Abstract
Emulsions and foams form the basis of an extensive variety of materials used in the beverage industry. One of the characteristics of beverage emulsions is that they are rather diluted, contain little amounts of a dispersed oil phase in the finished product, and must remain physically stable for long periods of time. Nowadays, the consumers ask for more than a drink. Thus, in the market, we can find a vast variety of beverages, where emulsion science seems to be the main factor for controlling flavor, color, the presence of constituents of technological or nutritional value, nutraceutical/bioactive components and, also, turbidity. This work intends to make an overview of the recent advances in beverage-emulsions technology. Some examples are given within the very large world of the beverage industry, from cream liqueurs, soft drinks, and functional beverages, to bottled water, fruit drinks, sparkling wine, and beer.
1. Introduction
The importance of colloidal systems is that they appear in a wide variety of products, and processes [1]. Food foams, emulsions, and suspensions form a major part of the foods and beverages produced and sold today [2]. For instance, edible foams can be found in beer, in bread, and in a chocolate mousse. Emulsions and foams form the basis of an extensive variety of materials used in food and drinks [3]. The main characteristics of beverage emulsions is that they are very dilute, containing as little as 20 mg/L of a dispersed oil phase in the finished product, and must remain physically stable for relatively long periods of time (4–12 months) [4]. One of the major concerns for dispersions is keeping the internal phase uniformly distributed during storage and consumption. This has led the food industry and many researchers to investigate the ability of hydrocolloids and proteins to stabilize emulsion and foam against creaming, flocculation, coalescence, drainage, and coarsening, depending on their intended application [5]. The use of food emulsifier began in 1869, in France. The French chemist Hippolyte Mège-Mouriès produced Margarine, a substitute for butter, made by emulsifying milk with lipids [6]. This technique was soon employed by the beverage industry.
Citrus flavor (essential oils extracted from the orange or lemon peel) is one of the most popular flavors used in soft drinks formulation. Since they are oily substances (insoluble in water), the drinks made with these oils are diluted oil/water (O/W) emulsions.
Different kinds of beverage colloids are presented in Figure 1. Due to their intended use, food colloids need to be non-toxic, non-carcinogenic, and non-allergenic. They also need to be stable for periods of months to years, including stability against destabilization processes such as aggregation, creaming, coalescence, and gelation. Innovative ingredients and solutions are emerging to help formulators add flavors, colors, and healthy ingredients and keep up with consumer demands for innovative beverages [6]. Chocolate drinks provide a related example of a beverage suspension.

Figure 1.
Examples of beverage emulsions (O/W)—Oil and water are immiscible, but with addition of stabilizing agents an emulsion can be achieved (A); and foams suspensions beverages—foam consists of a mass of small bubbles that are formed when air and a liquid are mixed together (B).
2. The Colloidal State
The word “colloid” has been used since 1862 to classify all the liquids that are colloidal dispersions. Chocolate, milk, and mayonnaise are examples of food colloids. Indeed, the colloidal state was recognized by Thomas Graham in 1861. This researcher, observed two classes of substances:
- -
- Crystalloid: readily pass through animal and vegetable membranes;
- -
- Colloids: diffused very slowly and cannot pass through membranes.
Fifty years later the colloidal state was described by Wolfgang Ostwald as a “world of neglected dimensions”, a reference to the world of systems in which the particles are extremely small [7]. Thus, a suspension of tiny particles in a continuous phase, the dispersion medium, is called a colloidal dispersion. The suspended particles are single large molecules or aggregates of molecules or ions ranging in size from 1 to 1000 nm [8]. Since a colloidal solution or substance is made up of scattered particles, light cannot pass straight through. This effect was observed and described by John Tyndall as the Tyndall Effect. The Tyndall effect is an easy way of determining whether a mixture is colloidal or not (Figure 2).

Figure 2.
The Tyndall effect [9]. When light is shined through a true solution, the light passes cleanly through the solution, however, when the light is passed through a colloidal solution, the substance in the dispersed phases scatters the light in all directions, making it readily seen.
The Tyndall effect is one property of colloid systems that distinguish them from true solutions. Due to colloidal particles being so small, their individual motion changes continually as a result of a random collision with the molecules of the dispersion medium. This random, zig-zagging movement is called Brownian movement and was first discovered by Robert Brown, a botanist, in 1827. Brownian movement is a phenomenon whereby small particles suspended in a liquid tend to move in pseudo-random paths through the liquid, even if the liquid has laminar movement. Brownian movement in colloidal sol arises due to the impact of the molecules of the dispersion medium with the colloidal particles. It has been postulated that the impact of the molecules of the dispersion medium on the colloidal particles are unequal [10], leading to the zig-zag (random) movement of the colloidal particles, mathematically studied by Smoluchowski [11]. This movement helps to keep the particles in suspension, which is another property of the colloidal systems. Absorption is another colloidal characteristic since the finely divided colloidal particles have a large surface area. However, the presence of colloidal particles has little effect on the colligative properties (boiling point, freezing point, etc.) of a solution [12].
Analogous to the identification of solution components as “solute” and “solvent”, the components of a colloidal dispersion are likewise classified according to their relative amounts [7]. The particles in a colloidal dispersion are sufficiently large for definite surfaces of separation to exist between the particles and the medium in which they are dispersed. Simple colloidal dispersions are, therefore, two-phase systems. The phases are distinguished by the terms dispersed phase (for the phase forming the particles, present in a relatively minor amount), and a dispersion medium (for the medium in which the particles are distributed) [13]. Colloids may involve virtually any combination of physical states (gas in liquid, liquid in solid, solid in gas, etc.). Colloids are characterized according to the states of the dispersed phase and the dispersing medium [14]. Table 1 summarizes various types of colloidal systems.

Table 1.
Types of colloidal systems. Adapted from Zumdahl and Zumdahl [14].
Although there are different types of colloidal dispersion sols and emulsions are the most important according to Shaw [13]. The term sol or hydrosol (when the dispersion medium is aqueous) was used for distinguish colloidal suspensions from macroscopic suspensions.
Colloids can be broadly classified as lyophobic or, more specifically, in aqueous systems, hydrophobic and as lyophilic or hydrophilic [7]. However, according to Shaw [13], this description is not correct, because if there is no affinity between the particles and the dispersion medium no dispersion would be formed. In addition, in a lyophilic colloidal system, lyophobic regions are often present, for example in proteins which may be partly hydrophobic (hydrocarbon regions) and partly hydrophilic (peptide linkages, and amino and carboxyl groups).
