Abstract
Pomegranate juice (PJ) has total antioxidant capacity which is reportedly higher compared to other common beverages. This short study aimed to assess the total antioxidant capacity of commercial PJ and pomegranate fruit using a newly described method for iron (III) reducing antioxidant capacity (iRAC) and to compare with the ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) and Folin–Ciocalteu assays. Commercial PJ, freeze-dried pomegranate, and oven-dried pomegranate were analyzed. The calibration results for iRAC were comparable to ABTS and Folin–Ciocalteu methods in terms of linearity (R2 > 0.99), sensitivity and precision. The total antioxidant capacity for PJ expressed as trolox equivalent antioxidant capacity (TEAC) was 33.4 ± 0.5 mM with the iRAC method and 36.3 ± 2.1 mM using the ABTS method. For dried pomegranates, total antioxidant capacity on a dry weight basis (DB) was 89–110 mmol/100 g DB or 76.0 ± 4.3 mmol/100 g DB using iRAC and ABTS methods, respectively. Freeze-dried pomegranate had 15% higher total antioxidant capacity compared with oven-dried pomegranate. In conclusion, pomegranate has high total antioxidant capacity as evaluated by the iRAC and ABTS methods, though variations occur due to the type of cultivar, geographic origin, processing and other factors. The study is relevant for attempts to refine food composition data for pomegranate and other functional foods.
1. Introduction
Pomegranate (Punica granatum L.) is an ancient food used as a traditional remedy against a variety of conditions including microbial infections. Pomegranate is perceived as a “superfood” due to its high antioxidant capacity [1,2,3,4,5,6,7,8]. Current databases show pomegranate juice (PJ) possesses total antioxidant capacity which is greater than many other beverages [9,10,11,12]. Although the total antioxidant capacity for pomegranate from different countries were reported, only few publications deal with commercial PJ as sold in the market [9,13,14]. The effect of drying on pomegranate seed, arils, and peels were examined [15,16], but oven-drying and freeze-drying effects on the total antioxidant capacity of whole pomegranate fruit has not been compared.
Recent Association of Analytical Communities (AOAC) guidelines indicated that total antioxidant capacity should be assessed by multiple methods and all findings expressed as trolox equivalent antioxidant capacity (TEAC) in order to enable comparisons between different assays [17]. We have reported a comparative study of honey using iron (III) reducing antioxidant capacity (iRAC), FRAP, ABTS, DPPH, and Folin methods, and found that solvent pH was a major influence on assay responses [18].
The aims of this short study were to reevaluate the total antioxidant capacity of pomegranate fruit and commercial PJ using a newly described iRAC assay [18,19] and to compare the findings with the well-established ABTS method [20]. Total phenol content (TPC) was evaluated, also, as this parameter correlated with total antioxidant capacity for plant derived foods [21]. The study is significant for attempts to refine food composition data for pomegranate and other functional foods for improved nutrition applications, product development, or international trade [22].
2. Materials and Methods
2.1. Preparation of Samples and Antioxidant Standard
Pomegranate fruit (Hicaz variety, Turkey) and commercial PJ (POM Wonderful 100% PJ; POM Wonderful LLC UK, Gent, Belgium) were purchased from a large supermarket in the United Kingdom (UK). The unpeeled pomegranates were washed, diced using a stainless steel knife and divided into two portions. One portion of pomegranate was oven-dried at 80 °C overnight and another was frozen at −80 °C for 48 h, then freeze-dried for 48 h using the HETO Power Dry PL6000 instrument (Thermo Fisher Scientific, Ltd., Dublin, Ireland). The dried pomegranate samples were ground using a blender (DeLonghi Coffee Grinder; Type KG40 EXA; Ningbo CIE Corporation, Zhejiang, China) and the resulting powders (5 g) were extracted by stirring with 100 mL of solvent (40:60 v/v methanol/water) for 2 h. The pomegranate solvent extract was centrifuged using a microcentrifuge (MSE Micro Centaur Centrifuge; MSE (UK) Ltd., London, UK) at a speed of 11,000 rpm (8871 g) for 5 min, and the supernatant stored at −18 °C. The solids content for PJ was determined by drying a known volume and weighing the residue. Gallic acid and trolox reference compounds were prepared as 1000 µM solution and diluted to 500 µM, 250 µM, 125 µM, and 62.5 µM daily before use. Pomegranate juice was analyzed as received after dilution (×100) with deionized water. Pomegranate extract was diluted (25–100 fold) before analysis.
