] [CrossRef] [PubMed]

| Extraction Condition | Extraction Time (min) | Temperature (°C) | Electric Field Intensity (kV cm−1) | Specific Energy (kJ kg−1) | Specific Energy Per Pulse (mJ kg−1) | PEF Energy (kWh) |
|---|---|---|---|---|---|---|
| PEF 1 | 20 | 24 | 2.0 | 0.457 | 0.381 | 3.33 × 10−6 |
| PEF 2 | 20 | 24 | 1.7 | 0.389 | 0.324 | 2.83 × 10−6 |
| PEF 3 | 20 | 24 | 1.4 | 0.320 | 0.267 | 2.33 × 10−6 |
| PEF 4 | 20 | 24 | 1.2 | 0.274 | 0.229 | 2.00 × 10−6 |
| Control | 20 | 24 | - | - | - | - |
| Rosa canina | ||||||||
|---|---|---|---|---|---|---|---|---|
| Extraction Condition | Individual Polyphenol Content, mg g−1 dw (Mean Values of Three Replicates, RSD 1 = 0.05–1%) | |||||||
| Quercetin 3-O-glucoside | Quercetin 3-O-rutinoside | Eriodictyol 7-O-rutinoside | Catechin | |||||
| Average 2 | Increase 3 (%) | Average | Increase (%) | Average | Increase (%) | Average | Increase (%) | |
| PEF 1 | 0.015 ± 0.002 a | 25 ± 3 a | 0.146 ± 0.007 b | 21 ± 0 a | 0.039 ± 0.003 a | 21 ± 8 a | 0.328 ± 0.024 b | 20 ± 1 a |
| PEF 2 | 0.015 ± 0.003 a | 24 ± 5 a | 0.149 ± 0.006 b | 23 ± 1 b | 0.040 ± 0.005 a | 24 ± 2 a | 0.344 ± 0.021 b | 26 ± 0 b |
| PEF 3 | 0.021 ± 0.002 b | 74 ± 9 b | 0.206 ± 0.009 d | 71 ± 0 d | 0.059 ± 0.006 b | 84 ± 9 b | 0.450 ± 0.032 c | 65 ± 2 d |
| PEF 4 | 0.020 ± 0.002 b | 66 ± 8 b | 0.188 ± 0.008 c | 55 ± 0 c | 0.054 ± 0.004 b | 69 ± 13 b | 0.420 ± 0.016 c | 54 ± 4 c |
| Control | 0.012 ± 0.002 a | - | 0.121 ± 0.005 a | - | 0.032 ± 0.005 a | - | 0.273 ± 0.017 a | - |
| Calendula officinalis | ||||||||
|---|---|---|---|---|---|---|---|---|
| Extraction Condition | Individual Polyphenol Content, mg g−1 dw (Mean Values of Three Replicates, RSD 1 = 0.05–1%) | |||||||
| Quercetin 3-O-glucoside | Quercetin 3-O-rutinoside | Isorhamnetin 3-O-rutinoside | Caffeic Acid | |||||
| Average 2 | Increase 3 (%) | Average | Increase (%) | Average | Increase (%) | Average | Increase (%) | |
| PEF 1 | 1.640 ± 0.075 b | 26 ± 11 a | 2.847 ± 0.279 a | 22 ± 1 a | 9.599 ± 0.657 b | 22 ± 3 a | 0.782 ± 0.074 a | 22 ± 14 a |
| PEF 2 | 1.706 ± 0.189 b | 30 ± 3 a | 2.918 ± 0.340 a | 25 ± 1 b | 10.071 ± 0.400 b | 28 ± 1 b | 0.822 ± 0.099 a | 28 ± 11 a |
| PEF 3 | 2.073 ± 0.133 c | 59 ± 11 b | 3.781 ± 0.359 b | 62 ± 2 c | 13.376 ± 0.479 c | 70 ± 2 c | 1.030 ± 0.096 b | 61 ± 19 b |
| PEF 4 | 2.125 ± 0.140 c | 63 ± 11 b | 3.851 ± 0.417 b | 65 ± 0 d | 13.612 ± 0.633 c | 73 ± 0 d | 1.069 ± 0.127 b | 66 ± 15 b |