Surfactant molecules, because of their affinity for water and oil and their consequent tendency to associate into micelles, form hydrophilic colloidal dispersions in water. Proteins, gums, gelatin, starch, and certain polymers (rubber) in organic solvents also form lyophilic colloidal systems [1]. Thus, while lyophilic dispersions (such as phospholipid vesicles and micelles) are inherently stable, lyophobic colloidal dispersions have a tendency to coalesce because they are thermodynamically unstable as a result of their high surface energy in their native state [7].
3. Classification of Emulsions, Foams, and Suspensions
3.1. Emulsions
Emulsions are colloidal dispersions in which a liquid is dispersed in a continuous liquid phase of different compositions [1,7]. Several classes of emulsions may be distinguished: oil-in-water (O/W), for oil droplets dispersed in water, water-in-oil (W/O), for water droplets dispersed in oil [1] and water-in-water (W/W), for two immiscible aqueous phases that are in thermodynamic equilibrium [15,16]. The W/W emulsions are very interesting colloidal dispersions much less known than conventional oil-in-water or water-in-oil emulsions, despite the fact that phase separation in aqueous mixtures is highly common. This kind of emulsions could result from mixtures of hydrophilic polymers [15]. Dilution tests may be carried out to find out whether an emulsion is oil-in-water or water-in-oil. These consist of a microscopic observation of a slide where a small amount of either oil or water is added to the emulsion sample [17]. It is also possible to formulate emulsions without water. Non-aqueous or anhydrous emulsions could be formulated as O1/O2 systems or oil in a polar solvent emulsion, as well as multiple oil-in-oil-in-oil systems or variants [7].
Most emulsions are not thermodynamically stable, they tend to separate into “oil” and “water” phases. Therefore, an emulsifying agent is usually required to form a stable emulsion [1,17].
Most meta-stable emulsions that will be encountered in practice contain oil, water, and an emulsifying agent (or stabilizing agents) which is usually a surfactant, a macromolecule, or finely divided solids. The emulsifying agent, or protective colloid, are surface-active, meaning that it reduces the surface tension of the liquid, and so tends to concentrate in boundary films [17].
3.2. Foams
A foam is a colloidal dispersion in which a gas is dispersed in a continuous liquid phase [1]. Foams are somewhat different from emulsions because, in foams, it is the dispersion medium which has colloidal dimensions [13]. Despite the fact that the bubbles in persistent foams are polyhedral and not spherical, it is nevertheless conventional to refer to the “diameter” of gas bubbles in foams as if they were spherical. Foam bubbles usually have diameters greater than 10 µm and may be larger than 1000 µm [1]. According to Calvert [17], in a liquid foam, a colloidal adsorptive agent forms a film that bounds the gas bubbles. The colloidal dimension in a foam is the thickness of the film, not the size of the bubble. The bubble is lighter than its surroundings and will rise to the top, where it joins the foam. When carbon dioxide is released, it uses the adsorptive agent to make the foam. Meringue is a dried foam using egg albumin. Marshmallows use sugared gelatin for the same purpose.
Foam stability is not necessarily a function of drop size, although there may be an optimum size for an individual foam type [1]. The stability of a foam depends upon two principal factors: the tendency for the liquid films to drain and become thinner, and their tendency to rupture as a result of random disturbances. Other factors which may significantly influence foam stability include evaporation and gas diffusion through the liquid films. All foams are unstable in the thermodynamic sense. However unstable and metastable foam structures are different. Unstable foams are formed from aqueous solutions of fatty acids or short chain alcohols which delay drainage and rupture of the film, not preventing such processes from occurring continuously until the foam disappears. The metastable foams are formed from solutions of soaps, synthetic detergents, proteins, etc. The balance of forces is such that the drainage of the liquid stops when a certain thickness of the film is reached, and in the absence of disturbing influences (such as vibration, draughts, evaporation, diffusion of gas from small bubbles too large bubbles, heat, temperature gradients, dust, and other impurities), these foams would persist almost indefinitely [13].
3.3. Suspensions
A suspension is a colloidal dispersion in which a solid is dispersed in a continuous liquid phase [1]. On the other hand, Florence and Attwood [7] define suspensions as being dispersions of an insoluble substance in an aqueous or non-aqueous continuous phase. Suspensions may be either aqueous or non-aqueous. The particles in suspensions are greater than 1000 nm, according to Schramm [1] usually have diameters greater than 0.2 µm, a spherical shape and may be visible to the naked eye or under a microscope.
If a suspension contains, in addition to solid particles and a continuous liquid phase, emulsified droplets and/or gas bubbles, two situations may occur: (i) if the solid particles are adsorbed on the emulsion droplets, then it is an emulsion that also contains solids; and (ii) if the particles and droplets are not mutually associated, then the system is at once a suspension and an emulsion. In these situations, the term used should be the one that best fits the context. Frequently one or the other is considered to be the primary dispersion while the other phase is considered to be an additive or a contaminant [1].
4. Recent Developments in Beverage Emulsions
4.1. Cream Liqueurs
Alcoholic emulsion creams, or cream liqueurs, are sweet semi-liquid alcoholic drinks that contain over 400 g/L of total extract and often 17% (v/v) in alcohol or high as 25% (v/v). Examples of alcoholic spirits used are brandy, whiskey and vodka [18]. Most products contain several other added ingredients which may include sugar, full-fat milk powder, non-fat milk solids, flavorings, colorings, preservatives and a thickening agent such as sodium caseinate, which also acts as a stabilizing agents to prevent the cream and alcohol from separating [1]. Examples of cream liqueurs are Irish Baileys and Carolans, Scotch Heather (made with whiskey). French chocolate rum Caprice, Dutch Bols, Italian and Czech liqueurs, as well as Polish egg-vanilla Advocaat also belong to that group [19].
Cream liqueurs are beverage emulsions where very small droplet sizes are needed to form the emulsion, so, a coarse emulsion is usually passed through a high-pressure homogenizer [1,20], which can produce small droplets (≈2 µm in diameter). High shear rates and high flow are needed for emulsification into small droplets and for good heat transfer or blending, respectively [1,21].