2.2. Iron (III) Reducing Antioxidant Capacity (iRAC) Assay
The iRAC assay was performed as described recently [18,19] with modification. The stock iRAC reagent comprised 20 mg of ferrozine dissolved with 18 mL of Tris buffer (0.1 M, pH 7.0) or potassium acetate buffer (0.1 M, pH 4.5) and mixed with 8 mg of iron (III) ammonium sulphate (8 mg) dissolved with 2 mL of deionized water. Typically, the final iRAC working solutions were prepared after the sample array to be analyzed was ready; 20 µL of pomegranate extract, PJ, or reference compound (trolox or gallic acid) were added to a 96-well microplate followed by 280 µL of the iRAC reagent. The reaction mixtures were incubated for 30 min at 37 °C. Absorbance was read at 562 nm (A562) using a microplate reader (VersaMax model reader; Molecular devices, Sunnydale, CA, USA). Several (25, 50, 100-fold) diluted samples were analyzed to determine the optimum dilution necessary for sample absorbance to fall within the linear range for analysis. Final samples were analyzed on two separate occasions using (n =) 12–16 wells of a microplate. For timecourse measurements, A562 readings were recorded at 2 min intervals for 30 min.
2.3. ABTS Assay
The ABTS assay was performed as described by Walker and Everette [20] with modifications. ABTS (27.4 mg) was added to 90 mL PBS buffer. Sodium persulfate (20 mg/mL PBS) was prepared separately, added to ABTS stock solution, and both were made up to 100 mL using PBS buffer. The mixture was stored in the dark for 16 h. The ABTS·+ solution was diluted with PBS buffer to obtain an absorbance of 0.85 at 734 nm (A734) using a 1 cm conventional spectrophotometer (Ultrospec 2000 UV/Visible spectrophotometer, Pharmacia Biotech. Ltd., Uppsala, Sweden). Thereafter, 20 µL of samples or reference compounds (trolox) were added to 96-well microplate followed by 280 µL ABTS·+ solution. The plates were incubated in the dark for 30 min at 37 °C and A734 was recorded using a microplate reader (VersaMax model reader; Molecular devices, Sunnydale, CA, USA). Pre-diluted samples were analyzed on two separate occasions using (n =) 12–16 wells of a microplate.
2.4. Folin–Ciocalteu Assay for Total Phenols
The Folin–Ciocalteu method of Singleton et al. [21] was used for TPC determination, with minor modification. Antioxidant standards or samples (50 µL) of were added to microcentrifuge tubes with 100 µL Folin–Ciocalteu reagent and 850 µL of sodium carbonate solution. The samples were vortexed briefly and incubated for 20 min at 37–40 °C. Thereafter, 200 µL of the reacted samples were transferred to a 96-well microplate (×4200 µL per sample) and absorbance was read at 760 nm (A760) using a microplate reader.
