Abstract
This study aimed to evaluate the blueberry and carrot juice blend as a fermentable substrate for Lactobacillus reuteri LR92, in order to develop a fermented non-dairy functional beverage. Analysis of cell viability, pH, and acidity were performed during the fermentation process. The resistance of the microorganism in the blend, under simulated gastrointestinal conditions and in storage at 4 °C for 28 days, was evaluated at the same time as the antioxidant potential of the fermented juice. After 40 h of fermentation, the L. reuteri population presented a logarithmic growth of three cycles, reaching count records of 10.26 ± 0.23 log CFU/mL and after 28 days of storage at 4 °C, the bacterial population maintained elevated numbers of viable cell (8.96 ± 0.08 log CFU/mL), with increase in the antioxidant capacity of the fermented blend. However, in the test of gastric simulation, the L. reuteri population had a logarithmic reduction of five cycles. In the presence of bile salts, the viability was maintained even after 150 min of incubation. This way, the results suggested that the blueberry and carrot blend juice can be considered as a good medium for the growth of L. reuteri, providing microbiological stability during refrigerated storage with elevated antioxidant capacity, which allows for the development of a non-dairy probiotic beverage.
1. Introduction
Fruits and vegetables are considered important nutritional sources for vitamins, minerals, soluble carbohydrate, and different nutrients. Therefore, juice blends of fruit and vegetable extracts can be elaborated with the aim of improving the sensory characteristics of the product and adding nutritional benefits to the different food in the blend [1,2].
Among the fruits that were already the object of more advanced studies, blueberry is one of the richest fruits in antioxidants due to its high phenolic compounds content, mainly anthocyanins [3,4,5]. Carrot is a vegetable with high nutritional value and is the main vegetable source of provitamin A carotenoids, specially α and β-carotene [6].
Probiotic bacteria can be defined as “live microorganisms that when consumed in adequate amounts, provide health benefits to the host” [7]. Although their consumption is mainly related to dairy products, some studies have demonstrated that the fermentation of these bacteria into non-dairy matrices, like in fruits and vegetables, can bring additional benefits to the consumer’s health [2,8,9,10,11,12].
However, the survival of the bacteria in fruit-based matrices is more complex than in dairy products, and the growth is affected by the species and fermentation conditions, such as pH, temperature, formulation of the medium, and others [9,10,11,12]. Therefore, protective agents can be added to the medium to maintain the viability of the microorganism under adverse conditions. Teixeira et al. [13] observed that the conversion of glutamine into glutamate by the Lactobacillus reuteri improved the survival rate of the bacterium under acidic conditions (pH = 4.00).
The L. reuteri is a lactic acid bacterium member of the gastrointestinal ecosystem of animals and humans, used to prevent infections caused by pathogenic agents and reduce effects of severe intestinal disorders and present a unique ability to excrete reuterin [14,15]. Besides this, it possesses other probiotic properties, such as the hypocholesterolemic effect [16] and it has been used to alleviate colic and diarrhea in pediatric patients [17]. Although this strain is usually used in dairy foods [15], few studies evaluated the adaptation and survival of this microorganism in fruit juices [18]. Perricone et al. [18] suggested that the viability of L. reuteri was strongly affected by the kind of juice. This way, more studies evaluating the applicability of the L. reuteri in non-dairy matrices are necessary and interesting due its beneficial potential.
The blueberry and carrot chemical composition seems to be a good medium for fermentation, since blueberry presents pH ca. 2.5 and significant quantities of sugars (9.96%), vitamins such as vitamin C (9.7 mg/100 g) and E (0.57 mg/100 g), and minerals. In the same manner, raw carrot (pH 6.2 ± 0.5) contains approximately 4.7% of total sugars, besides being rich in vitamin A (835 µg/100 g), and minerals like potassium (320 mg/100 g), sodium (69 mg/100 g), phosphorus (35 mg/100 g), and calcium (33 mg/100 g), which could contribute to microbial growth [19].
Kun et al. [8] demonstrated that Bifidobacterium sp. strains reached counts of 108 CFU/mL in pure carrot juice without the need for nutrient supplementation. In a similar manner, Tamminen et al. [20] obtained counts of 109 CFU/mL in pasteurized carrot juice. Besides, the fermentation of carrot juice can positively affect the bioavailability of some minerals (Ca, P, Fe) and increase the levels of vitamins, such as folate [21,22].
In this context, the objective of this study was to evaluate the fermentation and survival of the Lactobacillus reuteri LR92 in the blueberry and carrot blend supplemented with glutamine, for use as a new fermented non-dairy beverage with high antioxidant capacity, combining the nutritional properties of the fruits and vegetables with the beneficial properties of the bacteria. For this, the viability of the microorganism; pH and acidity during a fermentation process over 80 h; survival of the microorganism under acidity and bile salts conditions, and during refrigeration at 4 °C for 28 days; as well as the antioxidant capacity of the fermented juice, were evaluated.