Factors controlling the emulsion stability of cream liqueurs with high alcohol contents have been studied. Banks and Muir [22] found that, as the alcohol content of the cream liqueur increases, the emulsion becomes increasingly sensitive to the interaction between compositional parameters and processing conditions. The difficulties contributed by the alcohol content include making the aqueous phase a poorer solvent for proteins [1]. Banks and Muir [22] also found that the alcohol content of the liquid phase, rather than the total alcohol content, determines stability. However, as referred by Given [4], alcohol above 5% (v/v) is generally destructive to beverage flavor emulsions, especially for alcoholic beverages that need a hydrophobic flavor dispersion. By employing a polyglycerol fatty acid ester composed of at least 70% palmitic acid in conjunction with an ionic emulsifier, a stable acidified beverage containing a flavor emulsion and alcohol was achieved by the same author. Nonetheless, these problems have long been studied. For instances, the effect of adding low-molecular-weight surfactants, commercial glycerol monostearate or sodium stearyl lactylate, on the stability of simulated cream liqueurs was investigated by Dickinson and co-workers [23]. The presence of 0.4–0.5% (w/v) of glycerol monostearate or sodium stearyl lactylate led to a substantial reduction in creaming at room temperature as well as a longer shelf-life at 45 °C. Improved stability with respect to creaminess appeared to be associated with a change in emulsion rheological properties, probably arising from complex formation between the low-molecular-weight emulsifier and caseinate, both in bulk aqueous solution and at the surface of the fat droplets [23].
4.2. Soft Drinks
Soft drinks are called “soft” in contrast with “hard” alcoholic beverages. In a soft drink, small amounts of alcohol may be present, but the alcohol content must be less than 0.5% (v/v) of the total volume if the drink is to be considered non-alcoholic. Thus, the term “soft-drink” applies to beverages containing flavorings and/or fruit juices and other constituents of technological or nutritional value. A soft drink typically contains a sweetener and a flavoring. The sweetener may be fruit juice, sugar, high-fructose corn syrup, and sugar substitutes in the case of diet drinks. These drinks may also contain caffeine, colorings, preservatives, and other ingredients [24]. In order to meet current stringent quality and legislative controls, before going to the market, a new beverage is subjected to extensive trials to assess the suitability, performance, and quality of all its components, and as there are millions of liters sold per year, it is essential to reach the correct formula to achieve a product that could be reproducible.
Soft drinks may be served chilled, or at room temperature. In occasional cases, some soft drinks, such as Dr. Pepper, can be served warm [25]. Soft drinks are available, to consumers, in different containers, including cans, glass and plastic bottles, in a variety of sizes ranging from small bottles to large 2-liter containers.
The consumption of sugar-sweetened beverages (SSBs) is associated with obesity [26], type 2 diabetes (T2D) [27], dental caries and low nutrient levels. It could also be associated with many weight-related diseases, including diabetes, metabolic syndrome and cardiovascular risk factors and elevated blood pressure [28]. According to Sen [24], experimental studies support a causal role for sugar-sweetened soft drinks in these illnesses. “Sugar-sweetened” includes drinks that use high-fructose corn syrup, as well as those using sucrose. Many soft drinks contain ingredients that are themselves sources of concern like caffeine that is linked to anxiety and sleep disturbance. Bhupathiraju and co-workers [29] found that the association between higher SSBs consumption and a higher risk of T2D was potentially a result of the high content of sucrose or high-fructose and not to a joint effect of caffeine and sugar. These findings are significant because substituting caffeinated SSBs with caffeine-free SSBs, in the human diet, will not likely decrease the risk of T2D. Instead, the results suggest that replacement of SSB with coffee or tea may lower the risk of T2D.
Soft drinks are made by mixing dry ingredients and/or fruit juices with water. Production of soft drinks can be done at factories or simply at home by mixing either a syrup or dry ingredients with carbonated water. Carbonated water is made using a soda siphon or by dropping dry ice into water [30].
4.2.1. Carbonated Soft Drinks
Sparkling beverages are non-alcoholic, carbonated drinks containing flavorings, sweeteners, and other ingredients. Carbonated soft drinks are frequently prepared by diluting and carbonating “bottler’s”soft drink syrups, which are O/W emulsions of flavoring oils (about 10%, v/v) in aqueous solutions of sugars, coloring, and preservatives [31]. They come in many forms, including regular, low-calorie, no-calorie, caffeinated and caffeine-free drinks. These kinds of beverages are still being the object of study, due to health population risks of disease such as T2D [24].
Some flavors, like citrus oils, are prepared by formulation into an O/W emulsion [32], which must be stable to survive shipping and storage prior to bottling. Moreover, after purchase by consumers, they must also be stable after dilution and storage. The emulsifying agents used are of low molar-mass when preparing these formulations, such as polysaccharides [31]. One important visual characteristic of fruit juices is their turbidity due to suspended fragments of cell walls. Turbidity has become a product requirement by consumers. When the juices are not naturally turbid, during their formulation, suspended particles, or even emulsion droplets, can be introduced to visually simulate the natural juices turbidity.
4.2.2. Low-Calorie and Mid-Calorie Soft Drinks
Low or zero-calorie products are the beverages preferred by consumers, since overconsumption of sugary drinks is associated with obesity, consumers have a tendency to prefer low or zero-calorie products [33]. Low or zero-calorie drinks are not completely appreciated by consumers, due to their unpleasant taste. To overcome this issue the beverage industry has developed mid-calorie soft drinks by blending sugars (sucrose or high-fructose corn syrup) with high-potency sweeteners (acesulfame-potassium, aspartame, and sucralose). The amount of calories is reduced and the desirable flavor profile of the drinks is maintained. Moreover, tax may be imposed on sweetened beverages to discourage consumption of added sugar. Francis and co-workers [34], in a recent research report, concluded that “(…) taxing based on the amount of added sugar a drink contains, either by taxing sugar content directly or by levying higher volume taxes on drinks with more sugar, is feasible in many jurisdictions and reduces sugar consumption more effectively than comparable taxes on drink volume. Broad-based volume or sales taxes on all soft drinks, however, raise revenue more efficiently” [34].
