Effect of an In Vitro Digestion on the Antioxidant Capacity of a Microfiltrated Blackberry Juice (Rubus adenotrichos)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Blackberry Juice Preparation
2.3. In Vitro Digestion
2.4. Polyphenol Purification
2.5. Phenolic Characterization of the Blackberry Juice Digestion by HPLC
2.6. Antioxidant Assays
2.6.1. DPPH Radical-Scavenging Activity
2.6.2. Oxygen Radical Absorbance Capacity (ORAC)
2.6.3. Nitric Oxide Scavenging Activity
2.6.4. Inhibition of Lipid Peroxidation in Liposomes
2.6.5. Inhibition of Lipid Peroxidation in Liver Homogenates
2.6.6. Erythrocyte Cellular Antioxidant Activity (ERYCA)
2.6.7. Inhibition of Intracellular ROS
2.7. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Characterization of the Blackberry Juice Digestion Samples
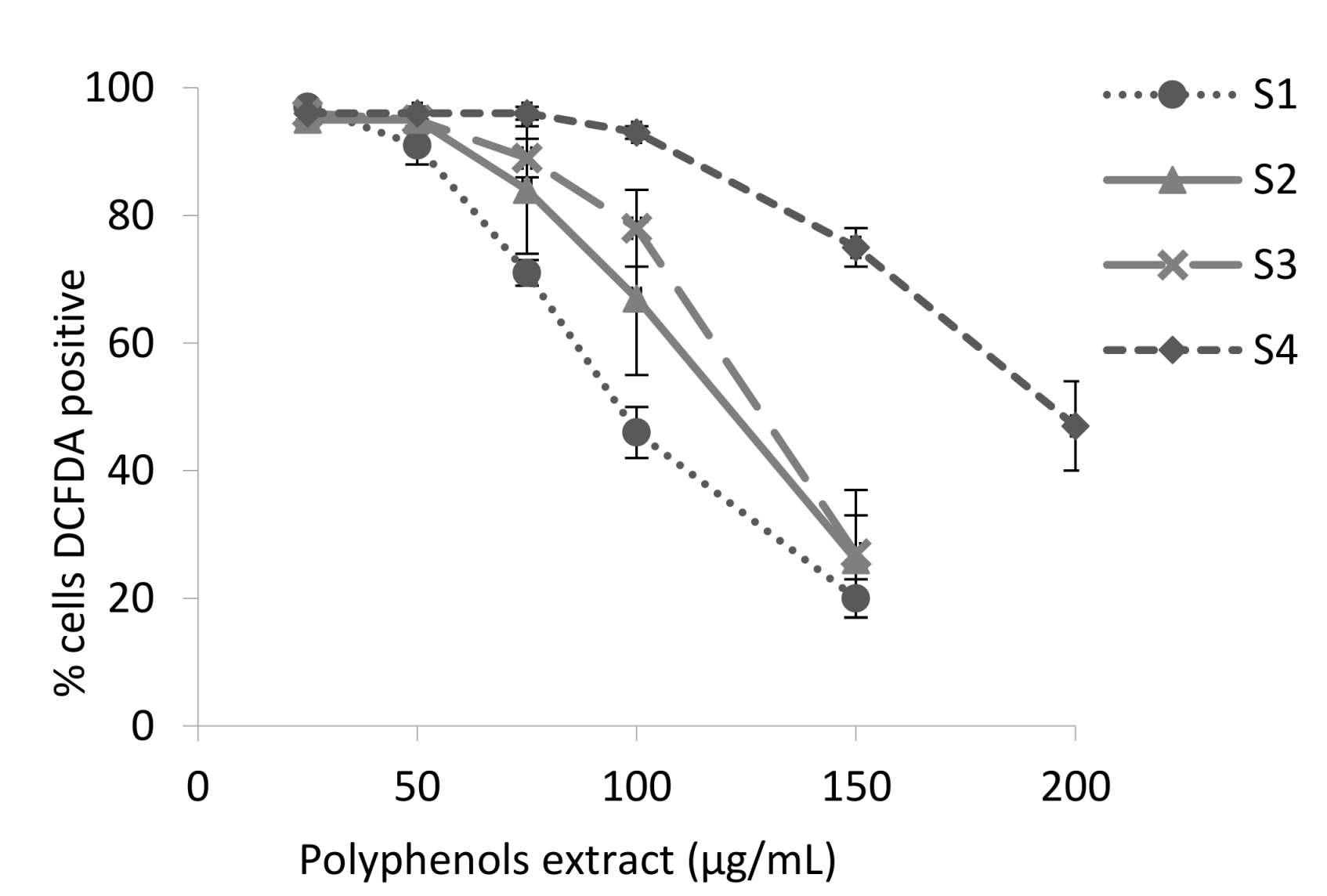
3.2. Antioxidant Activity of the Blackberry Juice Digestion Samples
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paredes-López, O.; Cervantes-Ceja, M.L.; Vigna-Pérez, M.; Hernández-Pérez, T. Berries: Improving Human Health and Healthy Aging, and Promoting Quality Life—A Review. Plant Foods Hum. Nutr. 2010, 65, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Tjelle, T.E.; Holtung, L.; Bøhn, S.K.; Aaby, K.; Thoresen, M.; Wiik, S.Å.; Paur, I.; Karlsen, A.S.; Retterstøl, K.; Iversen, P.O.; et al. Polyphenol-rich juices reduce blood pressure measures in a randomised controlled trial in high normal and hypertensive volunteers. Br. J. Nutr. 2015, 114, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Lyons, T.J. Strawberries, blueberries, and cranberries in the metabolic syndrome: Clinical perspectives. J. Agric. Food Chem. 2012, 60, 5687–5692. [Google Scholar] [CrossRef] [PubMed]
- Del Bo’, C.; Martini, D.; Porrini, M.; Klimis-Zacas, D.; Riso, P. Berries and oxidative stress markers: An overview of human intervention studies. Food Funct. 2015, 6, 2890–2917. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Mertz, C.; Cheynier, V.; Günata, Z.; Brat, P. Analysis of phenolic compounds in two blackberry species (Rubus glaucus and Rubus adenotrichus) by high-performance liquid chromatography with diode array detection and electrospray ion trap mass spectrometry. J. Agric. Food Chem. 2007, 55, 8616–8624. [Google Scholar] [CrossRef] [PubMed]
- Kaume, L.; Howard, L.R.; Devareddy, L. The Blackberry Fruit: A Review on Its Composition and Chemistry, Metabolism and Bioavailability, and Health Benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef] [PubMed]