2. Materials and Methods
2.1. Blueberry and Carrot Blend
The carrots were obtained from a supermarket store in the city of Londrina, state of Paraná, Brazil. They were washed, peeled, and cut into rounds of approximately 3 cm. In order to prepare the juice, 100 g of carrot were homogenized in a blender with 100 mL of sterilized distillate water (1:1 w/v). Then, a vacuum filtering was performed using a Büchner funnel for the removal of larger particles that could cause precipitates in the juice. The juice was packed into plastic bags hermetically closed and stored at −18 °C until the moment of use.
The blueberry juice was obtained from the purchase of the industrialized product (Pomar Vale do Dourado, city of Erechim, state of Rio Grande do Sul, Brazil), which contained a mix of cultivars.
The blend was prepared by mixing the juices in the 1:1 (v/v) proportion, added with 2 mM of glutamine and submitted to pasteurization at 80 °C for 1 min. The blueberry and carrot blend juice was characterized by a physical-chemical analysis through the determination of pH (Potentiometer–Ion pH 300), total sugars [23], lipids [24], acidity, proteins, and humidity, using the methods described by the AOAC [25].
2.2. Bacterial Strain
The commercial strain of Lactobacillus reuteri LR 92 (DSM 26866-Sacco-Italy) was maintained frozen in the pasteurized blueberry and carrot blend, with the addition of 2 mM of glutamine, containing 20% (v/v) of sterile glycerol and 0.1% (w/v) of powder culture. At the moment of use, the pre-inoculum was obtained from two activations of the blend at 32 °C for 24 h in batch system using anaerobic jars and placed into a laboratory oven (Novatecnica, Piracicaba, São Paulo, Brazil).
2.3. Fermentation Process
To evaluate the fermentation process of the blend by the L. reuteri LR92, 1% of the reactivated pre-inoculum was added to the pasteurized blueberry and carrot blend in 500 mL bottles and incubated in a anaerobic jar into a laboratory oven (Novatecnica, Piracicaba, São Paulo, Brazil), without stirring, at 32 °C for 80 h (conditions determined in preliminary tests). Each 8 h, samples were removed and an analysis of cell viability, pH and acidity expressed as lactic acid were performed in duplicate for calculation of the average from the two repetitions.
To keep the aseptic conditions during the removal of samples, at the determined times, the fermentation bottles were handled in a sterile chamber. Using a flame and sterile utensils, the samples were taken after homogenization, conditioned again in an anaerobic jar, and incubated in the laboratory oven until the next time point.
The specific growth rate (µN) and generation time (gt) were estimated at the time interval of the logarithmic phase. L. reuteri cell population gt was calculated as t/n, where (t) is the exponential growth time and the number of generations (n) was estimated from N = N02n, where N and N0 are the final and initial number of cells, respectively [26].
2.4. Resistance of L. reuteri in the Blend to Acidity and Bile Salts
The resistance of L. reuteri in the blueberry and carrot blend to acidity and bile salts was evaluated according to Krasaekoopt et al. [27]. The fermented blend was obtained after 40 h of fermentation, according to time determined at 2.3, and then the tolerance to the acid medium was verified by the addition of 1 mL of the product at 9 mL of sterile HCl (Synth) 0.08 M containing 0.2% of NaCl, pH 1.5, and incubated at 37 °C for 30, 60, 90, and 120 min. The resistance to the bile salt was analyzed with the addition of 1 mL of fermented blueberry and carrot blend at 9 mL of KH2PO4 0.05 M (Synth) containing 0.6% bile salt (Himedia), pH 7.4, and incubated at 37 °C for 150 min. At the end of each incubation period, the cell viability was determined by plate counting.
2.5. Stability in the Storage Period
The blueberry and carrot blend was fermented in glass bottles of 150 mL at 32 °C for 40 h, according to the conditions established in the study of fermentation process. After the fermentation, the sample was stored under refrigeration at 4 °C for 28 days, and analysis of the cell viability, total phenolics, and antioxidant activity (ABTS•+ and DPPH• methods), were performed each seven days, in triplicate to calculate the average of three repetitions.
2.6. Counting of Viable Cells
The viability of the Lactobacillus reuteri in the blueberry and carrot blend was established with the methodology of plate counting. Decimal dilutions of the fermented blend were performed in sterile Peptone Water 0.1% (w/v), followed by plating in MRS Agar and incubation at 37 °C for 48 h under anaerobic conditions.
2.7. Establishment of Antioxidant Capacity for the Fermented Blueberry and Carrot Blend
2.7.1. Extract Preparation
The extracts were prepared by adding the sample in 80% ethanol in the proportion of 1:10, followed by shaking it for 20 min at 200 rpm (MARCONI, MA 140/CFT, São Paulo, Brazil). Then, the samples were centrifuged (HARRIER, Castle Donington, UK) at 2000× g for 10 min, in order to obtain the supernatant. The extracts were stored at −22 °C until the time of use.
