Compound Analysis of Jing Liqueur and nrf2 Activation by Jing Liqueur—One of the Most Popular Beverages in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Preparation of Jing Liqueur
2.3. Concentration of Jing Liqueur
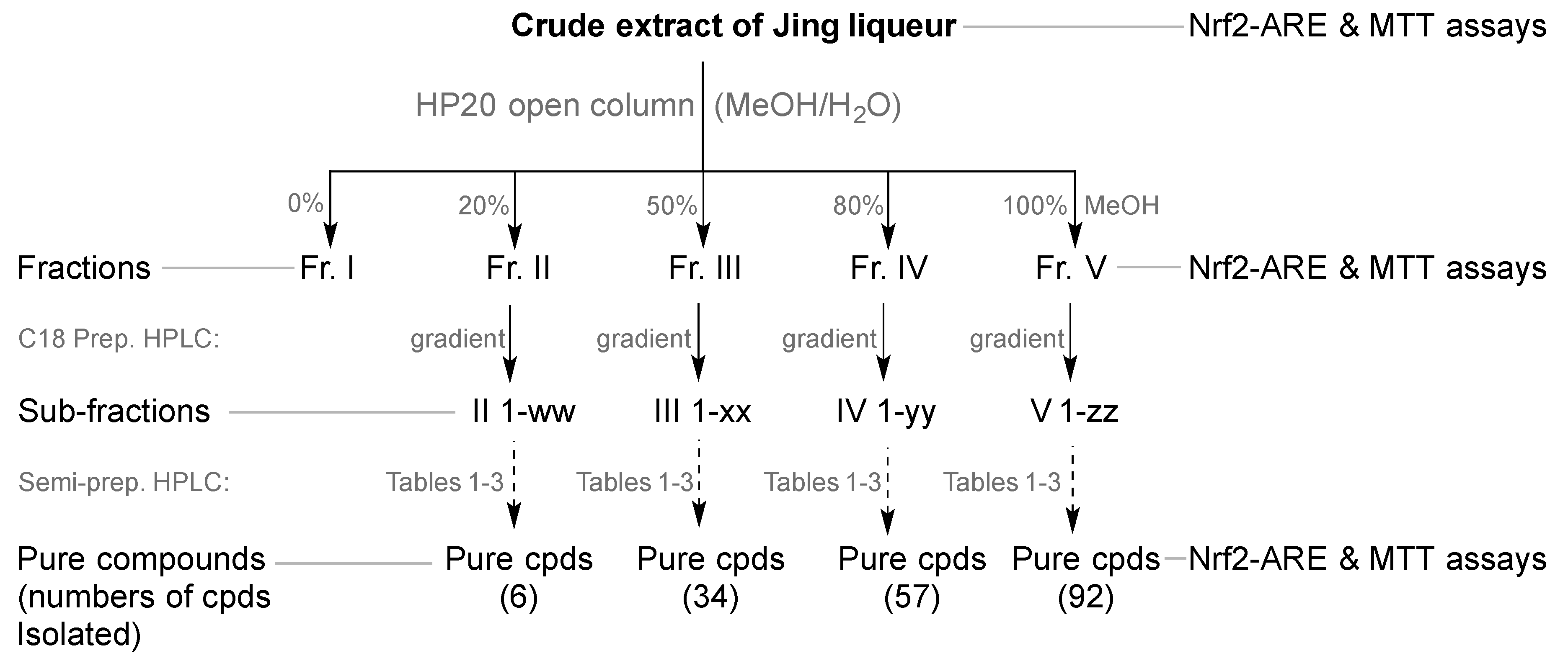
2.4. HP20 Open Column, Preparative and Semi-Preparative HPLC
2.5. LC/MS Condition for the Analysis
2.6. NMR Experiments
2.7. Analysis of Metals, Amino Acids, and Total Polysaccharides
2.8. Cell Culture and Condition
2.9. Chemicals Exposure and Luciferase Assay to Measure the Nrf2 Activation
2.10. Cell Viability Assay
2.11. Statistical Analysis
3. Results
3.1. Isolation and Structure Elucidation of Minor Compounds from Jing Liqueur
3.2. Analysis of Metals, Amino Acids, and Total Polysaccharides
3.3. Nrf2 Activation
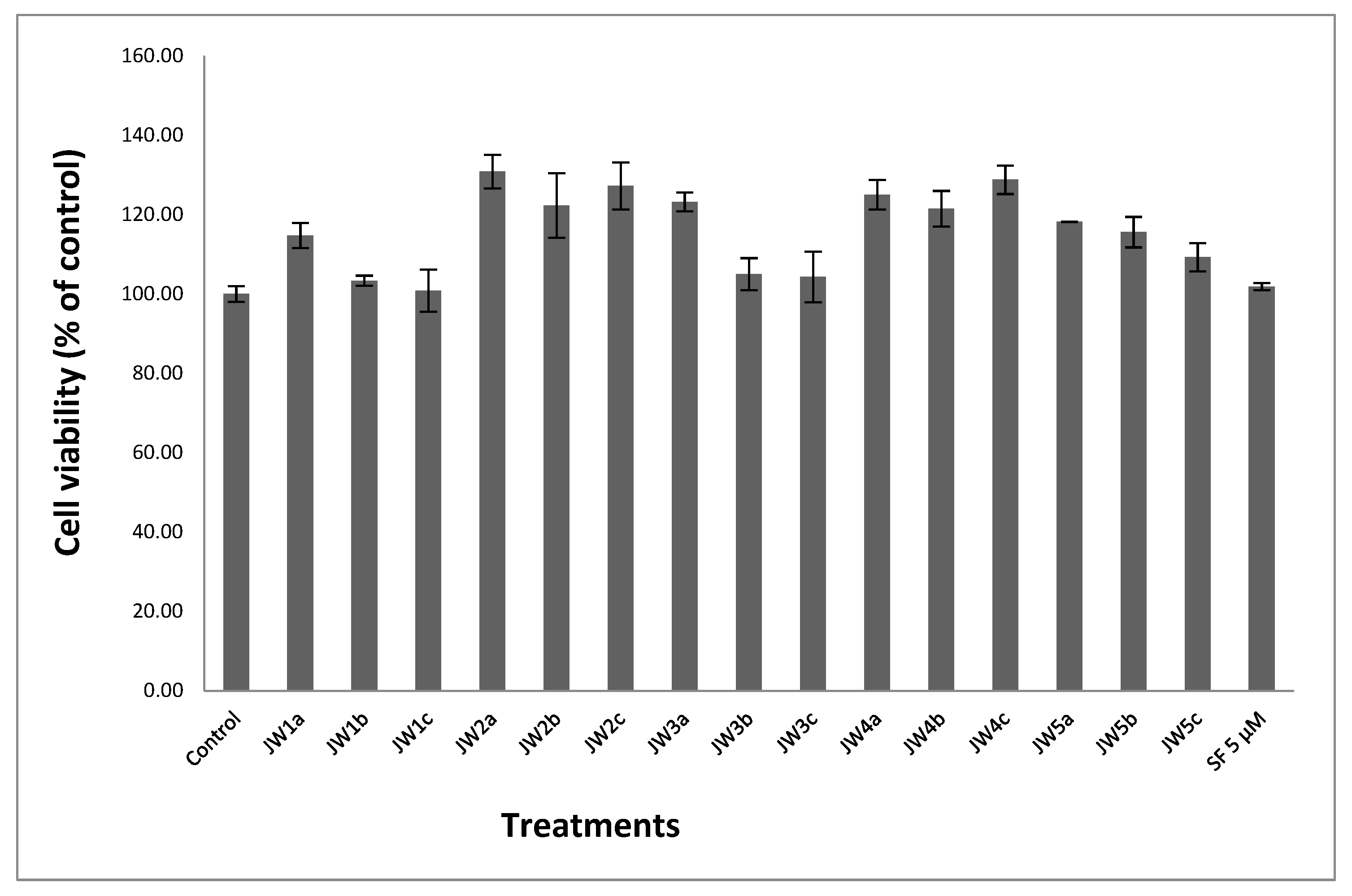
3.4. Cytotoxicity Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Feng, S.; Shan, Y.; Lu, S.; Liu, Y.; He, G. The anti-inflammatory effect of moderate drinking. Liquor-Mak. Sci. Tech. 2013, 229, 121–124. [Google Scholar]
- Lu, S.; Feng, S.; Li, J.; Yin, T.; Shi, Y.; Shi, J.; Chen, Y.; Liu, Y. Fatigue mitigating effect of Chinese Jing liqueur in sub-health patients. West. J. Trad. Chi. Med. 2017, 30, 82–84. [Google Scholar]
- Shan, Y.; Zhou, H.; Chen, M.; Chen, K.; Liu, Y.; Wang, L. Study on anti-fatigue, regulating immunity and enhancing sexual function of Chinese Jing liqueur. Trad. Chin. Pat. Med. 2018, 40, 1600–1603. [Google Scholar]
- Liu, J.; Zhao, Z.Z.; Chen, H.B. Review of astragali radix. Chin. Herb. Med. 2011, 3, 90–105. [Google Scholar]
- Wang, L.L.; Ding, H.; Yu, H.S.; Han, L.F.; Lai, Q.H.; Zhang, L.J.; Song, X.B. Cistanches herba: Chemical constituents and pharmacological effects. Chin. Herb. Med. 2015, 7, 135–142. [Google Scholar] [CrossRef]
- Li, Z.Q.; Cao, W.F. Research progress in Dioscorea opposita and major active components quick view other sources. Chin. J. Gerontol. 2013, 33, 1975–1976. [Google Scholar]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Plant. Med. 2010, 76, 7–19. [Google Scholar] [CrossRef]
- Chen, X.J.; Tang, Z.H.; Li, X.W.; Xie, C.X.; Lu, J.J.; Wang, Y.T. Chemical constituents, quality control, and bioactivity of Epimedii folium (Yinyanghuo). Am. J. Chin. Med. 2015, 43, 785–834. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, S.M.; Lu, Q.; Luo, J.; Cheng, Y.X. Identification of compounds from the water soluble extract of Cinnamomum cassia barks and their inhibitory effects against high-glucose-induced mesangial cells. Molecules 2013, 18, 10930–10943. [Google Scholar] [CrossRef]
- Mittal, M.; Gupta, N.; Parashar, P.; Mehra, V.; Khatri, M. Phytochemical evaluation and pharmacological activity of syzygium aromaticum: A comprehensive review. Int. J. Pharm. Pharm. Sci. 2014, 18, 10930–10943. [Google Scholar]
- Wei, W.L.; Zeng, R.; Gu, C.M.; Qu, Y.; Huang, L.F. Angelica sinensis in China-A review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. Int. J. Ethnopharmacol. 2016, 190, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, B.; Chou, G.; Yang, L.; Wang, Z. Chemical constituents from imperata cylindrical. China J. Chin. Mat. Med. 2012, 37, 2296–2300. [Google Scholar]