2.5. Data Analysis and Statistical Analysis
Microplate readouts were transferred to Excel for calculations and graphing. Calibration graphs for iRAC, ABTS, or Folin–Ciocalteu assays were generated by plotting absorbance changes (∆A) corrected for the sample-blank (B1) and zero-reagent blank (B2), e.g., ∆A = A − (B1 + B2) on the y-axis. The concentration of analyte (mol/L) in the assay vessel was plotted on the graph x-axis. For the ABTS assay, ∆A is A734 for ABTS reagent minus A734 for antioxidant samples. Calibration parameters (e.g., molar absorptivity, the minimum detectable concentration, upper limit of detection, regression coefficient) were determined by fitting a straight lines (y = mx) to the data, where m is the slope. The total antioxidant capacity for samples were determined from absorbance changes (∆As) using Beer’s relations Equations (1)–(3);
where, TAC = total antioxidant capacity e.g., mg-trolox/100 g of sample (mg-Tx/100 g), m = slope for the trolox calibration graph, Va = assay volume (µL; × 10−6 L), SV = samples sip volume assayed (µL; × 10−6 L), DF = dilution factor for samples before analysis (1 if undiluted), Vex = total volume of pomegranate extract, MW = molecular weight for the reference antioxidant (g/mole), W = weight of food sample (g). For the PJ samples W/Vex is the solid content as determined by drying. In consideration of food composition comparisons, data were adjusted from dry weight basis (DB) to fresh weight (FW) basis taking into account the initial percent moisture content (%M) of the product. Interconversions of FW to DB data was performed using the relation; DB = FW/(1 − θ) where θ = % M/100. Statistical significance was tested by using one-way ANOVA with Turkey post-hoc testing for separation of means. Significant differences were noted with p < 0.05. All analyses were carried out using IBM SPSS Statistics 24.
3. Results
3.1. Calibration Results of iRAC, ABTS, and Folin-Ciocalteu Methods
The assay time was fixed at 30 min based on the time course of A562 readings for the iRAC procedure (Figure 1); the other assays were also conducted over 30 min. Calibration responses for iRAC, ABTS and Folin–Ciocalteu assays (Table 1) were linear with the regression coefficient (R2) > 0.99. Other calibration parameters for iRAC and ABTS assays were broadly similar with respect to, lower limit of detection (LLD) and upper limits of detection (ULD), but the assay sensitivity (slope) and the precision (CV %) were higher in the former case (Table 1).
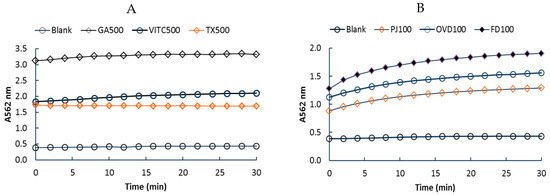
Figure 1.
Typical time course traces for absorbance readings at 562 nm (A562) for iron (III) reducing antioxidant capacity (iRAC) method; (A) Gallic acid (GA500), vitamin C (VITC500), or trolox (TX500) were reference compounds (500 uM), (B) Timed response for pomegranate juice or pomegranate extracts (5 g/100 mL) prepared with 40:60 v/v methanol/water) and diluted 100× before analysis. PJ100 = pomegranate juice, OVD100 = extract from oven-dried sample, FD100 = extract from freeze-dried sample.

Table 1.
Calibration parameters for iRAC, ABTS, and Folin–Ciocalteu assays.
3.2. Total Antioxidant Capacity for Pomegranate Samples
The total antioxidant capacity for PJ was 33.4 ± 0.5 mM or 24.5 ± 0.7 mM (mmol trolox equivalents per liter of PJ) determined by the iRAC method at pH 7.0 and pH 4.5, respectively. The ABTS assay for PJ at pH 7.0 showed a total antioxidant capacity was 36.3 ± 2.1 mM (Figure 2A).
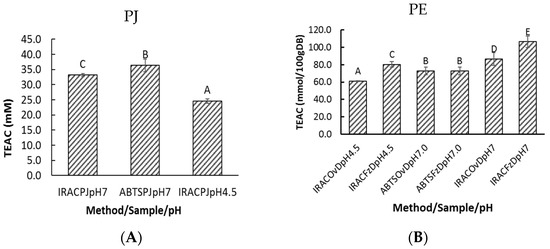
Figure 2.
Total antioxidant capacity for pomegranate samples. Pomegranate juice (A) was analyzed by iRAC at pH 7.0 & 4.5 (IRACPJpH7, IRACPJpH4.5) or ABTS method (ABTSPJpH7.0). Pomegranate extract (B) from freeze-dried (FzD) or oven-dried (OvD) powder was analyzed by iRAC or ABTS methods (iRACOvDpH4.5, ABTSFzDpH7.0 etc.). Data shows mean ± SD for n = 12 points. Bars with different letters show significant differences (p < 0.05).