2.7.2. Total Phenolics Analysis (Reduction of Folin–Ciocalteau Reagent)
In order to determine the total phenolic compounds, it was used the methodology described by Swain and Hills [28]. Briefly, 2.5 mL of 10% Folin-Ciocalteau reagent, 2.0 mL of 7.5% sodium carbonate and 0.5 mL of samples extracts were incubated for 5 min at 50 °C. After this period, the absorbance was read at 760 nm using a UV-visible spectrophotometer, model Libra S22 (Biochrom®, Cambourne, UK). The solution of Folin-Ciocalteau and sodium carbonate was used as a control. The quantification was performed with the standard curve of gallic acid (0.1 at 0.5 mM) and the results in mg equivalent of gallic acid/100 g (EAG) in fresh base.
2.7.3. Antioxidant Capacity: Capacity of Scavenging Free DPPH• Radicals
The capacity of scavenging DPPH• radicals was determined according to the methodology proposed by Brand-Williams et al. [29]. We added 50 µL of sample extract to 1 mL of acetate buffer solution (pH 5.5), 1 mL of absolute ethanol, and 0.5 mL of DPPH• solution 250 μmol/L, and stored it in the dark for 30 min. The absorbance was read in a spectrophotometer at 517 nm, using 1.0 mL of acetate buffer solution 100 mM pH 5.5 with 1.5 mL of absolute ethanol as a blank, and positive control with all solutions except the sample. The quantification was performed by Trolox standard curve (0.5 at 20 μmol/L), and the results were expressed in μmoL of Trolox per mL of sample.
2.7.4. Antioxidant Capacity: Capacity of Scavenging Free Radicals (ABTS•+)
The capacity of scavenging the free radical ABTS•+(2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid)) was determined according to the methodology described by Sanchez-Gonzalez et al. [30]. The solution 7 mM ABTS•+ with 2.45 mM potassium persulfate was prepared in the dark and 16 h before the analysis. This solution was diluted with sodium phosphate (pH 7.4) until it presented an absorbance of 0.7 ± 0.02 at 730 nm, followed by the addition of 10 μL of the sample and 4 mL of ABTS diluted solution. After 6 min of reaction, the absorbance of the samples was read at 730 nm. The quantification was performed based on the Trolox standard curve (0.5 at 20 μmol/L), and the results were expressed in μmoL of Trolox per gram of sample in fresh base.
2.8. Statistical Analysis
The data were submitted to the Analysis of Variance (ANOVA), Student’s t-test, or Tukey’s range test, for the comparison of averages at a 5% significance level, using the program STATISTICA 7.0. The results were expressed in average ± standard deviation.
3. Results
3.1. Centesimal Composition
The centesimal composition of the fermented and unfermented blueberry and carrot blend used in this work is presented by the Table 1. The fermented and unfermented samples did not present relevant differences (p > 0.05) in regard to protein, lipids, sugars, ashes, and humidity (Table 1). Some differences were observed between the pH and total sugars of the unfermented sample in comparison to the fermented one (p ≤ 0.05).

Table 1.
Centesimal composition and pH of blueberry and carrot blend unfermented and fermented by Lactobacillus reuteri. Results are expressed as mean values of three repetitions followed by the standard deviation.
3.2. Fermentation Process
In previous studies (data not published but demonstrated in Figure S1), only the L. reuteri growth in blueberry juice was evaluated. It was possible to observe that the microorganism presented low adaptability and a low capacity of multiplication in this medium, and after 36 h of fermentation, no viable cells were detected (Figure S1). The low pH of the juice was measured during the fermentation time and can be one of the reasons of the low growing rate in blueberry juice.
In this way, the blend of blueberry juice with carrot was an alternative to favor the growth conditions for the bacteria, combining the nutritional properties of the blueberry and the carrot.
The L. reuteri growth in the blueberry and carrot blend for 80 h of fermentation is shown in Figure 1A. An exponential growth of the microorganism up to 40 h was observed, reaching the counting of 10.26 ± 0.2 log CFU/mL. After 48 h of fermentation, the microorganism viability started to reduce. However, the values were maintained at 9.32 ± 0.2 log CFU/mL until the end of the fermentation time, which are higher than the minimum value recommended of 106 CFU/mL of viable cells for probiotic products [31].
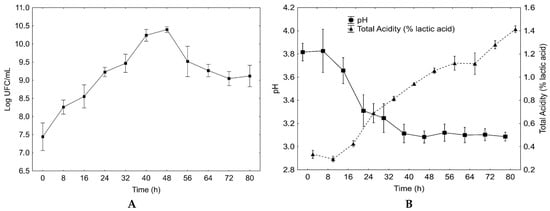
Figure 1.
Viability of Lactobacillus reuteri (A), and pH (■) and acidity (% lactic acid) (▲) (B) for 80 h of fermentation in blueberry and carrot blend at 32 °C. The results are presented as the mean of three repetitions and standard deviation.