- Zhang, M.; An, C.; Gao, Y.; Rehana, K.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Liby, K.T. NRF2 and cancer: The good, the bad and the importance of context. Nat. Rev. Cancer 2012, 12, 564–571. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Lee, H.; Rangasamy, T.; Reddy, S.P.; Yamamoto, M.; Kensler, T.W.; Biswal, S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Investig. 2006, 116, 984–995. [Google Scholar] [CrossRef]
- Matzinger, M.; Fischhuber, K.; Heiss, E.H. Activation of Nrf2 signaling by natural products-can it alleviate diabetes? Biotechnol. Adv. 2018, 36, 1738–1767. [Google Scholar] [CrossRef]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef]
- Guo, N.; Bai, Z.; Jia, W.; Sun, J.; Wang, W.; Chen, S.; Wang, H. Quantitative Analysis of Polysaccharide Composition in Polyporus umbellatus by HPLC-ESI-TOF-MS. Molecules 2019, 24, 2526. [Google Scholar] [CrossRef]
- Li, W.-K.; Xiao, P.-G.; Liao, M.-C.; & Zhang, R.-Y. Caohuoside-C from the aerial parts of Epimedium koreanum Nakai. Gaodeng Xuexiao Huaxue Xuebao 1995, 16, 230–233. [Google Scholar]
- Kanwal, N.; Siddiqui, A.J.; Haq, F.U.; El-Seedi, H.R.; Musharraf, S.G. Two-stage mass spectrometry approach for the analysis of triterpenoid glycosides in Fagonia indica. RSC Adv. 2018, 8, 41023–41031. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Chin-Dusting, J.; Alexander, C.; Arnold, P.; Hodgson, W.; Lux, A.; Jennings, G. Effects of In Vivo and In Vitro L-Arginine Supplementation on Healthy Human Vessels. J. Cardiovasc. Pharm. 1996, 28, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Prasad, A.S. Zinc in Human Health: Effect of Zinc on Immune Cells. Mol. Med. 2008, 14, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Beto, J.A. The Role of Calcium in Human Aging. Clin. Nutr. Res. 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Cashman, K.D. Calcium intake, calcium bioavailability and bone health. Br. J. Nutr. 2002, 87 (Suppl. 2), S169–S177. [Google Scholar] [CrossRef]
- Grossman, R.; Ram, Z. The dark side of Nrf2. World Neurosurg. 2013, 80, 284–286. [Google Scholar] [CrossRef]





| NO | Fr. | tR, HPLC Condition | MF | Compound Name | Reference (See SM) |
|---|---|---|---|---|---|
| 1 | V | C.1, ACN, 33% | C20H18O6 | Noranhydroicaritin | Komatsu et al. 1970 |
| 2 | V | Sepherdex LH20 | C25H26O6 | Broussoflavonol F | Fang et al. 1995 |
| 3 | IV | 71 min, C.1, ACN, 18% 110 min | C26H28O11 | Epimedoside C | Li et al. 1990 |
| 4 | IV | 68 min, C.2, ACN, 30–35%, 80 min | C27H30O11 | Icariside I | Mizuno et al. 1987 |
| 5 | V | 56 min, C.1, ACN, 24% | C33H40O15 | Ieariline | Liang et al. 1988 |
| 6 | V | C.1, ACN, 33% | C27H30O11 | Caohuoside C | Li et al. 1995 |
| 7* | V | C.1, ACN, 30% | C26H28O11 | 4′-O-demethyl caohuoside C (New) | New |
| 8 | V | C.1, ACN, 35% | C26H28O11 | Phelodendrozide | Wang et al. 2010 |