- Felgines, C.; Talavera, S.; Texier, O.; Gil-Izquierdo, A.; Lamaison, J.L.; Remesy, C. Blackberry anthocyanins are mainly recovered from urine as methylated and glucuronidated conjugates in humans. J. Agric. Food Chem. 2005, 53, 7721–7727. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Giusti, M.M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Ann. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; González-Barrio, R.; Cerdá, B.; López-Bote, C.; Rey, A.I.; Tomás-Barberán, F.A. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J. Agric. Food Chem. 2007, 55, 10476–10485. [Google Scholar] [CrossRef] [PubMed]
- García-Muñoz, C.; Hernández, L.; Pérez, A.; Vaillant, F. Diversity of urinary excretion patterns of main ellagitannins’ colonic metabolites after ingestion of tropical highland blackberry (Rubus adenotrichus) juice. Food Res. Int. 2014, 55, 161–169. [Google Scholar] [CrossRef]
- Cerdá, B.; Periago, P.; Espín, J.C.; Tomás-Barberán, F.A. Identification of urolithin A as a metabolite produced by human colon microflora from ellagic acid and related compounds. J. Agric. Food Chem. 2005, 53, 5571–5576. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, F.; Pérez, A.M.; Acosta, O.; Dornier, M. Turbidity of pulpy fruit juice: A key factor for predicting cross-flow microfiltration performance. J. Membr. Sci. 2008, 325, 404–412. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Zafrilla, P.; Tomás-Barberán, F.A. An in vitro method to simulate phenolic compound release from the food matrix in the gastrointestinal tract. Eur. Food Res. Technol. 2002, 214, 155–159. [Google Scholar] [CrossRef]
- Coates, E.M.; Popa, G.; Gill, C.I.; McCann, M.J.; McDougall, G.J.; Stewart, D.; Rowland, I. Colon-available raspberry polyphenols exhibit anti-cancer effects on in vitro models of colon cancer. J. Carcinog. 2007, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Montoya, Ó.; Vaillant, F.; Cozzano, S.; MertzbAn, C.; Pérez, M.; Castro, M.V. Phenolic content and antioxidant capacity of tropical highland blackberry (Rubus adenotrichus Schltdl.) during three edible maturity stages. Food Chem. 2010, 119, 1497–1501. [Google Scholar] [CrossRef]