The specific growth rate obtained for L. reuteri in the blueberry and carrot juice blend was 5.64 h with a µN of 0.005 h−1.
The Figure 1B shows the results for acidity and pH for the blueberry and carrot blend during the L. reuteri growth at 32 °C for 80 h. It was possible to observe that, during fermentation, the acidity of the medium increased from 0.34% ± 0.02% of lactic acid in the beginning to 1.42% ± 0.01% after 80 h, and the pH decreased from 3.82 to 3.11 with the microorganism growth and variation of this values during the fermentation process, which suggests that the L. reuteri used the sugars present in the blend to produce lactic acid.
3.3. Stability in the Storage Period
After establishing the growth kinetics, the blend fermented for 40 h was stored for 28 days at 4 °C, and the results for the counting of viable cells are presented in the Figure 2.
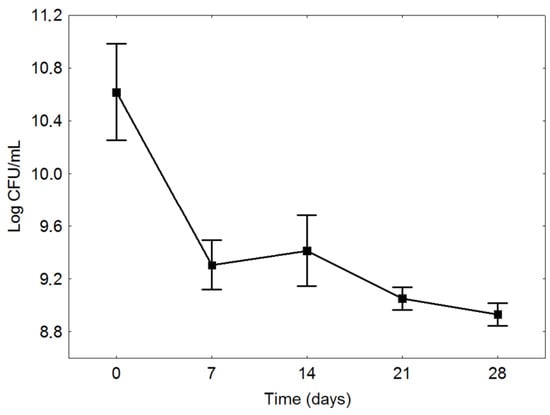
Figure 2.
Survival of Lactobacillus reuteri of fermented blueberry and carrot blend during storage for 28 days at 4 °C. The results are shown in the average of three repetitions and standard deviation.
The L. reuteri population decreased from 10.77 ± 0.14 log CFU/mL in the beginning of storage to 9.23 ± 0.18 log CFU/mL at the end of the first week of storage. After seven days, no relevant alteration was observed (≤0.05) in the number of viable cells, which shows the stability of the bacteria under storage conditions. After 28 days of storage, the L. reuteri population reached a survival of 8.96 ± 0.08 log CFU/mL, remaining as a product with microbial population compatible to the level of bacteria for functional product consumption [32].
3.4. Resistance of L. reuteri in the Blend to Acidity and Bile Salts
Besides being able to survive in the food product, the microorganisms must resist the acidity and bile of the gastrointestinal tract. Aiming to evaluate the tolerance of the L. reuteri under these conditions in the fermented blueberry and carrot blend, an in vitro test was performed and the results are presented in the Figure 3. During the test, significant differences in the microorganism viability were observed from 0 to 120 min (p ≤ 0.05). Initially, the average value of viable cells was of 10.27 ± 0.21 log CFU/mL, and after 120 min the microorganism population had a logarithmic reduction of five cycles in relation to the 0 min time, reaching the counting of 4.23 ± 0.03 log CFU/mL.
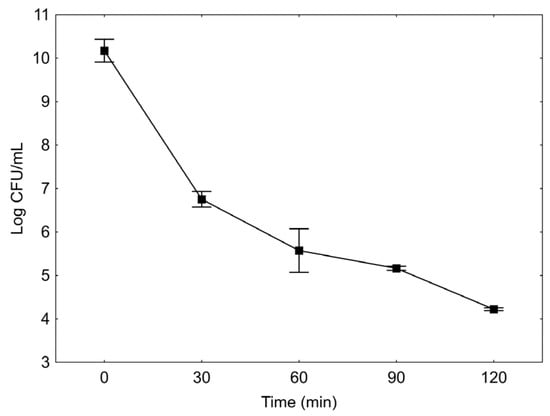
Figure 3.
Survival of Lactobacillus reuteri in fermented blueberry and carrot blend, during simulated gastric in pH 1.5 with 0, 30, 60, 90, and 120 min of incubation. The results are expressed as the average of three replicates.
After 150 min of incubation in the presence of bile salts in pH 7.4, the average number of L. reuteri viable cells was of 9.17 ± 0.07 log CFU/mL, indicating that the microorganism can tolerate the intestinal conditions when fermented in blueberry and carrot blend.
3.5. Antioxidant Capacity under Storage
In order to verify the antioxidant activity in the fermented product, tests were performed to determine the total phenolic compounds and scavenging DPPH• and ABTS•+ radicals during a storage period of 28 days at 4 °C, which are presented in the Table 2. According to Roginski and Lissi [33], in order to estimate the antioxidant activity in matrices such as food and biological fluids, the use of more than one analysis method is recommended, as they are limited and have different methodologies.

Table 2.
Total phenolic compounds and antioxidant activity of blueberry and carrot blend fermented by L. reuteri during storage for 28 days at 40 °C.