| 9 | V | C.1, ACN, 33% | C26H28O10 | baohuoside II | Dong et al. 1994 |
| 10 | IV | 53.5 min, C.2, ACN, 17% 100 min | C32H38O16 | Hexandraside E | Leu et al. 2006 |
| 11 | V | C.2, ACN, 40–50%, 48 min | C26H28O10 | ||
| 12 | V | 49 min, C.2, ACN, 34% | C32H38O14 | Baohuoside IV | Li and Liu 1990 |
| 13 | V | C.1, ACN, 35% | C32H38O15 | Icarisoside B | Fukai et al. 1988 |
| 14 | V | C.1, ACN, 35% | C32H38O15 | Zhao et al. 2010 | |
| 15 | V | C.1, MeOH, 64% | C34H40O15 | Tu et al. 2011 | |
| 16 | V | C.1, ACN, 33% | C31H36O14 | Ikarisoside F | Fukai et al. 1988 |
| 17 | IV | 75 min, C.2, ACN, 30–35%, 80 min | C33H40O14 | Ueda et al. 1992 | |
| 18 | V | 35.5 min, C.2 ACN, 36% | C32H38O14 | Zhao et al. 2010 | |
| 19 | V | 39 min, C.2, ACN, 34% | C33H40O15 | Baohuoside VII | Li et al. 1988 |
| 20 | V | Sepherdex LH20 | C25H26O5 | 5,7, 4′-trihydroxy-8, 3′-diprenylflavone | Guo et al. 2006 |
| 21 | V | C.1, MeOH, 65% | C27H30O10 | Icariside II | Zhang et al. 2006 |
| 22 | V | C.1, MeOH, 65% | C32H38O14 | Sagittatoside B | Mizuno et al. 1988 |
| 23 | V | C.1, ACN, 24% | C39H50O19 | Epimedin C | Mizuno et al. 1988 |
| 24 | V | C.1, ACN, 24% | C33H40O15 | Sagittatoside A | Mizuno et al. 1988 |
| 25 | V | C.1, MeOH, 65% | C33H40O14 | 2″-O-rhamnosyl icariside II | Zhao et al. 2016 |
| 26 | V | C.1, MeOH, 64% | C33H38O14 | 3″′-carbonyl-2″-β-l-quinovosyl icariside II | Zhang et al. 2006 |
| 27 | V | C.1, ACN, 33% | C16H12O4 | Asahina et al. 1935 | |
| 28 | IV | 85.5 min, C.1, ACN, 18% 110 min | C39H50O20 | Das and Tripathi 2002 | |
| 29 | V | Sepherdex LH20 | C15H10O5 | Versulin | Geissman et al. 1946 |
| 30 | V | Sepherdex LH20 | C15H10O7 | Xanthaurine | Bao et al. 2004 |
| 31 | V | C.1, ACN, 35% | C27H30O11 | Koreanoside E | Li et al. 2015 |
| 32 | V | C.1, MeOH, 64% | C34H42O15 | Hu et al. 2010 | |
| 33 | V | C.1, MeOH, 64% | C34H42O14 | ||
| 34 | V | C.1, ACN, 24% | C39H50O20 | Epimedin A | Han, Lee 2017 |
| 35 | V | C.1, ACN, 24% | C39H50O20 | Maohuoside B | Li et al. 2006 |
| 36 | V | C.1, ACN, 24% | C38H48O19 | Epimedin B | Guo and Xiao 2003 |
| 37 | V | C.1, ACN, 24% | C39H50O19 | Hexandraside D | Mizuno et al. 1991 |
| 38 | V | C.1, ACN, 28% | C39H48O19 | Zhao et al. 2008 | |
| 39 | V | C.1, ACN, 28% | C39H50O19 | Ueda et al. 1992 | |
| 40 | V | 29 min, C.1, ACN, 22–33%, 60 min | C39H50O20 | Hexandraside F | Wang et al. 2007 |
| 41 | V | 33 min, C.1, ACN, 22–33%, 60 min | C38H48O19 | Zhao et al. 2010 | |
| 42 | IV | 85.5 min, C.2, ACN, 18% 110 min | C16H12O6 | Diosmetin | Takeda et al. 2007 |
| 3 | V | 77.5 min, C.2, ACN, 16–20%, 100 min | C38H48O20 | Rouhuoside | Li et al. 1990 |
| 44 | V | 47 min, C.2, ACN, 22–33%, 60 min | C38H48O20 | Diphylloside A/Ikarisoside C | Jia et al. 1998 |
| 45 | IV | 39 min, C.2, MeOH, 50–60% 80 min | C39H48O21 | Jin et al. 2013 | |
| 46 | V | C.1, ACN, 28% | C39H48O20 | Jin et al. 2013 | |
| 47 | III | 41.5 min, C.1, MeOH, 6–8.5%, 65 min | C34H44O20 | Li et al. 2012 | |