4.3. Milk-Coffee and Rich-Milk-Coffee Beverages
Milk coffee beverages contain about 1% milk fat, forming an oil-in-water emulsion. To emphasize the coffee flavor, many milk coffee beverages contain coffee extracts; these are the so-called “rich milk coffee” beverages. When the content of the coffee extracts increases, milk coffee beverages become unstable and since the storage period for these milk coffee beverages is 6 to 12 months, the milk fat gradually floats towards the air–emulsion interface. Aggregation or flocculation [35,36] follows, and a milk ring is formed at the air–emulsion interface. The milk ring formation, or “oiling off”, is accelerated in these drinks. Moreover, the average drop size distribution of the combined emulsifier system containing protein (in this case, milk proteins) increases because the protein induces an attractive depletion interaction between droplets, thereby inducing flocculation and coalescence [37]. This situation is aggravated by the fact that the plastic bottle is transparent: consumers can see that the milk fat has separated, so the product value depreciates. When flocculation happens, even if the milk coffee beverage is repeatedly shaken, the milk ring will not re-disperse and some lumps of the milk fat will float on the surface. As explained by Ogawa and Cho [38], if the milk fat sticks to the can or plastic bottle, consumers will get the impression that these beverages are of a low quality and will no longer purchase them. These authors [36] found that excessive roasted coffee beans will make the milk coffee unstable. The addition of emulsifiers, like polyglycerol esters with long hydrophilic moieties, is effective for ensuring emulsion’s stability.
4.4. Beverage Concentrates
Beverage concentrates are products that are sold in fluid or powdered form, which consumers then dilute with water prior to consumption. These formulations may contain ingredients, such as flavors, sweeteners, color additives, nutrients or others, which altogether form a drinkable beverage after dilution. According to Klein and co-workers [39], beverage concentrates, also known as “cloudy emulsions”, are usually prepared as concentrates and are diluted at a later stage to the final product. The cloudy emulsion provides flavor, color, and cloud (turbidity) to the soft drink.
Beverage concentrates due to the fact of having ingredients present in a highly concentrated form (high acid, sweetener, flavor, buffer, and nutraceuticals) can suffer spoilage which reduce shelf-life, once accelerated chemical reactions occur [40]. Thus, they must be stabilized by emulsifiers and/or amphiphilic polysaccharides such as whey protein isolate and arabic gums which, in aqueous solution, interact via electrostatic force and improve the emulsion stability [39].
4.5. Functional Beverages
The functional beverage category includes isotonic (beverages that rehydrate), lifestyle/wellness drinks, meal replacements, and medicinal teas. Nowadays, there is an increasing consumer demand for products beneficial to health and human wellness [40]. There is a high demand to fortify many beverage products with ingredients that are perceived by the consumers as giving added health benefits, such as vitamins, proteins, antioxidants, minerals, dietary fibers, and ω-3 fatty acids [40].
4.5.1. Dairy-Based Beverages
Fresh milk, fermented milk, and yogurt drinks are the most common dairy-based beverages. They are vehicles for probiotics [41], live microorganisms which when administered in adequate amounts confer a health benefit to the host, such as: alleviation of lactose-intolerance symptoms and treatment of diarrhea. In children, they enhance the immune response and can be used to treat atopic dermatitis symptoms, alleviation of symptoms of inflammable bowel disease and irritable bowel syndrome, among others [42]. Most dairy industries now add probiotic bacteria (Lactobacillus spp. and Bifidobacterium spp.) to some of their products [43].
Other nutraceutical/bioactive components that can be added are: ω-3 fatty acids (α-linoleic acid, eicosapentaenoic acid and docosahexanoic acid) [44]; Plant sterols like phytosterols and phytostanols that, when added to foods, reduce the absorption of all sterols from the digestive tract, leading to the decrease of cholesterol levels in the blood [44,45]; proteins, like milk caseins, that may act as a precursor of biologically-active peptides with different physiological effects [46]; conjugated linoleic acid that has been demonstrated to have anti-oxidative and anticancer effects and melatonin, a naturally occurring hormone, that controls the body’s day-and-night rhythm and is effective for insomnia [44]. Some dairy and milk industries also add vitamins and minerals, to compensate for vitamin and mineral losses during milk processing; added minerals typically include calcium, magnesium, and iron [44].
4.5.2. Milk-Like Beverages
Lactose intolerance and cholesterol content are major disadvantages related to functional dairy products [46,47]. So, new products containing probiotic strains have been launched in the market, particularly, beverages based on fruits, vegetables, cereals, and soybeans [42]. Soybeans and “soybean-milk” are an interesting alternative due to some important characteristics. They are rich in fibers, vitamin K, riboflavin, thiamine, folic acid, minerals like magnesium and phosphorus, isoflavones and other flavonoids, which present a strong antioxidant potential [48]. Some cereals could also be fermentable substrates for the growth of probiotic microorganisms [49] acting as vehicles for functional compounds [50].
Finally, fruit juices made with cranberry, blueberry, pomegranate, apple, blackcurrant, acai, acerola, guarana, mango, bilberries, grapes, cherries, kiwifruits, strawberries, feijoa, peach, and plums, could also be ideal media for probiotic, due to their content of essential nutrients [48]. Moreover, Gaanappriya and co-workers [51], in 2013, also studied juices from watermelon, sapodilla, and orange as suitable carriers for lactobacilli for preparing beverage for consumers who are allergic to dairy products.
4.5.3. Sport and Energy Drinks
Sports drinks prevent dehydration, supply carbohydrates and electrolytes (sodium, potassium, calcium, magnesium) [52]. They are usually consumed before or during exercise and training. Some considerations must be taken when formulating these drinks. Due to their high sugar content (glucose, fructose, sucrose, and maltodextrin/glucose polymers) they could be perceived “as extremely sweet”. Thus, the addition of maltodextrins and glucose polymers, as alternatives for sucrose or glucose, is recommended [48]. However, sports drinks must be consumed with moderation, once excessive consumption of carbohydrate increases overall daily caloric intake that could lead to weight gain, and poor diet quality [48].
Energy drinks have become popular among young people mainly college students, athletes, and active individuals between the ages of 21 and 35 years [53]. Their objective is to provide sustenance and improved performance, concentration, and endurance [54]. There are many energy-drinks on the market; the most common ingredient between all of them is caffeine, often combined with taurine, glucuronolactone, guarana, and B vitamins to form an “energy blend” that gives an “energy-boost” [55]. However, serious concerns have arisen about the safety of these drinks or some of their major components (caffeine, guarana, and ginseng). Explicitly, caffeine has been shown to enhance physical performance in adults by increasing aerobic endurance and strength, improving reaction time, and delaying fatigue. However, it should be noted that these effects are dose-dependent, and their effects have not been studied in children and adolescents. Interestingly guarana also contains significant amounts of caffeine [52], thus, its presence in an energy drink is a cause of concern because it increases the total caffeine level in the beverage. Ginseng has multiple and important drug interactions [54]. In young people, of particular concern is the increasing use of alcohol mixed with energy drinks, which may increase alcohol dependence and the likelihood of alcohol-related accidents and injuries [56].