- Azofeifa, G.; Quesada, S.; Boudard, F.; Morena, M.; Cristol, J.P.; Pérez, A.M.; Vaillant, F.; Michel, A. Antioxidant and anti-inflammatory in vitro activities of phenolic compounds from tropical highland blackberry (rubus adenotrichos). J. Agric. Food Chem. 2013, 61, 5798–5804. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, N.; Panda, A.B.; Raj, N.R.; Shrivastava, A.; Prathani, R. The Evaluation of Nitric Oxide Scavenging Activity of Acalypha Indica Linn root. Asian J. Res. Chem. 2009, 2, 148–150. [Google Scholar]
- Pérez, R.; Vargas, R.; Martínez, F.; García, E.V. Antioxidant activity of alkaloids from Bocconia arborea. A study on six testing methods. ARS Pharm. 2003, 44, 5–21. [Google Scholar]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- González, E.; Vaillant, F.; Rojas, G.; Pérez, A.M. Novel semiautomated method for assessing in vitro cellular antioxidant activity using the light-scattering properties of human erythrocytes. J. Agric. Food Chem. 2010, 58, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, Y.; Zhang, L.; Su, H.; Zheng, X. Blackberry subjected to in vitro gastrointestinal digestion affords protection against Ethyl Carbamate-induced cytotoxicity. Food Chem. 2016, 212, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Su, H.; Xu, Y.; Bao, T.; Zheng, X. Protective effect of wild raspberry (Rubus hirsutus Thunb.) extract against acrylamide-induced oxidative damage is potentiated after simulated gastrointestinal digestion. Food Chem. 2016, 196, 943–952. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. J. Agric. Food Chem. 2005, 53, 5896–5904. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Pérez-Vicente, A.; Gil-Izquierdo, A.; García-Viguera, C. In vitro Gastrointestinal Digestion Study of Pomegranate Juice Phenolic Compounds, Anthocyanins, and Vitamin C. J. Agric. Food Chem. 2002, 50, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- McGhie, T.K.; Ainge, G.D.; Barnett, L.E.; Cooney, J.M.; Jensen, D.J. Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolized by both humans and rats. J. Agric. Food Chem. 2003, 51, 4539–4548. [Google Scholar] [CrossRef] [PubMed]
- Stanisavljevic, N.; Samardzic, J.; Jankovic, T.; Šavikin, K.; Mojsin, M.; Topalović, V.; Stevanović, M. Antioxidant and antiproliferative activity of chokeberry juice phenolics during in vitro simulated digestion in the presence of food matrix. Food Chem. 2015, 175, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Felgines, C.; Texier, O.; Garcin, P.; Besson, C.; Lamaison, J.L.; Scalbert, A. Tissue distribution of