During the storage period of the fermented blueberry and carrot blend, the phenolic compounds content and the antioxidant activity by the scavenging of DPPH• radical did not show significant differences (p ≤ 0.5). In regard to the antioxidant activity, a significant increase with the methodology of the ABTS•+ radical was observed.
4. Discussion
4.1. Centesimal Composition
The results obtained by centesimal composition analysis, showed that protein, lipids, sugars, ashes, and humidity contents were close to the values found in the literature for the blueberry fruit and carrot root in separate manner [19]. The small differences observed for the blueberry and carrot blend in regard to the mentioned studies can be explained by the addition of water in the preparation of the juices, besides the use of different varieties of blueberry and carrot.
In relation to fermented and non-fermented products, the differences could be explained by the bacterial metabolism, with consumption of sugars present in the medium for the growth and production of lactic acid [11], according to what was observed in the fermentation process.
4.2. Fermentation Process
In a non-adapted media—e.g., non-dairy products—lactic acid bacteria take more time to reach highest log cycle counting, making a full study of the bacterial strain growth necessary. This way, the 0 h of fermentation was chosen, to know how the cells growth and lose its viability.
In the present study, the L. reuteri viability above 1010 CFU/mL was obtained with 40 h of fermentation, and for this reason we chose to execute the other analysis.
Those values were higher than the ones found by Mousavi et al. [34] and Perricone et al. [18], when they fermented pomegranate juice with L. plantarum and red fruits juice with L. reuteri, respectively. Perricone et al. [18] suggested that some species of lactic acid bacteria present lower growth speeds in fruit juices with high phenolic compound contents. As well as the fruits mentioned, the blueberry is a fruit that is rich in these compounds [3,4,5], which is allied to the low juice pH, could explain the longer time (40 h) to reach the highest cell count for L. reuteri in the blend.
The high viable cell counts suggest that both blueberry and carrot supplied sufficient nutrients to the multiplication of L. reuteri. Besides that, the addition of the carrot juice could have decreased the deleterious effects present in the blueberry juice, and the presence of phenolic compounds and antioxidants could have contributed for a higher survival through the creation of anaerobic conditions in ideal proportions that favored the multiplication of L. reuteri [35].
Although some lactic bacteria can tolerate low pH values [12], the use of blends can be an alternative for the pH adjustment in an acidic food through the addition of another with less acidity; in this case, blueberry with a pH between 2.56 and 2.67 [36] and carrot with a pH between 6.5 and 6.84 [6], could have had made the medium more favorable for L. reuteri growth. In this way, the mixture of blueberry and carrot juice is a more favorable medium for the L. reuteri growth, while combining the benefits from the both foods.
In relation to kinetics parameters, the low values of µN (0.005 h−1) can be correlated with the gt of 5.63 h. Specific growth rate relates the instantaneous velocity in a given time interval with the number of cells and it is expected that the lower specific value is, the lower the microorganism generation time will be. Generation time is subject to environmental factors and genetic and growing conditions. Yáñez et al. [37] obtained a growth rate for Lactobacillus rhamnosus ranging from 0.11 h−1 to 0.22 h−1, according to acid concentration. They observed that, as the concentration of the acid increases, the growth rate decreases, which can explain the low values from the present study.
4.3. Stability in the Storage Period
Cell viability is the main factor to be considered in functional products. In the present study, L. reuteri reached the maximum exponential growth rate at time 40 h, in which was possible to observe the beginning of the stationary phase. According to Meng et al. [38], when bacteria enter the stationary phase, they generally develop a resistance to stress, becoming more resistant during process conditions and storage period. Also, the fermentation for 40 h maintains a low pH, which increases the product safety during storage and prevents the development of the pathogenic bacteria [11]. This way, it was chosen to interrupt the process and perform the analysis of storage and resistance to acidity and bile salts.
In regard to the evaluation during the refrigerated storage, this study presented superior results in comparison to those presented by Mousavi et al. [34], which did not obtain viable cells of Lactobacillus plantarum, L. acidophilus, L. paracasei, and L. delbruekii from the third week of storage in pomegranate juice.
Dimitroviski et al. [2] observed an increase, within 16 days, in the survival of L. plantarum when 30% blueberry juice was added to the formulation of artichoke juice in comparison to the same formulation without the blueberry. According to those authors, the presence of this juice also contributed to the increase of sensory acceptability.
Some components present in juices—like fibers, sugars, and proteins—can contribute to the increase of survival of probiotic cultures in these foods [39]. Besides that, according to Vasiljevic and Shah [40], the microorganism viability in the food matrix depends on several factors, such as the strain used, food pH, bacterial metabolism, and others, and, therefore, the adaptation of the probiotic to the substrate is a crucial criterion for the evaluation of the final product.