4.6. Bottled Water
Recently, there has been an increase in the global sales of flavored bottled waters, and fruit-flavored waters. Utilization of conventional emulsions in bottled waters to deliver oil-soluble flavors and nutraceuticals is limited due to the increase in turbidity caused by light scattering from the oil droplets [48]. However, microemulsions and nanoemulsions that contain very fine droplets that do not scatter light strongly may be used for this purpose [57] (Figure 3). In fact, microemulsions are potentially excellent carriers for bioactive molecules. They offer the advantage of spontaneous formation, ease of manufacture, thermodynamic stability, and improved solubilization of bioactive materials. In fact, Spernath and Aserin [58] found that even W/O microemulsions, which are expected to break upon dilution in the digestive tract, increase the permeability and bioavailability of drugs.
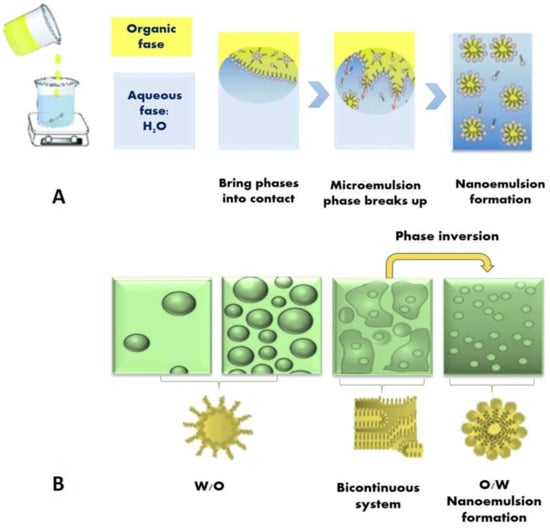
Figure 3.
Schematic diagram of a potential mechanism for the formation of nanoemulsions: (A) by the spontaneous emulsification method. When the organic phase (oil + hydrophilic surfactant) and aqueous phase (water) are brought into contact a bicontinuous microemulsion is formed at the boundary, which breaks up and forms tiny oil droplets; (B) by the emulsion phase inversion method. In this case, water is titrated into a surfactant–oil mixture with constant stirring.
4.7. Fruit Drinks
Today’s consumer’s demand for beverage with added health benefits is adding another level of complexity in the process of beverages formulation and development. The beverage industry is responding to these requests by reducing calories and minimizing ingredient lists. For the consumer, a beverage communicates functionality by its cloudy appearance and thickness in the mouth [59]. To invoke the idea of freshly-squeezed juice, fruit juices, incorporate specific cloud emulsions in product formulations, suggesting that the juice is made of ripe, real fruit, and vegetable ingredients [59].
Xanthan gum (Figure 4) is a polysaccharide used as a food additive. It is a powerful thickening agent, and could also be used as a stabilizer. It can be produced from a range of simple sugars using a fermentation process performed by a strain of Xanthomonas campestris. Xanthan gum is used in fruit-flavored drinks as a stabilizer for odor and flavor, and at the same time, it contributes to the texture.

Figure 4.
Powdery form of xanthan gum and carboxymethyl cellulose (CMC).
Some fruit-juices, with pulp, may precipitate after long storage periods, being difficult to maintain a homogeneous suspension of the fruit pulp. To circumvent separation, Carboxymethyl cellulose (CMC—Figure 4) or mixed hydrocolloids, are added to maintain turbidity and suspension [60,61]. CMC, also called cellulose gum, is a cellulose derivative with carboxymethyl groups (-CH2-COOH) bound to some of the hydroxyl groups of the glucopyranose monomers that make up the cellulose backbone and it is often used as its sodium salt derivative (sodium carboxymethyl cellulose) [62]. The amount of CMC added depends on the soluble solid content of the drink and the level of dilution before consumption. Large amounts of CMC can be used to improve texture and as a stabilizing agent. CMC reduces/prevents the formation of oil rings around the neck of the bottle. CMC is usually added after the addition of preservatives, coloring or flavoring agents. The concentration of CMC that is typically used is in the range of 0.1–0.4% [60]. In some cases, CMC is used together with other types of gum [61].
4.8. Sparkling Wine and Beer
Sparkling wine, is a wine that undergoes a second alcoholic fermentation to induce carbonation and the consequent creation of bubbles. These bubbles (foam) are evanescent and not really very stable. However, it is thought that proteins, due to their surfactant properties, and polysaccharides from the grapes used for wine-making are important factors in foam formation and stability [63,64,65].
The foam formation and foamability parameters seem to be dependent on: (1) the variety of grape used due to differences in the concentrations and types of proteins; (2) aging time and contact time with the yeast of the second fermentation due to the release of glycoproteins, yeast mannoproteins released during fermentation and autolysis that have been described as the major foam promoters due to their structure, which favors adsorption to the foam bubbles gas/liquid interface [64,66]; (3) concentration of iron in the wine, due to the ability of iron cations to complex with the proteins [67]; (4) wine anthocyanins (mainly different forms of malvidin) and amino acids (mainly β-alanine that confer hydrophobicity to the peptides and improve the quality of the foam) [65].
Consumer preferences for beer foams vary but can be characterized in terms of foam stability, quantity, lacing (adhesion to a glass surface), whiteness, “creaminess” (bubble texture), and concentration. The foam “head” created when the beer is poured or dispensed, is also an important aspect of consumer approval of a particular beer product. Also, unlike for sparkling wine, whose foam film lifetimes are short (hydrodynamic control), beer foam has a slower drainage rate due to the adsorption of proteins at the interfaces and the generation of a significant disjoining pressure between bubbles [1].
Beer foam formation and stability are influenced by both raw materials, namely malt, and brewing process. Evans et al. [68] established that the foam stability depends on the malt source. However, the “head” character cannot be predicted. According to several authors, the beer foam stability is determined by the interaction of a number of components present in beer, mainly proteins/polypeptides originated from malt and iso-α-acids from hop [69,70,71].
According to Kordialik-Bogacka and Antczak [72] a more stable beer foam is the one that presents smaller bubbles of uniform size. By increasing concentrations of malt proteins foam stability could increase. The same phenomenon occurs when adding propylene glycol alginate, metal cations (e.g., Mn+2, Al+3, Ni+2), and hop iso-α-acids. Increasing the amount of lipids, protein modification and ethanol will lead to the opposite effect—decrease foam stability. Moreover, according to Evans and Sheehan [73], in unpasteurized beer, proteinase A also reduces beer foam stability.