| 48 | V | 38.5 min, C.2, ACN, 22–24%, 75 min | C15H10O4 | Isoaurostatin/4′,7-Dihydroxyisoflavone | Xu et al. 1979 |
| 49 | V | Sepherdex LH20 | C16H12O4 | Formononetin | Reiners 1966 |
| 50 | V | 47 min, C.2, ACN, 22–24%, 75 min | C16H12O5 | Biochanin A, Olmelin | Nilsson 1961 |
| 51 | V | 47 min, C.2, ACN, 22–24%, 75 min | C16H12O5 | Calycosin, Cyclosin | Markham et al. 1968 |
| 52 | V | C.2, ACN, 20–22%, 75 min 55.5 min | C16H12O5 | 7,4′-Dihydroxy-3′-methoxyisoflavone | Hirakura et al. 1997 |
| 53 | V | C.2, ACN, 20–22%, 75 min 55.5 min | C23H24O10 | 8-O-Methylretusin-7-O-β-d-glucopyranoside | Rukachaisirikul 2002 |
| 54 | V | 69 min, C.1, MeOH, 35%, 80 min | C23H24O10 | Clarke et al. 2004 | |
| 55 | IV | 17 min, C.2, ACN, 15–18%, 80 min | C21H20O9 | Daidzoside | Xiao et al. 2016 |
| 56 | IV | 17min, C.2, ACN, 15–18%, 80 min | C22H22O10 | 3′-Methoxydaidzin | Hirakura et al. 1997 |
| 57 | IV | 38 min, C.2, ACN, 20%, 90 min | C21H20O10 | Genistoside | Yuan et al. 2008 |
| 58 | V | 66.5 min, C.2, ACN, 22–24%, 75 min | C22H22O10 | Calycosin 7-glucoside | Markham et al. 1968 |
| 59 | IV | 59 min, C.1, ACN, 18% 110 min | C22H22O9 | Ononoside | Lebreton et al. 1967 |
| 60 | IV | 14.5 min, C.2, MeOH, 20% | C27H30O14 | Daidzein 7,4′-diglucoside | Li et al. 2014 |
| 61 | IV | 33.5 min, C.2, MeOH, 28%, 100 min | C26H28O14 | Neobacin | Breytenbach 1986 |
| 62 | IV | 19 min, C.2, ACN, 15–18%, 80 min | C22H22O10 | Caragiside B | Nisar et al. 2011 |
| 63 | V | C.1, MeOH, 35%, 75 min | C22H22O9 | Isoononin | Liu et al. 2005 |
| 64 | IV | 24 min, C.2, MeOH, 20% | C26H28O14 | Ambocin | Breytenbach 1986 |
| 65 | IV | C.2, ACN, 15–18%, 80 min | C21H20O9 | Neopuerarin | Zhang et al. 2009 |
| 66 | IV | 42 min, C.2, ACN, 20%, 90 min | C21H20O9 | Ma et al. 2017 | |
| 67 | IV | 18 min, C.2, MeOH, 20% | C21H20O10 | 8-C-Glucosyl-7,3′,4′-trihydroxy isoflavone | Wong et al. 2017 |
| 68 | IV | 63 min, C.2, MeOH, 20%, 56 min, 20–30% 40 min | C26H28O13 | Chen et al. 2009 | |
| 69 | IV | C.2, ACN, 15–18%, 80 min | C21H20O9 | Neopuerarin A | Zhang et al. 2009 |
| 70 | III | 39 min, C.2, MeOH, 6–8.5%, 65 min | C28H32O15 | Wang et al. 2006 | |
| 71 | III | 26 min, C.2, MeOH, 28%, 50 min | C21H20O10 | Pistelli et al. 1998 | |
| 72 | IV | 54 min, C.2, ACN, 20%, 90 min | C22H22O10 | Ohshima et al. 1988 | |
| 73 | IV | 58.5 min, C.2, ACN, 20%, 90 min | C26H28O13 | Puerarin apioside | Ingham et al. 1986 |
| 74 | IV | 67.5 min, C.2, ACN, 20%, 90 min | C26H28O13 | Kinjo et al. 1987 | |
| 75 | III | 45 min, C.2, MeOH, 6–8.5%, 65 min | C26H28O14 | Peng et al. 2011 | |
| 76 | IV | 73 min, C.2, MeOH, 28% 100 min | C23H22O10 | Acetyldaidzin | Ohta et al. 1979 |
| 77 | IV | 24 min, C.2, ACN, 15–18%, 80 min | C23H22O11 | Zhou et al. 2013 | |
| 78 | IV | 74 min, C.1, ACN, 18% 110 min | C23H28O11 | Astraganoside | Liu et al. 2007 |
| NO | Fr. | tR, HPLC Condition | MF | Compound | Reference (See SM) |
|---|---|---|---|---|---|
| 79 | V | 58 min, C.2, ACN, 57%, 30 min, 57–62% 20 min, 62–70% 29 min | C30H48O3 | Oleanolic acid | Tan et al. 2002 |