The method of drying and type of assay affected values for total antioxidant capacity (Figure 2B). Freeze-dried pomegranate showed a higher iRAC response compared with oven-dried pomegranate, but no differences were observable using the ABTS assay, perhaps because the two assays detect different antioxidant principles, i.e., iron (III) reduction and free radical quenching. Past studies showed that drying pomegranate arils at moderate drying temperatures (55–75 °C) produced losses of TPC, total anthocyanin, flavonoids, and TAC determined using the DPPH method [15]. Clearly, the effect of processing on bioactive components of PJ requires further study.
The order of total antioxidant capacity for whole pomegranate fruit was freeze-dried pomegranate > oven-dried pomegranate and also iRAC (pH 7.0) ≥ ABTS (pH7.0) > iRAC (pH 4.5). Limited conclusions can be drawn from the limited data, however, the effect of pH (iRAC pH 4.5 and pH 7.0) and the slight differences between ABTS and iRAC are consistent with our previous studies [18,19]
3.3. Total Phenols Content of Pomegranate Samples by Folin–Ciocalteu Assay
Values for the TPC ranged from 5.8% to 6.9% GAE for dried pomegranate (Table 2). The order of decreasing values for TPC was, freeze-dried pomegranate > oven-dried pomegranate > PJ on per dry weight basis. A one-way ANOVA test showed the TPC for freeze-dried and oven-dried pomegranate samples were significantly different (p < 0.05).

Table 2.
Total phenol content for pomegranate samples per dry weight basis .
In this study, percent moisture (% M) obtained by drying PJ and pomegranate whole fruit was 84% and 80%, respectively. Expressed on an as is basis, the TPC for PJ corresponds to 250 ± 12 mg GAE/100 mL or 238.1 mg GAE/100 g FW (assuming a PJ density of 1.05 g/mL). The TPC for oven-dried pomegranate whole fruit expressed on a FW basis is 1166 mg GAE/100 g FW. The TPC for freeze-dried pomegranate whole fruit expressed on a FW basis is 1383 mg GAE/100 g FW.
4. Discussion
The health benefits of PJ are attributed partly to its high antioxidant capacity and TPC [1,2,3,4,5,6,7,8]. Currently, pomegranate is listed as one the highest sources of dietary antioxidants amongst many beverages, including red wine, green tea, grape, apple, orange or cranberry juices [9,10,11,12]. Nonetheless, published total antioxidant capacity values for pomegranate vary considerably (Table 3). In this paper, we examined total antioxidant capacity for pomegranate in terms of a newly described iRAC method [18,19], and compared values with the ABTS method [20] in accordance with AOAC recommendations [17].

Table 3.
Reported total antioxidant capacity and TPC values for pomegranate juice.
When comparing honey analysis using iRAC method with the FRAP, ABTS, DPPH, and Folin methods, it was found that solvent pH is a major factor influencing different assay responses [18]. The choice of the ABTS method as the reference in this study is based on the similar neutral pH used for this and the iRAC method. The previous study found also that the iRAC and ABTS gave the closest readings for honey. Folin–Ciocalteu assay was employed as a “dual purpose” assay widely used for TPC and total antioxidant capacity measurements for plant-derived foods [21].
A simple study design that compared oven-dried or freeze-dried pomegranate and juice extracted from the same fruit would provide interesting data related to the effect of processing, as reported elsewhere [9,14]. By contrast, we elected to study a commercially available juice sample in order to draw comparisons with data published for a similar brand of juice (see Table 3; [10]). Food composition data for pre-dried pomegranate were converted to for the equivalent FW value by adjusting for the moisture content; freeze drying is a common strategy for analyzing fresh matter, whereby rapid freezing can be employed to avoid side-reactions, such as enzymatic browning.