anthocianins in rats fed a blackberry anthocyanin-enriched diet. Mol. Nutr. Food Res. 2009, 53, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Borges, G.; Crozier, A. Berry flavonoids and phenolics: Bioavailability and evidence of protective effects. Br. J. Nutr. 2010, 104, S67–S90. [Google Scholar] [CrossRef] [PubMed]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Lamaison, J.L.; Rémésy, C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J. Nutr. 2003, 133, 4178–4182. [Google Scholar] [CrossRef] [PubMed]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Manach, C.; Lamaison, J.L.; Rémésy, C. Anthocyanins are efficiently absorbed from the small intestine in rats. J. Nutr. 2004, 134, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.-M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Tomás-Barberán, F.A.; Espín, J.C. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J. Nutr. Biochem. 2006, 17, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Boxin, O.U.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Antioxidant capacity: Which capacity and how to assess it? J. Berry Res. 2011, 1, 169–176. [Google Scholar] [CrossRef]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Rhone, M.; Lyons, T.J. Berries: Emerging impact on cardiovascular health. Nutr. Rev. 2010, 68, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Do antioxidants impair signaling by reactive oxygen species and lipid oxidation products? FEBS Lett. 2012, 586, 3767–3770. [Google Scholar] [CrossRef] [PubMed]
- Serram, N.P.; Nair, M. Inhibition of Lipid Peroxidation and Structura-Activity-Related Studied of the dietary constituents Anthoyanins, Anthocyanidins, and Actechins. J. Agric. Food Chem. 2002, 50, 5308–5312. [Google Scholar] [CrossRef]
- Reddy, M.K.; Gupta, S.K.; Jacob, M.R.; Khan, S.I.; Ferreira, D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007, 73, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Dobroslawa, B.; Kasimsetty, S.G.; Khan, S.I.; Daneel, F. Urolithins, intestinal microbial metabolites of pomegranate ellagitannins, exhibit potent antioxidant activity in a cell-based assay. J. Agric. Food Chem. 2009, 57, 10181–10186. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.; Chaturvedi, M.; Aggarwal, B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2011, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Muñoz, C.; Vaillant, F. Metabolic fate of ellagitannins: Implications for health, and research perspectives for innovative functional foods. Crit. Rev. Food Sci. Nutr. 2014, 54, 1584–1598. [Google Scholar] [CrossRef] [PubMed]