| 80 | V | 26 min, C.2, ACN, 57%, 30 min, 57–62% 20 min, 62–70% 29 min | C30H48O4 | Sumaresinolic acid | Chan et al. 1992 |
| 81 | V | C.1, MeOH, 72% | C36H58O8 | 3-O-β-Glc-oleanolic acid | Dubois et al. 1990 |
| 82 | IV | 34 min, C.2, MeOH, 60–70%, 60 min | C48H76O19 | Calendulaglycoside B | Vidal-Ollivier et al. 1989 |
| 83 | V | C.1, MeOH, 72% | C36H56O9 | Calenduloside E | Zhang et al. 2013 |
| 84 | V | C.2, ACN, 30–35%, 60 min, 35–40% 20 min | C48H74O18 | Papyrioside LG | |
| 85 | V | 27 min, C.1, ACN, 35–42%, 80 min, 42–100% 5 min | C42H66O14 | Chikusetsusaponin Iva | Yang et al. 1995 |
| 86 | V | C.2, ACN, 30–35%, 60 min, 35–40% 20 min | C42H64O14 | Kuroda et al. 2006 | |
| 87 | IV | 36.5 min, C.2, MeOH, 65–68%, 40 min | C54H86O23 | Scheffleraside II | Mshvildadze et al. 2001 |
| 88 | V | C.2, ACN, 30–35%, 60 min, 35–40% 20 min | C49H78O19 | Chikusetsusaponin V methyl ester | Kondo et al. 1971 |
| 89 | V | C.2, ACN, 30–35%, 60 min, 35–40% 20 min | C48H76O19 | Ginsenoside Ro | Matsuda et al. 1990 |
| 90 | V | C.2, ACN, 30–35%, 60 min, 35–40% 20 min | C54H86O24 | Calendulaglycoside A | Vidal-Ollivier et al. 1989 |
| 91 | V | C.2, ACN, 30–35%, 60 min, 35–40% 21 min | C53H84O23 | Elatoside C | Yoshikawa et al. 1993 |
| 92 | V | 24 min, C.1, ACN, 35–42%, 80 min, 42–100% 5 min | C47H74O18 | Pseudoginsenoside RT1 | Morita et al. |
| 93 | V | 55 min, C.1, ACN, 35–42%, 80 min, 42–100% 5 min | C43H68O14 | Silphioside A | Jiang et al. 1992 |
| 94 | V | 46 min, C.1, ACN, 35–42%, 80 min, 42–100% 5 min | C48H76O18 | Umbellatoside B | Sosa et al. 2011 |
| 95 | V | 20 min, C.2, ACN, 36%, 40 min | C42H66O14 | Wedelin | Matos et al. 1983 |
| 96 | V | 27.8 min, C.2, ACN, 57%, 30 min, 57–62% min, 62–70% 29 min | C30H48O4 | 6β-Hydroxyursolic acid | Sakakibara et al. 1983 |
| 97 | V | C.2, ACN, 35% | C30H48O6 | 3β,6β,19α,24-tetrahydro xyurs-12-en-28-oic acid | Fang et al. 1996 |
| 98 | IV | 71 min, C.2, ACN, 18% 110 min | C36H58O11 | Abe et al. 1987 | |
| 99 | V | 38 min, C.2, ACN, 20–22%, 75 min | C36H58O11 | Abe et al. 1987 | |
| 100 | V | 20min, C.2, ACN, 36%, 40 min | C42H66O14 | Wedelin | Matos et al. 1983 |
| 101 | IV | 8 min, C.2, MeOH, 10–14%, 40 min | C37H55N3O16 | Lycibarbarspermidine L | Zhou et al. 2016 |
| 102 | IV | C.2, MeOH, 10–14%, 40 min | C37H55N3O16 | Lycibarbarspermidine M | Zhou et al. 2016 |
| 103 | IV | C.2, MeOH, 10–14%, 40 min | C37H53N3O16 | Lycibarbarspermidine E | Zhou et al. 2016 |
| 104 | IV | C.2, MeOH, 10–14%, 40 min | C37H53N3O16 | Jin et al. 2015 | |
| 105 | IV | C.2, MeOH, 10–14%, 40 min | C31H43N3O11 | Lycibarbarspermidine D | Zhou et al. 2016 |
| 106 | IV | C.2, MeOH, 10–14%, 40 min | C31H43N3O11 | Lycibarbarspermidine A | Zhou et al. 2016 |
| 107 | IV | 26.5min, C.2, ACN, 20%, 90 min | C13H14N2O2 | Tetrahydroharman-3-carboxylic acid | Tsuchiya et al. 1999 |
| 108 | III | 23 min, C.2, MeOH, 15%, 95 min | C13H14N2O3 | Herraiz et al. 2004 | |
| 109 | III | 39 min, C.2, MeOH, 17%, 80 min | C13H14N2O2 | Herraiz et al. 1993 | |