Another consumer-driven objective, in addition to stable foam, is the so-called “lace curtains” on the wall of a glass and cling patterns (Figure 5). The adhesiveness needed by the foam to produce this effect appears to be derived from the hops [71].
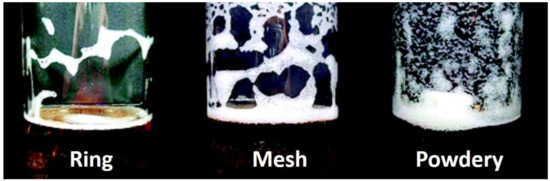
Figure 5.
Three major types of cling that can be created by hop acids in lager beer. Foam-stabilizing properties and cling formation patterns of iso-α-acids and reduced iso-α-acids, investigated by Kunimune and Shellhammer [69], using an unhopped lager beer. The unhopped beer was dosed with iso-α-acid (Iso), rho-iso-α-acid (Rho), tetrahydro-iso-α-acid (Tetra), and hexahydro-iso-α-acid (Hexa), separately, over a range of concentrations from 2 to 10 ppm. Cling formation patterns could be categorized into three groups: “ring”, “mesh”, and “powdery”. The control beer had the lowest foam stability and did not yield any foam cling [71].
5. Final Remarks
There are many challenges associated with the development of beverage emulsions. They are prepared from various ingredients with different formulations and processing operations, thus subjected to undesirable changes in their physical and sensory properties. To reduce risks and maintain product overall quality it is mandatory to develop a robust initial formulation and manufacturing processes.
In some drinks, consumer appreciate the foam that appears in the glass, which is the case of beer and sparkling wine. The foams could be characterized by stability, quantity, lacing, whiteness, “creaminess”, and concentration. These parameters are influenced by both raw materials, namely malt and iso-α-acids from hops (in beer) and the yeast fermentation process and yeasts proteins (in sparkling wine).
To meet changing market and consumer’s demands, the beverage industry needs to seek for new products formulation that may include constituents of technological, nutritional, and nutraceutical/bioactive characteristics and at the same time, remove ingredients which are undesirable from the consumer’s perspective. For completion, a list with the permitted emulsifying, gelling, stabilizing or thickening agents, last revised on 25 September 2017, and approved by the Government of Canada is included (Table 2) [74].

Table 2.
List of permitted emulsifying, gelling, stabilizing, or thickening agents [74].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schramm, L.L. Emulsions, Foams, and Suspensions: Fundamentals and Applications; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; ISBN 3-527-30743-5. [Google Scholar]
- Green, A.J.; Littlejohn, K.A.; Hooley, P.; Cox, P.W. Formation and stability of food foams and aerated emulsions: Hydrophobins as novel functional ingredients. Curr. Opin. Colloid Interface Sci. 2013, 18, 292–301. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-based delivery systems for lipophilic bioactive components. J. Food Sci. 2007, 72, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Given, P.S. Encapsulation of Flavors in Emulsions for Beverages. Curr. Opin. Colloid Interface Sci. 2009, 14, 43–47. [Google Scholar] [CrossRef]
- Dickinson, E. Interfacial structure and stability of food emulsions as affected by protein-polysaccharide interactions. Soft Matter 2008, 4, 932–942. [Google Scholar] [CrossRef]
- European Food Emulsifiers Manufacturers Association (EFEMA). Available online: http://www.emulsifiers.org/ViewDocument.asp?ItemId=9 (accessed on 6 December 2017).
- Florence, A.T.; Attwood, D. Physicochemical Principles of Pharmacy: In Manufacture, Formulation and Clinical Use, 6th ed.; Pharmaceutical Press: London, UK, 2016; ISBN 978 0 85711 174 6. [Google Scholar]
- Eagland, D. The colloidal state. Contemp. Phys. 1973, 14, 119–148. [Google Scholar] [CrossRef]
- Petrucci, R.; William, H.; Herring, F.; Madura, J. General Chemistry: Principles and Modern Applications, 9th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2007; ISBN-13: 978-0132388269. [Google Scholar]
- Urbina-Villaba, G.; Toro-Mendoza, J.; Lozsan, A.; Garcia-Sucre, M. Brownian Dynamics simulation of emulsions stability. In Emulsions: Structure, Stability and Interactions; Elsevier Academic Press: Amsterdam, The Netherland, 2004; ISBN 9780080472652. [Google Scholar]
- Smoluchowski, M. Mathematical Theory of the Kinetics of the Coagulation of Colloidal Solutions. Z. Phys. Chem. 1917, 19, 129–135. [Google Scholar]
- Lagasse, P.; Columbia University. Colloid. In The Columbia Encyclopedia, 7th ed.; Columbia University Press: New York, NY, USA, 2017. [Google Scholar]
- Shaw, D.J. Introduction to Colloid and Surface Chemistry, 4th ed.; Butterworth-Heinemann: Boston, MA, USA, 1992; pp. 1–20. ISBN 0 7506 1182 0. [Google Scholar]
- Zumdahl, S.S.; Zumdahl, S.A. Chemistry, 7th ed.; Houghton Mifflin Company: Boston, MA, USA, 2007; ISBN-13: 978-0618528448. [Google Scholar]
- Esquena, J. Water-in-water (W/W) emulsions. Curr. Opin. Colloid Interface Sci. 2016, 25, 109–119. [Google Scholar] [CrossRef]
- Nicolai, T.; Murray, B. Particle stabilized water in water emulsions. Food Hydrocoll. 2017, 68, 157–163. [Google Scholar] [CrossRef]
- Calvert, J.B. Colloids. 2002. Available online: https://mysite.du.edu/~jcalvert/phys/colloid.htm#Emul (accessed on 22 December 2017).