While determining the survival of the L. reuteri, Jones et al. [15] obtained counting of 1 × 1010 CFU per dose of yogurt consumed. Perricone et al. [18] observed that the survival of the L. reuteri was highly affected by the type of juice, the highest ones being obtained with the pineapple, orange, and apple juices; and the lowest with the red fruit juices. Pallin et al. [41] evaluated the growth, metabolism, and production of bioactive compounds during the fermentation of barley by Lactobacillus reuteri and identified the importance of the strain used during the fermentation process.
Therefore, it is safe to affirm that the L. reuteri LR92 showed capacity of adaptation in low pH for 28 days, which shows that the use of this microorganism is viable even in products with high acidity.
4.4. Resistance of L. reuteri in the Blend to Acidity and Bile Salts
The tolerance to acidity is one of the most important properties of lactic acid bacteria, as they enable the cells to survive in gastric conditions and exert beneficial health effects in the intestine. However, in the present study, a decrease on the microbial population during acidity condition was observed, which can be explained by the stressful conditions of the environment with prolonged exposure to the pH of gastric acid.
Céspedes et al. [42] investigated resistance to simulated gastric acid in commercial nondairy drinks added with lactobacilos (Lactobacillus casei LC-01 and L. casei BGP 93) during storage conditions. These authors suggested that although the changes in resistance in the different juices were observed, the juices with a pH around 3 had a cell decay of ca. 3 log cycles after 90 min, which was lower compared to the present study (ca. 4 log cycles). They concluded that the survival of bacterial strains in fruit matrices can be very variable and product-dependent and also may be a characteristic of the lactic acid bacteria in low pH juices.
Krasaekoopt and Watcharapoka [43] evaluated the application of inulin or galactooligossaccharides (GOS) for microencapsulation of L. acidophilus and L. casei during simulated digestive system using the same method used in the present study. These authors concluded that the use of prebiotics could be an alternative to increasing the number of viable cells in acid conditions, which could be tested in the future for the blueberry and carrot blend.
4.5. Antioxidant Capacity under Storage
During the storage period, a slight increase in the antioxidant capacity was also observed. This can be attributed to the increase of aglycone levels, which are formed from the conversion of anthocyanins by enzymes such as β-glucosidase, originated from the bacteria even under low temperatures (4 °C) [44].
The blueberry has one of the highest antioxidant capacities when compared to other fruits and vegetables, but those values may change in different varieties of the fruit [3]. The carrot was analyzed in regard to the antioxidant activity by Melo et al. [45], which concluded that, from 15 vegetables analyzed, the carrot was one of the vegetables that presented the lowest capacity for scavenging DPPH• radical.
Reque et al. [4] evaluated the antioxidant activity of blueberry juice stored for 10 days under refrigeration at 4 °C. In this work, the ABTS•+ content at day 0 of the storage was of 11.14 ± 0.18 µM of trolox/mL, and at day 8 was of 6.98 ± 0.12 µM of trolox/mL, which are superior results than the ABTS•+ content found in the present study. According to Skrede et al. [5], the extraction of blueberry can provoke oxidation reactions from the enzyme polyphenol oxidase, with consequent degradation of phenolic compounds present in the juice.
5. Conclusions
Although there are several studies evaluating the use of microorganisms in food, there are few data related to the survival of the L. reuteri in fruit and vegetable juices. The results of this work suggest that the blueberry and carrot blend allows L. reuteri growth in sufficient levels for a possible probiotic product. When stored at 4 °C, the L. reuteri viability presents a slight reduction after seven days, which was maintained until the end of 28 days of storage. Besides that, the fermentation contributes to the increase of the antioxidant activity of the blend during the storage period, indicating that the L. reuteri can be used in fruit and vegetable juices, with an improvement of nutritional aspects.
Supplementary Materials
The following are available online at www.mdpi.com/2306-5710/2/4/37/s1, Figure S1: Lactobacillus reuteri LR92 viability and pH during 80 h of fermentation in blueberry juice only.
Acknowledgments
We would like to thank the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq) Brazil, for financial support by Process number: 479623/2012-0 and in the form of scholarship for K.B.G.