| 110 | III | 36.5 min, C.2, MeOH, 15%, 95 min | C14H17NO3 | 3α-Benzoyloxynortropan-6β-ol | Al-Said et al. 1986 |
| 111 | IV | 12 min, C.2, ACN, 20%, 90 min | C17H21NO5 | Confoline | Aripova et al. 1996 |
| 112 | II | 25 min, C.2, MeOH, 5%, 40 min | C6H7NO2 | Hiermann et al. 2002 | |
| 113 | III | 47.5 min, C.2, MeOH, 17–20%, 100 min | C10H13NO4 | Chin et al. 2003 | |
| 114 | III | 7.5 min, C.2, MeOH, 5–15%, 60 min | C6H5NO2 | Nicotinic acid | Krehl et al. 1946 |
| 115 | IV | 18 min, C.2, MeOH, 20% | C8H15NO2 | Singer et al. 1935 | |
| 116 | IV | 8 min, C.2, MeOH, 10–14%, 40 min | C14H22NO4+ | Codonopyrrolidium B | He et al. 2014 |
| 117 | III | 17 min, C.2, MeOH, 10%, 80 min | C8H17N3O3 | Gegauer et al. 2003 | |
| 118 | III | 19 min, C.2, MeOH, 10%, 80 min | C6H13NO2 | Perrin et al. 2000 | |
| 119 | IV | 20 min, C.2, ACN, 15–18%, 80 min | C10H8O4 | Scopoletin | Best 1948 |
| 120 | IV | 23 min, C.2, ACN, 15–18%, 80 min | C20H24O8 | Vellein | Maruyama et al. 2009 |
| 121 | IV | 33.5min, C.2, MeOH, 28%, 100 min | C8H10O2 | Phenethyl alcohol | Wang et al. 1982 |
| 122 | IV | 35.5 min, C.2, ACN, 20%, 90 min | C8H8O4 | Pisolithin B | Benecke et al. 1984 |
| 123 | V | 43.5 min, C.2, ACN, 22–33%, 60 min | C9H8O2 | trans-β-Carboxystyrene | Billmann et al. 1909 |
| 124 | IV | 21 min, C.2 ACN, 15–18%, 80 min | C10H10O4 | Ferulic acid | Henderson and Farmer 1955 |
| 125 | V | 60 min, C.2 ACN, 22–24%, 75 min | C11H12O4 | Oonuma et al. 1993 | |
| 126 | V | C.1, ACN, 28% | C11H12O3 | Newman et al. 1952 | |
| 127 | V | C.1, ACN, 28% | C12H14O4 | Ethyl ferulate | Nakayama et al. 1996 |
| 128 | III | 71 min, C.1, ACN, 12–25%, 43 min, 25–30% 12 min | C9H8O4 | Caffeic acid | Baerheim 1951 |
| 129 | IV | 55 min, C.1, MeOH, 45–48%, 80 min | C11H12O4 | Ethyl caffeoate | Mao et al. 2011 |
| 130 | III | 37min, C.2, MeOH, 28%, 50 min | C9H8O3 | trans-p-Hydroxycinnamic acid | King et al. 1952 |
| 131 | III | 40 min, C.2, MeOH, 28%, 50 min | C10H10O4 | Isoferulic acid | Qiao and Chen 1991 |
| 132 | III | 48.5 min, C.2, MeOH, 15%, 95 min | C15H18O8 | p-Coumaric acid β-glucoside | Runeckles, Woolrich 1963 |
| 133 | III | 48.5 min, C.2, MeOH, 15%, 95 min | C16H20O9 | Glucosidoferulic acid | Ibrahim et al. 1970 |
| 134 | IV | 22 min, C.2, MeOH, 20% | C10H10O4 | Muratake et al. 2013 | |
| 135 | III | 30 min, C.2, MeOH, 6–8.5%, 65 min | C14H20O7 | Fujimatu et al. 2003 | |
| 136 | IV | 73.5 min, C.2, MeOH, 20%, 56 min, 58.5 min, 20–30%, 40 min | C35H46O20 | Echinacoside | Frezza et al. 2017 |
| 137 | III | 29.5 min, C.2, MeOH, 30–40%, 90 min | C37H48O21 | Tubuloside A | Kobayashi et al. 1987 |
| 138 | IV | 80 min, C.2, MeOH, 28% 100 min | C29H36O15 | Verbascoside | Pham et al. 1988 |
| 139 | V | 60.5 min, C.2, ACN, 16–20%, 100 min | C20H26O12 | Wende and Fry 1997 |
| NO | Fr. | tR, HPLC Condition | MF | Compound | Reference (See SM) |
|---|---|---|---|---|---|
| 140 | V | C.1, ACN, 60% | C16H14O4 | Letcher and Nhamo 1973 | |
| 141 | V | C.2, ACN, 40–50%, 48 min | C17H16O4 | Batatasin I | Gonnet et al. 1973 |