- Tarko, T.; Tuszyński, T. Influence of Selected Additives on Colloid Stability of Alcoholic Emulsion Creams. Pol. J. Food Nutr. Sci. 2007, 57, 17–24. [Google Scholar]
- Nowicki, Z.T. Liquors fashionable again. Rynki Alkoholowe 1997, 5, 18–20. [Google Scholar]
- Dalgleish, D.G. Food Emulsions in Emulsions and Emulsion Stability; Sjoblom, J., Ed.; Marcel Dekker: New York, NY, USA, 1996; pp. 287–325. [Google Scholar]
- Miner, P.E. Emulsion Rheology: Creams and Lotions in Rheological Properties of Cosmetics and Toiletries; Laba, D., Ed.; Marcel Dekker: New York, NY, USA, 1993; pp. 313–370. [Google Scholar]
- Banks, W.; Muir, D.D. Effect of alcohol content on emulsion stability of cream liqueurs. Food Chem. 1985, 18, 139–152. [Google Scholar] [CrossRef]
- Dickinson, E.; Narhan, S.K.; Stainsby, G. Stability of Cream Liqueurs Containing Low-Molecular-Weight Surfactants. J. Food Sci. 1989, 54, 77–81. [Google Scholar] [CrossRef]
- Sen, D.J. Fizzy Yummy Liquid: The Most Hankering Item in Age Eight to Eighty. Int. J. Pharm. Res. Bio-Sci. 2014, 3, 532–559. [Google Scholar]
- Stryer, L.; Berg, J.M.; Tymoczko, J.L. Biochemistry, 5th ed.; W. H. Freeman: San Francisco, CA, USA, 2002; ISBN 0-7167-3051-0. [Google Scholar]
- Woodward-Lopez, G.; Kao, J.; Ritchie, L. To what extent have sweetened beverages contributed to the obesity epidemic? Public Health Nutr. 2010, 14, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Manson, J.A.E.; Ludwig, D.S.; Colditz, G.A.; Stampfer, M.J.; Willet, W.C.; Hu, F.B. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. J. Am. Med. Assoc. 2004, 292, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Vartanian, L.R.; Schwartz, M.B.; Brownell, K.D. Effects of soft drink consumption on nutrition and health: A systematic review and meta-analysis. Am. J. Public Health 2007, 97, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Pan, A.; Malik, V.S.; Manson, J.E.; Willett, W.C.; van Dam, R.M.; Hu, F.B. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am. J. Clin. Nutr. 2013, 97, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Ashurst, P. Soft Drink and Fruit Juice Problems Solved; Woodhead Publishing Limited: Oxford, UK, 2009; ISBN 978-1-84569-326-8. [Google Scholar]
- Taylor, B. Ingredients, and formulation of carbonated soft drinks. In Carbonated Soft Drinks: Formulation and Manufacture; Steen, D.P., Ashurst, P.R., Eds.; Blackwell Publishing: Oxford, UK, 2006; pp. 48–86. [Google Scholar]
- Tan, C.T. Physical Chemistry in Flavor Products Preparation in Flavor Technology, Physical Chemistry, Modification, and Process; Ho, C.-T., Tan, C.-T., Tong, C.-H., Eds.; American Chemical Society: Washington, DC, USA, 1995; pp. 1–17. [Google Scholar]
- Slavin, J. Beverages and body weight: Challenges in the evidence-based review process of the Carbohydrate Subcommittee from the 2010 Dietary Guidelines Advisory Committee. Nutr. Rev. 2012, 70, S111–S120. [Google Scholar] [CrossRef] [PubMed]
- Francis, N.; Marron, D.; Rueben, K. The Pros, and Cons of Taxing Sweetened Beverages Based on Sugar Content; Research Report; Urban Institute: Washington, DC, USA, 2016; 30p. [Google Scholar]
- McClements, D.J. Food Emulsions: Principles, Practices and Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9781498726689. [Google Scholar]
- Dickinson, E.; Lorient, D. Food Macromolecules, and Colloids; The Royal Society of Chemistry: Cambridge, UK, 1995; ISBN-10: 085404700X. [Google Scholar]
- Kong, Y.; Nikolov, A.; Wasan, D.; Ogawa, A. Emulsion Texture, and Stability: Role of Surfactant Micellar Interactions in the Presence of Proteins. Ind. Eng. Chem. Res. 2008, 47, 9108–9114. [Google Scholar] [CrossRef]
- Ogawa, A.; Cho, H. Role of food emulsifiers in milk coffee beverages. J. Colloid Interface Sci. 2015, 449, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Aserin, A.; Svitov, I.; Garti, N. Enhanced stabilization of cloudy emulsions with gum Arabic and whey protein isolate. Colloids Surf. B Biointerfaces 2010, 77, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Piorkowski, D.T.; McClements, D.J. Beverage emulsions: Recent developments in formulation, production, and applications. Food Hydrocoll. 2014, 42, 5–41. [Google Scholar] [CrossRef]
- Gürakan, G.C.; Cebeci, A.; Özer, B. Probiotic dairy beverages: Microbiology and technology. In Development and Manufacture of Yogurt and Other Functional Dairy Products; Yildiz, F., Ed.; Taylor & Francis Group: Florence, SC, USA, 2010; pp. 165–195. [Google Scholar]
- Saarela, M. Probiotics as ingredients in functional beverages. In Functional and Speciality Beverage Technology; Paquin, P., Ed.; Woodhead Publishing Limited: Cambridge, UK; CRC Press LLC: Boca Raton, FL, USA, 2009; pp. 55–70. [Google Scholar]
- Talwalkar, A.; Kailasapathy, K. A review of oxygen toxicity in probiotic yogurts: Influence on the survival of probiotic bacteria and protective techniques. Compr. Rev. Food Sci. Food Saf. 2004, 3, 117–124. [Google Scholar] [CrossRef]
- Özer, B.; Kirmaci, H.Y. Functional milks and dairy beverages. Int. J. Dairy Technol. 2010, 63, 1–15. [Google Scholar] [CrossRef]
- Alemany-Costa, L.; González-Larena, M.; García-Llatas, G.; Alegría, A.; Barberá, R.; Sánchez-Siles, L.M.; Lagarda, M.J. Sterol stability in functional fruit beverages enriched with different plant sterol sources. Food Res. Int. 2012, 48, 265–270. [Google Scholar] [CrossRef]
- Prado, F.C.; Parada, J.L.; Pandey, A.; Soccol, C.R. Trends in non-dairy probiotic beverages. Food Res. Int. 2008, 41, 111–123. [Google Scholar] [CrossRef]
- Davoodi, H.; Esmaeili, S.; Mortazavian, A.M. Effects of milk and milk products consumption on cancer: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 249–264. [Google Scholar] [CrossRef]