Author Contributions
C.S.I.M: Performed the tests and contributed to the manuscript writing. K.B.G: Contributed to the project design and to the manuscript writing. S.G: Contributed to the project design and to the manuscript writing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Quinteros, E.T.T. Processamento e Estabilidade de Néctares de Acerola-Cenoura. Master’s Thesis, Faculty of Food Engineering, State University of Campinas, Campinas, Brazil, 1995. [Google Scholar]
- Dimitrovski, D.; Velickova, E.; Dimitrovska, M.; Langerholc, T.; Winkelhausen, E. Synbiotic functional drink from Jerusalem artichoke juice fermented by probiotic Lactobacillus plantarum PCS26. J. Food Sci. Technol. 2016, 53, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Cardeñosa, V.; Girones-Vilaplana, A.; Muriel, J.L.; Moreno, D.A.; Moreno-Rojas, J.M. Influence of genotype, cultivation system and irrigation regime on antioxidant capacity and selected phenolics of blueberries (Vaccinium corymbosum L.). Food Chem. 2016, 202, 276–283. [Google Scholar]
- Reque, P.M.; Steffens, R.S.; Jablonski, A.; Flôres, S.H.; Rios, A.O.; Jong, E.V. Cold storage of blueberry (Vaccinium spp.) fruits and juice: Anthocyanin stability and antioxidant activity. J. Food Compos. Anal. Res. 2013, 33, 111–116. [Google Scholar] [CrossRef]
- Skrede, G.; Wrolstad, R.E.; Durst, R.W. Changes in anthocyanins and polyphenolics during juice processing of Highbush blueberries (Vaccinium corymbosum L.). J. Food Sci. 2000, 55, 357–364. [Google Scholar] [CrossRef]
- Lima, K.S.C.; Lima, A.L.S.; Luchese, R.H.; Godoy, R.L.O.; Sabaa-Srur, A.U.O. Minimally processed carrots in modified atmosphere packaging and gama irradiation treatment: Microbiological, fisical-chemistry and chemistry evaluation. Food Sci. Technol. 2003, 23, 240–250. [Google Scholar]
- Food and Agriculture Organization (FAO); World Health Organization (WHO). Guidelines for the Evaluation of Probiotics in Food: Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: London, ON, Canada, 2002. [Google Scholar]
- Kun, S.; Rezessy-Szabo, J.M.; Nguyes, Q.D.; Hoschke, A. Changes of microbial population and some components in carrot juice during fermentation with selected Bifidobacterium strains. Process Biochem. 2008, 43, 816–821. [Google Scholar] [CrossRef]
- Rivera-Espinoza, Y.; Gallardo-Navarro, Y. Non-dairy probiotic products. Food Microbiol. 2010, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mestry, A.P.; Mujumdar, A.S.; Thorat, N. Optimization of Spray Drying of an Innovative Functional Food: Fermented Mixed Juice of Carrot and Watermelon. Dry. Technol. 2011, 29, 1121–1131. [Google Scholar] [CrossRef]
- Costa, M.G.M.; Fonteles, T.V.; de Jesus, A.L.T.; Rodrigues, S. Sonicated pineapple juice as substrate for L. casei cultivation for probiotic beverage development: Process optimisation and product stability. Food Chem. 2013, 139, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Perricone, M.; Bevilacqua, A.; AltierI, C.; Sinigaglia, M.; Corbo, M.R. Challenges for production of probiotic fruit juices. Beverages 2015, 1, 95–103. [Google Scholar] [CrossRef]
- Teixeira, J.S.; Seeras, A.; Sanchez-Maldonado, A.F.; Zhang, C.; Su, M.S.; Gänzle, M.G. Glutamine, glutamate, and arginine-based acid resistance in Lactobacillus reuteri. Food Microbiol. 2014, 42, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Coccorullo, P.; Strisciuglio, C.; Martinelli, M.; Miele, E.; Greco, L.; Staiano, A. Lactobacillus reuteri (DSM 17938) in infants with functional chronic constipation: A double-blind, randomized, placebo-controlled study. J. Pediatr. 2010, 157, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Martoni, C.J.; Tamber, S.; Parent, M.; Prakash, S. Evaluation of safety and tolerance of microencapsulated Lactobacillus reuteri NCIMB 30242 in a yogurt formulation: A randomized, placebo-controlled, double-blind study. Food Chem. Toxicol. 2012, 50, 2216–2223. [Google Scholar] [CrossRef] [PubMed]
- Taranto, M.P.; Medici, M.; Perdigón, G.; Ruiz Holgado, A.P.; Valdez, G.F. Effect of Lactobacillus reuteri on the prevention of hypercholesterolemia in ice. J. Dairy Sci. 2000, 83, 401–403. [Google Scholar] [CrossRef]
- Dinleyici, E.C.; Dalgic, N.; Guven, S.; Metin, O.; Yasa, O.; Kurugol, Z.; Turel, O.; Tanir, G.; Yazar, A.S.; Arica, V.; et al. Lactobacillus reuteri DSM 17938 shortens acute infectious diarrhea in a pediatric outpatient setting. J. Pediatr. 2015, 91, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Perricone, M.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Bevilacqua, A. Viability of Lactobacillus reuteri in fruit juices. J. Funct. Foods 2014, 10, 421–426. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). Agricultural Research Service. USDA Food Composition Databases. Available online: https://ndb.nal.usda.gov/ndb/search (accessed on 25 June 2016).