| 142 | V | C.1, ACN, 30% | C15H10O4 | Morton et al. 1941 | |
| 143 | V | C.1, ACN, 30% | C16H12O5 | Wang et al. 2011 | |
| 144 | V | C.1, ACN, 33% | C16H12O5 | Wu et al. 2003 | |
| 145 | V | C.1, ACN, 30% | C15H10O5 | Lee et al. 1994 | |
| 146 | V | C.1, ACN, 30% | C16H12O4 | Digitolutein | Koumaglo et al. 1992 |
| 147 | V | 34 min, C.2, ACN, 40% | C15H10O3 | Bistrzycki and Zen-Ruffinen 1920 | |
| 148 | V | 34 min, C.2, ACN, 40% | C15H10O5 | Yang et al. 1992 | |
| 149 | IV | 19 min, C.2, MeOH, 20%, 56 min, 20–30% 40 min | C14H8O4 | Varbanov et al. 1986 | |
| 150 | V | 29 min, C.2, ACN, 22–27%, 60 min | C13H8O4 | 1,3-Dihydroxyxanthone | Liang et al. 1982 |
| 151 | III | 39 min, C.2, MeOH 6–8.5%, 65 min | C12H16O8 | Baba et al. 1995 | |
| 152 | IV | 15 min, C.2, MeOH, 20% | C16H18O9 | Biflorin | Zhang et al. 1997 |
| 153 | III | 46 min, C.2, MeOH, 15%, 95 min | C16H18O9 | Isobiflorin | Tanaka et al. 1993 |
| 154 | III | 37.5 min, C.2, MeOH, 6–8.5%, 65 min | C16H18O9 | Undulatoside A | Itoh et al. 2003 |
| 155 | III | 37.5 min, C.2, MeOH, 6–8.5%, 65 min | C16H18O9 | Wang et al. 2011 | |
| 156 | III | 55 min, C.2, MeOH, 17%, 80 min | C26H34O12 | Yan et al. 2008 | |
| 157 | V | 89 min, C.2, ACN, 16–20%, 100 min | C22H26O8 | Syringaresinol | Abu Zarga 1986 |
| 158 | V | 54 min, C.2, ACN, 22–33%, 60 min | C18H16O5 | Shi et al. 2007 | |
| 159 | IV | 25.5 min, C.2, ACN, 15–20%, 50 min | C18H14O8 | Prolithospermic acid | Dai et al. 2010 |
| 160 | III | 29 min, C.2, MeOH, 28%, 50 min | C18H14O8 | 7-Epiblechnic acid | Wang et al. 2010 |
| 161 | III | 29 min, C.2, MeOH, 28%, 50 min | C18H14O8 | Blechnic acid | Wada et al. 1992 |
| 162 | V | 69 min, C.2, MeOH, 35%, 80 min | C23H26O10 | Guo et al. 2014 | |
| 163 | V | 36 min, C.2, ACN, 22–24%, 75 min | C7H6O3 | p-Salicylic acid | Sager and Schooley 1945 |
| 164 | V | 63.5 min, C.2, ACN, 22–24%, 75 min | C9H10O3 | Heim et al. 1957 | |
| 165 | V | 41 min, C.2, ACN, 20–22%, 75 min | C8H8O3 | p-Methoxy benzoic acid | Reitberg and Schentag 1983 |
| 166 | III | 67 min, C.2, MeOH, 12–25%, 43 min, 25–30% 12 min | C8H8O4 | Parham et al. 1954 | |
| 167 | III | 49.5 min, C.2, ACN, 13–15%, 90 min | C8H8O4 | Vanillic acid | Sammons and Williams 1946 |
| 168 | IV | 20min, C.2, MeOH, 20% | C7H6O3 | Rancinamycin IV | Li et al. 2004 |
| 169 | IV | 44 min, C.2, MeOH, 20%, 56 min, 20–30% 40 min | C7H6O2 | Rivers 1947 | |
| 170 | II | 39.5 min, C.2, MeOH, 7–9%, 70 min | C14H18O9 | Yang et al. 2013 | |
| 171 | V | 57 min, C.2, ACN, 20–22%, 75 min | C9H12O3 | 1,3,5-Trimethoxybenzene | Allain et al. 1980 |
| 172 | IV | 24min, C.2, MeOH, 20% | C8H10O2 | p-Hydroxyphenetole | Rosenwald 1951 |
| 173 | II | 22 min, C.2, MeOH, 2%, 60 min | C13H18O8 | Tachioside | Sano et al. 1991 |
| 174 | IV | 73 min, C.2, MeOH, 28% 100 min | C9H10O4 | ||
| 175 | III | 77 min, C.2, MeOH, 12–25%, 43 min, 25–30% 12 min | C7H6O3 | Salicylic acid | Ichniowski and Hueper 1946 |
| 176 | III | 78 min, C.2, MeOH, 25–30%, 60 min, 30–35% 40 min | C16H26O8 | Bodinierin | Xie et al. 2006 |