In Figure 1B, samples are diluted as indicated in the graph. In Figure 2, the sample profiles provide a qualitative view of the timecourse, and the y-axes are not intended to identical; precise measurements of the TPC and TAC for samples are given elsewhere in the paper. The assay time was elected as 30 min for this study, which is the time used for the ABTS assay. The kinetics for absorbance changes show nearly complete reactions for the pomegranate samples, but there may be scope for changing the assay times. It is not yet possible to declare the iRAC method as either a kinetic method or an endpoint method.
4.1. Total Antioxidant Capacity and TPC of Pomegranate Juice
The basic principles behind the iRAC assay is that an excess amount of iron (III) is reduced to iron (II) by antioxidants. The concentration of iron (II) is then detected with ferrozine as a complexing agent [18,19]. The iRAC method is a modification of the FRAP method [23], but the former is performed at pH 7.0 rather than pH 3.6; the iRAC method was also useable at pH 4.5 (Figure 2). Interestingly, PJ total antioxidant capacity was ~8% lower using the iRAC method compared with the ABTS method, whilst the former was ~20% higher overall after the dried pomegranate samples are also considered (see below).
The total antioxidant capacity for commercial PJ in this study (33.5 mM) was higher than reported for PJ obtained from 23 other pomegranate cultivars analyzed using the ABTS method (Table 3). By contrast, our sample for POMW 100%PJ manufactured in the United Kingdom had 19.4% lower total antioxidant capacity compared a POMW 100%PJ brand produced and analyzed in California (USA) 10 years earlier [10]. The former PJ contained 120 mg vitamin C per liter (0.7 mM) which is ~2% of the total antioxidant capacity.
The TPC for commercial PJ (250 ± 12 mg GAE/100 mL; this study) compares with range of 140–380 mg GAE/100 mL reported frequently with the exception of one study [26] (Table 3). In general, TPC for PJ prepared from whole fruit is higher than the TPC for PJ extracted from frozen arils or peeled pomegranate; processing whole fruit leads to the transfer of hydrolysable tannin from pomegranate peels to the PJ [9]. About 29% of TPC for pomegranate was associated with PJ compared with 69% associated with pomegranate peel [27]. Significant process losses for TPC (and antioxidant capacity) were reported also when manufacturing pomegranate nectar from whole fruit [14]; under such circumstances, about 37% TPC was associated with pasteurized PJ compared with 47% associated with peel [14]. There were no TPC differences were reported for PJ extracted using organically grown versus conventionally grown pomegranate fruits [28].
The Folin–Ciocalteu assay is known as a dual-purpose assay for TPC as well as total antioxidant capacity [21], with sensitivity to a wide range of reducing compounds [29]. Therefore, antioxidant capacity tends to be correlated with TPC for plant-derived foods which contain phenols as the major antioxidants [21,29]. In a recent study, we found that Folin, DPPH, ABTS, FRAP, and iRAC methods were highly correlated, with a Pearson’s correlation coefficient (R) equal 0.83–0.99 [19]. Overall, the data surveyed in this paper [9,10,14,24,25,26,27] suggests that the total antioxidant capacity and TPC for PJ may vary considerably as a result of processing factors.
4.2. Total Antioxidant Capacity and TPC for Pomegranate Fruit
In this study, whole pomegranate fruit was pre-processed (washing, dicing, freeze drying/oven drying, grinding to form powder, and solvent extraction with methanol/water (40:60%)) prior to analysis. The observed total antioxidant capacity and TPC values are for unpeeled whole fruit, and values might also be moderated by drying and the efficiency of the extraction. In other studies, juice is extracted from fresh whole pomegranates, or fruits were homogenized or macerated directly with solvent, and the extract subjected to analysis; typically, such data were then adjusted for moisture content [14,26,27,28,29,30,31]. There has been no concerted investigation to examine whether analysis of fresh or dried sample extracts (two alternative sample treatment regimens) affect the final results materially. Some data available on the TPC for whole pomegranate fruit, seeds, and peel are summarized in Table 4; data were converted from FW to DB by the authors as necessary (see above).