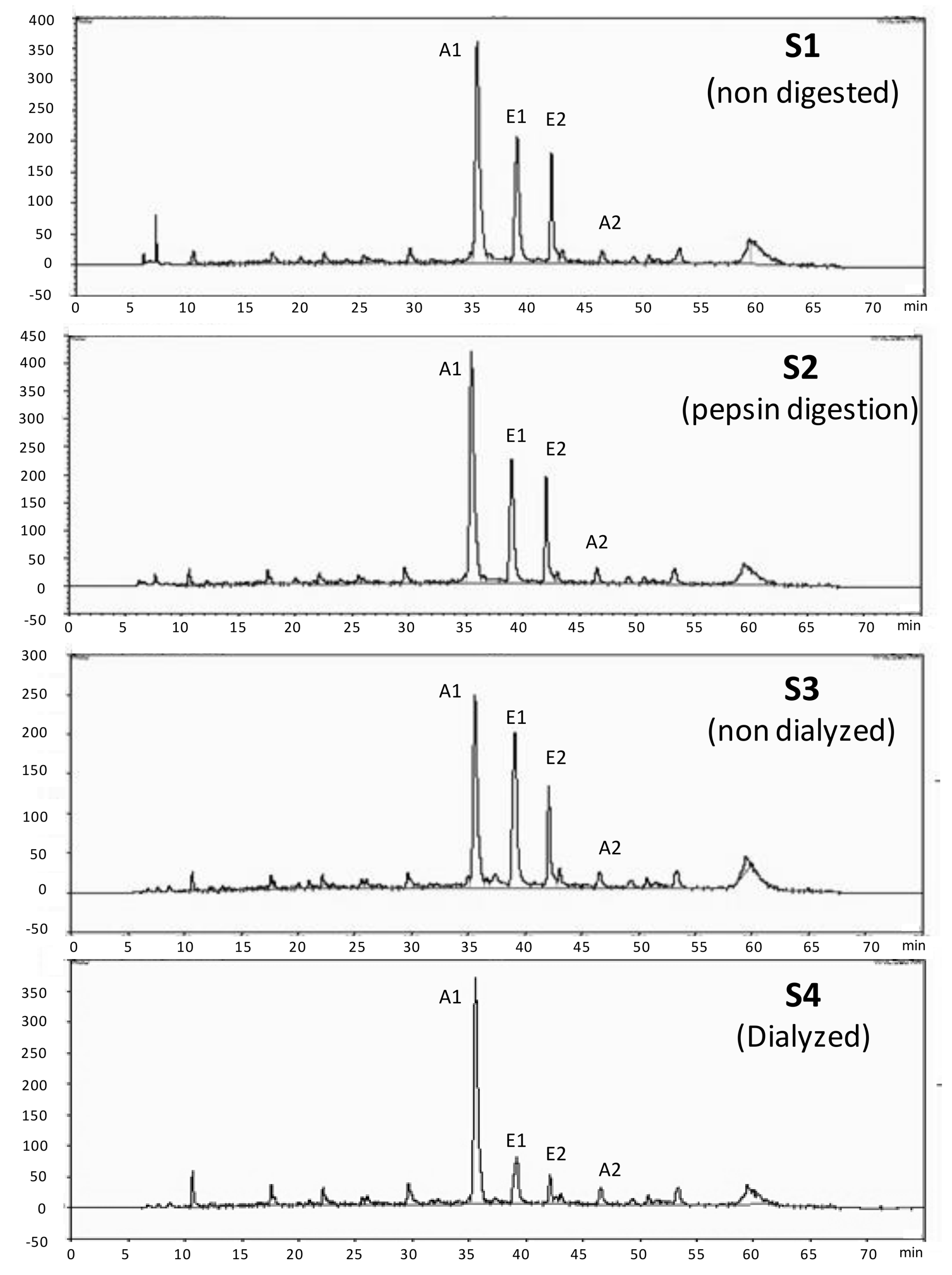
| Blackberry Digestion Samples | HPLC-Anthocyanins mg C3G/L | HPLC-Ellagitannins mg EA/L |
|---|---|---|
| S1 (Non-digested) | 480 ± 37 a | 353 ± 13 a |
| S2 (Pepsin digestion) | 580 ± 15 a | 394 ± 24 a |
| S3 (Non-dialyzed) | 220 ± 19 b | 233 ± 6 b |
| S4 (Dialyzed) | 91 ± 4 c | 24 ± 3 c |
| Blackberry Digestion Samples | DPPH IC50 (μg/mL) | ORAC mmol TE/g of Extract | NO Scavenging IC50 (μg/mL) |
|---|---|---|---|
| S1 (Non-digested) | 5.10 ± 0.20 a | 5.94 ± 0.41 a | 197 ± 19 a |
| S2 (Pepsin digestion) | 4.75 ± 0.03 a | 6.30 ± 0.42 a | 183 ± 14 a |
| S3 (Non-dialyzed) | 5.30 ± 0.15 a | 5.86 ± 0.27 a | 217 ± 13 ab |
| S4 (Dialyzed) | 6.79 ± 0.06 b | 6.56 ± 0.18 a | 267 ± 21 b |
| Blackberry Digestion Samples | Liposomes | Rat Liver Homogenate | ERYCA |
|---|---|---|---|
| IC50 (µg/mL) | IC50 (µg/mL) | mmol QE/g of Extract | |
| S1 (Non-digested) | 5.03 ± 0.49 a | 32.7 ± 4.8 a | 3.87 ± 0.19 a |
| S2 (Pepsin digestion) | 4.27 ± 0.27 a | 29.2 ± 5.0 a | 3.88 ± 0.41 a |
| S3 (Non-dialyzed) | 5.36 ± 0.62 a | 37.0 ± 7.1 a | 3.80 ± 0.25 a |
| S4 (Dialyzed) | 5.84 ± 0.41 a | 39.1 ± 6.3 a | 3.54 ± 0.25 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azofeifa, G.; Quesada, S.; Pérez, A.M.; Vaillant, F.; Michel, A. Effect of an In Vitro Digestion on the Antioxidant Capacity of a Microfiltrated Blackberry Juice (Rubus adenotrichos). Beverages 2018, 4, 30. https://doi.org/10.3390/beverages4020030
Azofeifa G, Quesada S, Pérez AM, Vaillant F, Michel A. Effect of an In Vitro Digestion on the Antioxidant Capacity of a Microfiltrated Blackberry Juice (Rubus adenotrichos). Beverages. 2018; 4(2):30. https://doi.org/10.3390/beverages4020030
Chicago/Turabian StyleAzofeifa, Gabriela, Silvia Quesada, Ana M. Pérez, Fabrice Vaillant, and Alain Michel. 2018. "Effect of an In Vitro Digestion on the Antioxidant Capacity of a Microfiltrated Blackberry Juice (Rubus adenotrichos)" Beverages 4, no. 2: 30. https://doi.org/10.3390/beverages4020030
APA StyleAzofeifa, G., Quesada, S., Pérez, A. M., Vaillant, F., & Michel, A. (2018). Effect of an In Vitro Digestion on the Antioxidant Capacity of a Microfiltrated Blackberry Juice (Rubus adenotrichos). Beverages, 4(2), 30. https://doi.org/10.3390/beverages4020030