| 177 | III | 80 min, C.2, MeOH, 25–30%, 60 min, 30–35% 40 min | C16H26O8 | Kankanoside O | Liu et al. 2016 |
| 178 | III | 75 min, C.2, MeOH, 20–25%, 80 min | C16H28O7 | Fan et al. 2011 | |
| 179 | V | 21 min, C.2, ACN, 16–20%, 100 min | C27H44O8 | (25R)-20,26-Dihydroxyecdysone | Suksamrarn 1998 |
| 180 | III | 43min, C.2, MeOH, 30–40%, 90 min | C27H44O7 | 26-Hydroxyecdysone | Li et al. 2006 |
| 181 | IV | C.2, ACN, 15–18%, 80 min | C27H44O7 | 3-epi-20-Hydroxyecdysone | Thompson et al. 1974 |
| 182 | III | 47.5 min, C.2, MeOH, 6–8.5%, 65 min | C16H22O10 | Li et al. 1999 | |
| 183 | II | 51 min, C.2, MeOH, 2%, 60 min | C16H22O11 | Desacetylasperulosidic acid | Inouye et al. 1974 |
| 184 | II | 47.5 min, C.2, MeOH, 7–9%, 70 min | C16H24O10 | Mussaenosidic acid | Kohda et al. 1989 |
| 185 | III | 9.5 min, C.2, MeOH, 5–15%, 60 min | C11H13N3O5 | 9-Deazainosine | Liu et al. 2005 |
| 186 | V | 38 min, C.2, MeOH, 35%, 80 min | C12H16O2 | Sedanonic acid lactone | Mitsuhashi and Nomura 1966 |
| 187 | V | 46 min, C.2, ACN, 20–22%, 75 min | C12H16O4 | Senkyunolide I | Huang et al. 2013 |
| 188 | IV | 65.5 min, C.2, ACN, 17% 100 min | C12H16O4 | Senkyunolide H | Huang et al. 2013 |
| 189 | V | C.2, ACN, 60% | C17H24O2 | Falcarindiol | Lechner et al. 2004 |
| Cpd | Fr. | Relative Nrf2 Activity (Fold Induction) | Cpd | Fr. | Relative Nrf2 Activity (Fold Induction) | Cpd | Fr. | Relative Nrf2 Activity (Fold Induction) |
|---|---|---|---|---|---|---|---|---|
| Control | - | 1.0 | 125 | V | 9.5 | 145 | V | 3.0 |
| 50 | V | 4.0 | 126 | V | 16.0 | 146 | V | 3.0 |
| 51 | V | 11.5 | 127 | V | 10.5 | 168 | IV | 3.0 |
| 53 | V | 4.0 | 128 | V | 3.0 | 171 | V | 3.5 |
| 55 | IV | 3.0 | 129 | IV | 2.2 | 186 | V | 3.5 |
| 58 | V | 6.5 | 143 | V | 6.0 | SF (5 μM) | - | 15.0 |
| 78 | IV | 4.0 | 144 | V | 3.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.-S.; Xu, J.; Chen, M.; Wang, D.; Yang, Y.; Manavalan, A.; Wu, X.; Liu, Y.; Cao, S. Compound Analysis of Jing Liqueur and nrf2 Activation by Jing Liqueur—One of the Most Popular Beverages in China. Beverages 2020, 6, 1. https://doi.org/10.3390/beverages6010001
Cai Y-S, Xu J, Chen M, Wang D, Yang Y, Manavalan A, Wu X, Liu Y, Cao S. Compound Analysis of Jing Liqueur and nrf2 Activation by Jing Liqueur—One of the Most Popular Beverages in China. Beverages. 2020; 6(1):1. https://doi.org/10.3390/beverages6010001
Chicago/Turabian StyleCai, You-Sheng, Jian Xu, Mosi Chen, Daoqing Wang, Yuejun Yang, Arulmani Manavalan, Xiaohua Wu, Yuancai Liu, and Shugeng Cao. 2020. "Compound Analysis of Jing Liqueur and nrf2 Activation by Jing Liqueur—One of the Most Popular Beverages in China" Beverages 6, no. 1: 1. https://doi.org/10.3390/beverages6010001
APA StyleCai, Y.-S., Xu, J., Chen, M., Wang, D., Yang, Y., Manavalan, A., Wu, X., Liu, Y., & Cao, S. (2020). Compound Analysis of Jing Liqueur and nrf2 Activation by Jing Liqueur—One of the Most Popular Beverages in China. Beverages, 6(1), 1. https://doi.org/10.3390/beverages6010001