Table 4.
Reported total phenols content for whole pomegranate fruit, seeds, and peels ↕.
The TPC value for the Hicaz pomegranate from this study (approx. 6372 mg GAE/100 g DB; Table 2) was about 2.6× higher than the average values reported for other PG whole fruit samples (Lefan, Sweet, & Sour PG; Table 4). Further general difference in TPC were noted (Table 4) with different cultivars, fruit parts (whole fruit > peel >> seeds or arils) and extraction solvent (methanol, methanol/water > water solvent [27,32]. The Hicaz variety of pomegranate had a high TPC, but comparisons with other varieties are not possible owing to the various experimental approaches used. The TPC for pomegranate declines with increasing maturation and ripening [30]. The total antioxidant capacity for pomegranate fruit using iRAC method ((72–106.3 mmol/100 g DB; Figure 2B) agreed closely with the ABTS analysis (this study) and ABTS results reported previously as 122.9 mmol/100 g DB [14]. Past studies showed that total antioxidant capacity of pomegranate was strongly correlated with TPC, tannins, and flavonoids [4,30].
5. Conclusions
The iron (III) reducing antioxidant capacity (iRAC) for pomegranate and juice was similar to values for ABTS free radical quenching capacity, both expressed as TEAC units. Both iRAC and ABTS assays confirm the previously reported high total antioxidant capacity values for PJ. Some differences in the total antioxidant capacity and TPC values for pomegranate and PJ were evident due to varying cultivars and processing factors. Such results have relevance for attempts to refine food composition data for pomegranate and other functional foods.
Author Contributions
Conceptualization, R.O.-A., P.S.N. and B.S.; Methodology, R.O.-A., B.S. and P.S.N.; Software, R.O.-A.; Formal Analysis, H.C.W.; Investigation, H.C.W.; Data Curation, H.C.W.; Writing-Original H.C.W., R.O.-A.; Writing, Review and Editing, R.O.-A.; Visualization, H.C.W., R.O.-A.; Supervision, R.O.-A.; Project Administration, R.O.-A.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Keshvari, M.; Sahebkar, A.; Sarrafzadegan, N. Pomegranate consumption and blood pressure: A review. Curr. Pharm. Des. 2017, 23, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Calhau, C. The bioactivity of pomegranate: Impact on health and disease. Crit. Rev. Food Sci. Nutr. 2011, 51, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Kalaycioglu, Z.; Erim, F.B. Total phenolic contents, antioxidant activities, and bioactive ingredients of juices from pomegranate cultivars worldwide. Food Chem. 2017, 221, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Ferri, C.; Giorgini, P.; Bo, S.; Nachtigal, P.; Grassi, D. Effects of pomegranate juice on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2017, 115, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; McClees, S.F.; Afaq, F. Pomegranate for prevention and treatment of cancer: An update. Molecules 2017, 22, 177. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, A.; Elavarasu, S.; Sundaram, R.; Kumar, T.; Rajendran, D.; Prem, F. Ancient seed for modern cure-pomegranate review of therapeutic applications in periodontics. J. Pharm. Bioallied Sci. 2017, 9 (Suppl. 1), S11–S14. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tian, L. Diverse phytochemicals and bioactivities in the ancient fruit and modern functional food pomegranate (Punica granatum). Molecules 2017, 22, 1606. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.J.; Wei, J.Y.; Yang, J.J.; Xu, J.; Pang, W.; Jiang, Y.G. Pomegranate juice is potentially better than apple juice in improving antioxidant function in elderly subjects. Nutr. Res. 2008, 28, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bohn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Tezcan, F.; Gultekin-Ozguven, M.; Diken, T.; Ozcelik, B.; Erim, F.B. Antioxidant activity and total phenolic, organic acid and sugar content in commercial pomegranate juices. Food Chem. 2009, 115, 873–877. [Google Scholar] [CrossRef]
- Surek, E.; Nilufer-Erdil, D. Changes in phenolics and antioxidant activity at each step of processing from pomegranate into nectar. Int. J. Food Sci. Nutr. 2014, 65, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; DerMarderosian, A.; Porter, J.R. Anthocyanins and polyphenol oxidase from dried arils of pomegranate (Punica granatum L.). Food Chem. 2013, 118, 11–16. [Google Scholar] [CrossRef]
- Baslar, M.; Karasu, S.; Kilicli, M.; Us, A.A.; Sagdiç, O. Degradation kinetics of bioactive compounds and antioxidant activity of pomegranate arils during the drying process. Int. J. Food Eng. 2014, 10, 839–848. [Google Scholar] [CrossRef]
- Anon, AOAC SMPR 2011.011. Standard method performance requirements for in vitro determination of total antioxidant activity in foods, beverages, food ingredients, and dietary supplements. J. AOAC Int. 2012, 956, 1557.