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional Beverages: The Emerging Side of Functional Foods. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Nyanzi, R.; Jooste, P.J. Cereal-based functional foods. In Probiotics; Rigobelo, E.C., Ed.; InTech: Rijeka, Croatia, 2012; pp. 161–196. [Google Scholar]
- Luana, N.; Rossana, C.; Curiel, J.A.; Kaisa, P.; Marco, G.; Rizzello, C.G. Manufacture and characterization of a yogurt-like beverage made with oat flakes fermented by selected lactic acid bacteria. Int. J. Food Microbiol. 2014, 185, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Gaanappriya, M.; Guhankumar, P.; Kiruththica, V.; Santhiya, N.; Anita, S. Probiotication of fruit juices by Lactobacillus acidophilus. Int. J. Adv. Biotechnol. Res. 2013, 4, 72–77. [Google Scholar]
- Heckman, M.A.; Sherry, K.; Gonzalez de Mejia, E. Energy drinks: An assessment of their market size, consumer demographics, ingredient profile, functionality, and regulations in the United States. Compr. Rev. Food Sci. Food Saf. 2010, 9, 303–317. [Google Scholar] [CrossRef]
- Dikici, S.; Saritas, A.; Kilinc, S.; Guneysu, S.; Gunes, H. Does an energy drink cause a transient ischemic attack? Am. J. Emerg. Med. 2015, 33, 129. [Google Scholar] [CrossRef] [PubMed]
- Gunja, N.; Brown, J.A. Energy drinks: Health risks and toxicity. Res. MJA 2012, 196, 46–49. [Google Scholar] [CrossRef]
- Higgins, J.P.; Tuttle, T.D.; Higgins, C.L. Energy beverages: Content and safety. Mayo Clin. Proc. 2010, 85, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Azagba, S.; Langille, D.; Asbridge, M. An emerging adolescent health risk: Caffeinated energy drink consumption patterns among high school students. Prev. Med. 2014, 62, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Komaiko, J.S.; McClements, D.J. Formation of Food-Grade Nanoemulsions Using Low-Energy Preparation Methods: A Review of Available Methods. Compr. Rev. Food Sci. Food Saf. 2016, 15, 331–352. [Google Scholar] [CrossRef]
- Spernath, A.; Aserin, A. Microemulsions as carriers for drugs and nutraceuticals. Adv. Colloid Interface Sci. 2006, 128–130, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, D. All-Natural Cloudy Beverage Emulsions. 2017. Available online: https://sensientfoodcolors.com/en-ap/research-development/natural-cloudy-beverage-emulsions/ (accessed on 24 January 2018).
- Akkarachaneeyakorn, S.; Tinrat, S. Effects of types and amounts of stabilizers on physical and sensory characteristics of cloudy ready-to-drink mulberry fruit juice. Food Sci. Nutr. 2015, 3, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Zecher, D.; Van Coillie, R. Cellulose derivatives. In Thickening and Gelling Agents for Food; Imeson, A., Ed.; Blackie Academic & Professional: London, UK, 1992; pp. 40–65. [Google Scholar]
- Codex Alimentarius Commission. Sodium Carboxymethyl Cellulose (Cellulose gum); GFSA Online; FAO: Rome, Italy, 2016. [Google Scholar]
- Andres-Lacueva, C.; Gaillard, M.; Lopez-Tamames, E.; Lamuela-Raventos, R.M. Influence of variety and aging on foaming properties of sparkling wine (Cava). J. Agric. Food Chem. 1996, 44, 3826–3829. [Google Scholar] [CrossRef]
- Andres-Lacueva, C.; Lamuela-Raventos, R.M.; Buxaderas, S.; Torre-Boronat, M.C. Influence of variety and aging on foaming properties of cava (sparkling wine). J. Agric. Food Chem. 1997, 45, 2520–2525. [Google Scholar] [CrossRef]
- Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Pérez-Magariño, S. Role of major wine constituents in the foam properties of white and rosé sparkling wines. Food Chem. 2015, 174, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, S.; Crapisi, A.; Curioni, A. Foamability of Prosecco wine: Cooperative effects of high molecular weight glycocompounds and wine PR-proteins. Food Hydrocoll. 2014, 34, 202–207. [Google Scholar] [CrossRef]
- Perkowitz, S. Gloriously bubbly. New Sci. 2000, 164, 58–61. [Google Scholar]
- Evans, D.E.; Robinson, L.H.; Sheehan, M.C.; Hill, A.; Skerritt, J.S.; Barr, A.R. Application of immunological methods to differentiate between foam-positive and haze-active proteins originating from malt. J. Am. Soc. Brew. Chem. 2003, 61, 55–62. [Google Scholar]
- Ferreira, M.P.L.V.O.; Jorge, K.; Nogueira, L.C.; Silva, F.; Trugo, L.C. Effects of the combination of hydrophobic polypeptides, ISO-alpha acids, and maltooligosaccharides on beer foam stability. J. Agric. Food Chem. 2005, 53, 4976–4981. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.E.; Surrel, A.; Sheehy, M.; Stewart, D.C.; Robinson, L.H. Comparison of foam quality and the influence of hop alpha-acids and proteins using five foam analysis methods. J. Am. Soc. Brew. Chem. 2008, 66, 1–10. [Google Scholar] [CrossRef]
- Kunimune, T.; Shellhammer, T.H. Foam-Stabilizing Effects and Cling Formation Patterns of ISO-α-acids and Reduced ISO-α-acids in Lager Beer. J. Agric. Food Chem. 2008, 56, 8629–8634. [Google Scholar] [CrossRef] [PubMed]
- Kordialik-Bogacka, E.; Antczak, N. Prediction of Beer Foam Stability from Malt Components. Czech J. Food Sci. 2011, 29, 243–249. [Google Scholar] [CrossRef]
- Evans, D.E.; Sheehan, M.C. Don’t be fobbed off: The substance of beer foam—A review. J. Am. Soc. Brew. Chem. 2002, 60, 47–57. [Google Scholar] [CrossRef]
- Government of Canada. List of Permitted Emulsifying, Gelling, Stabilizing or Thickening Agents [2017-09-25] Document Reference Number: NOM/ADM-0101; NOM/ADM-0088; NOM/ADM-0085; NOM/ADM-0083; NOM/ADM-0069; NOM/ADM-0060; NOM/ADM-0048; NOM/ADM-0044; NOM/ADM-0040; NOM/ADM-0036; NOM/ADM-0015, NOM/ADM-0014, NOM/ADM-0005. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/food-additives/lists-permitted/4-emulsifying-gelling-stabilizing-thickening-agents-2015-09-28.html (accessed on 5 March 2018).
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).