| Control | 1.312 ± 0.174 a | - | 2.334 ± 0.253 a | - | 7.868 ± 0.377 a | - | 0.652 ± 0.134 a | - |
| Castanea sativa | ||||||||
|---|---|---|---|---|---|---|---|---|
| Extraction Condition | Individual Polyphenol Content, mg g−1 dw (Mean Values of Three Replicates, RSD 1 = 0.05–1%) | |||||||
| Quercetin 3-O-glucoside | Quercetin 3-O-rutinoside | Hyperoside | Ellagic Acid | |||||
| Average 2 | Increase 3 (%) | Average | Increase (%) | Average | Increase (%) | Average | Increase (%) | |
| PEF 1 | 1.499 ± 0.117 b | 30 ± 1 a | 6.869 ± 0.357 b | 20 ± 2 a | 4.269 ± 0.256 b | 25 ± 0 a | 3.444 ± 0.182 b | 27 ± 4 a |
| PEF 2 | 1.545 ± 0.110 b | 34 ± 2 b | 6.926 ± 0.375 b | 21 ± 2 a | 4.371 ± 0.253 b | 28 ± 0 b | 3.526 ± 0.199 b | 30 ± 3 a |
| PEF 3 | 1.960 ± 0.055 c | 71 ± 10 c | 8.700 ± 0.315 c | 52 ± 5 b | 5.396 ± 0.336 c | 58 ± 0 c | 4.068 ± 0.218 c | 50 ± 4 b |
| PEF 4 | 2.098 ± 0.156 c | 82 ± 2 c | 9.273 ± 0.309 c | 62 ± 6 b | 5.874 ± 0.362 c | 72 ± 0 d | 4.665 ± 0.298 d | 72 ± 3 c |
| Control | 1.153 ± 0.098 a | - | 5.724 ± 0.397 a | - | 3.415 ± 0.210 a | - | 2.712 ± 0.224 a | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakka, A.; Bozinou, E.; Stavropoulos, G.; Samanidis, I.; Athanasiadis, V.; Dourtoglou, V.G.; Makris, D.P.; Lalas, S.I. Enhancement of Polyphenols Recovery from Rosa canina, Calendula officinalis and Castanea sativa Using Pulsed Electric Field. Beverages 2021, 7, 63. https://doi.org/10.3390/beverages7030063
Lakka A, Bozinou E, Stavropoulos G, Samanidis I, Athanasiadis V, Dourtoglou VG, Makris DP, Lalas SI. Enhancement of Polyphenols Recovery from Rosa canina, Calendula officinalis and Castanea sativa Using Pulsed Electric Field. Beverages. 2021; 7(3):63. https://doi.org/10.3390/beverages7030063
Chicago/Turabian StyleLakka, Achillia, Eleni Bozinou, Giorgos Stavropoulos, Iordanis Samanidis, Vassilis Athanasiadis, Vassilis G. Dourtoglou, Dimitris P. Makris, and Stavros I. Lalas. 2021. "Enhancement of Polyphenols Recovery from Rosa canina, Calendula officinalis and Castanea sativa Using Pulsed Electric Field" Beverages 7, no. 3: 63. https://doi.org/10.3390/beverages7030063
APA StyleLakka, A., Bozinou, E., Stavropoulos, G., Samanidis, I., Athanasiadis, V., Dourtoglou, V. G., Makris, D. P., & Lalas, S. I. (2021). Enhancement of Polyphenols Recovery from Rosa canina, Calendula officinalis and Castanea sativa Using Pulsed Electric Field. Beverages, 7(3), 63. https://doi.org/10.3390/beverages7030063