- Yusof, H.I.M.; Owusu-Apenten, R.; Nigam, P.S. Determination of iron (III) reducing antioxidant capacity for manuka honey and comparison with ABTS and other methods. J. Adv. Biol. Biotechnol. 2018, 18. [Google Scholar] [CrossRef]
- Kirkpatrick, G.; Nigam, P.; Owusu-Apenten, R.K. Total phenols, antioxidant capacity and antibacterial activity of Manuka honey chemical constituents. J. Adv. Biol. Biotechnol. 2017, 15. [Google Scholar] [CrossRef]
- Walker, R.B.; Everette, J.D. Comparative reaction rates of various antioxidants with ABTS radical cation. J. Agric. Food Chem. 2009, 57, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Charrondiere, U.R.; Rittenschober, D.; Nowak, V.; Stadlmayr, B.; Wijesinha-Bettoni, R.; Haytowitz, D. Improving food composition data quality: Three new FAO/INFOODS guidelines on conversions, data evaluation and food matching. Food Chem. 2016, 193, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, M.; Durgac, C.; Serce, S.; Kaya, C. Chemical and antioxidant properties of pomegranate cultivars grown in the Mediterranean region of Turkey. Food Chem. 2008, 111, 703–706. [Google Scholar] [CrossRef]
- Cam, M.; Hisil, Y.; Durmaz, G. Classification of eight pomegranate juices based on antioxidant capacity measured by four methods. Food Chem. 2009, 112, 721–726. [Google Scholar] [CrossRef]
- Hmid, I.; Elothmani, D.; Hanine, H.; Oukabli, A.; Mehinagic, E. Comparative study of phenolic compounds and their antioxidant attributes of eighteen pomegranate (Punica granatum L.) cultivars grown in Morocco. Arab. J. Chem. 2017, 10, S2675–S2684. [Google Scholar] [CrossRef]
- Gozlekci, S.; Saracoglu, O.; Onursal, E.; Ozgen, M. Total phenolic distribution of juice, peel, and seed extracts of four pomegranate cultivars. Pharmacogn. Mag. 2011, 7, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Nuncio-Jáuregui, N.; Cano-Lamadrid, M.; Hernández, F.; Carbonell-Barrachina, Á.A.; Calín-Sánchez, Á. Comparison of fresh and commercial pomegranate juices from Mollar de Elche cultivar grown under conventional or organic farming practices. Beverages 2015, 1, 34–44. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin−Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Changes in physical properties, chemical and elemental composition and antioxidant capacity of pomegranate (cv. Ruby) fruit at five maturity stages. Sci. Hort. 2013, 150, 37–46. [Google Scholar] [CrossRef]
- Elfalleh, W.; Hannachi, H.; Tlili, N.; Yahia, Y.; Nasri, N.; Ferchichi, A. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J. Med. Plants Res. 2012, 6, 4724–4730. [Google Scholar] [CrossRef]
- Rababah, T.M.; Banat, F.; Rababah, A.; Ereifej, K.; Yang, W. Optimization of extraction conditions of total phenolics, antioxidant activities, and anthocyanin of oregano, thyme, terebinth, and pomegranate. J. Food Sci. 2010, 75, C626–C632. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).