- Tamminen, M.; Salminen, S.; Ouwehand, A.C. Fermentation of carrot juice by probiotics: Viability and preservation of adhesion. Int. J. Biotechnol. Wellness Ind. 2013, 2, 10–15. [Google Scholar] [CrossRef]
- Jägerstad, M.; Jastrebova, J.; Svensson, U. Folates in fermented vegetables—A pilot study. LWT Food Sci. Technol. 2006, 37, 603–611. [Google Scholar] [CrossRef]
- Bergqvist, S.W.; Sandberg, A.S.; Calrlsson, N.G.; Andid, T. Improved iron solubility in carrot juice fermented by homo- and hetero-fermentative lactic acid bacteria. Food Microbiol. 2005, 22, 53–61. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method form Determination of Sugars and Related Substances. Nature 1956, 28, 350–356. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Physiol. Pharmacol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists (AOAC). Official Methods of Analysis, 15th ed.; AOAC: Washington, DC, USA, 2006. [Google Scholar]
- Spinosa, W.A.; dos Júnior, V.S.; Galvan, D.; Fiorio, J.L.; Gomez, R.J.H.C. Fermentation kinetics of rice syrup, with high contente of dextrose equivalente, by Saccharomyces cerevisae and characterization of volatile compounds from wine. J. Food Process. Pres. 2016, 40, 1199–1205. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. Int. Dairy J. 2004, 14, 737–743. [Google Scholar] [CrossRef]
- Swain, T.; Hills, W.E. The phenolic constituents of Prunnus domestica. The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 19, 63–68. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sánchez-Gonzalez, I.; Jiménez-Escrig, A.; Saura-Calixto, F. In vitro antioxidant activity of coffees brewed using different procedures (Italian, espresso and filter). Food Chem. 2005, 90, 133–139. [Google Scholar] [CrossRef]
- Shah, N.P. Functional cultures and health benefits. Int. Dairy J. 2007, 17, 1262–1277. [Google Scholar] [CrossRef]
- Charteris, W.P.; Kelly, P.M.; Morelli, L.; Collins, J.K. Ingredient selection criteria for probiotic microorganisms in functional dairy foods. Int. J. Dairy Technol. 1998, 51, 123–136. [Google Scholar] [CrossRef]
- Ronginski, V.; Lissi, E.A. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem. 2005, 92, 235–254. [Google Scholar]
- Mousavi, Z.E.; Mousavi, S.M.; Razavi, S.H.; Emam-Djomeh, Z.; Kiani, H. Fermentation of pomegranate juice by probiotic lactic acid bacteria. World J. Microbiol. Biotechnol. 2011, 27, 123–128. [Google Scholar] [CrossRef]
- Lima, I.F.P.; Lindner, J.D.; Soccol, V.T.; Parada, J.L.; Soccol, C.R. Development of an innovative nutraceutical fermented beverage from Herbal Mate (Ilex paraguariensis A. St.-Hil.) extract. Int. J. Mol. Sci. 2012, 12, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.O.; Pertuzatti, P.B.; Corrêa, F.V.; Salas-Mellado, M.L.M. Study of rabbiteye blueberry (Vaccinium ashei Reade) in the process of food products. Food Sci. Technol. 2007, 27, 18–22. [Google Scholar]
- Yáñez, R.; Marques, S.; Gírio, F.M.; Roseiro, J.C. The effect of acid stress on lactate production and growth kinetics in Lactobacillus rhamnosus culture. Process Biochem. 2008, 43, 356–361. [Google Scholar] [CrossRef]
- Meng, X.C.; Stanton, G.F.; Fitzgerald, C.D.; Ross, R.P. Anydrobiotics: The challenges of drying probiotic cultures. Food Chem. 2007, 106, 1406–1416. [Google Scholar] [CrossRef]
- Nualkaekul, S.; Charalampopouloos, D. Survival of Lactobacillus plantarum in model solution and fruit juices. Int. J. Food Microbiol. 2011, 146, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Vasiljevic, T.; Shah, N.P. Probiotics—From Metchnikoff to bioactives. Int. Dairy J. 2008, 18, 714–728. [Google Scholar] [CrossRef]
- Pallin, A.; Agback, P.; Jonsson, H.; Roos, S. Evaluation of growth, metabolism and production of potentially bioactive components during fermentation of barley with Lactobacillus reuteri. Food Microbiol. 2016, 57, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Céspedes, M.; Cárdenas, P.; Staffolani, M.; Ciappini, M.C.; Vinderola, G. Performance in Nondairy Drinks of Probiotic L. casei Strains Usually Employed in Dairy Products. J. Food Sci. 2013, 78, M756–M762. [Google Scholar] [CrossRef] [PubMed]
- Krasaekoopt, W.; Watcharapoka, S. Effect of addition of inulin and galactooligosaccharide on the survival of microencapsulated probiotics in alginate beads coated with chitosan in simulated digestive system, yogurt and fruit juice. LWT Food Sci. Technol. 2014, 57, 761–766. [Google Scholar] [CrossRef]
- Moraes Filho, M.L.; Hirozawa, S.S.; Prudencio, S.H.; Ida, E.I.; Garcia, S. Petit suisse from black soybean: bioactive compounds and antioxidant properties during development process. Int. J. Food Sci. Nutr. 2014, 65, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Melo, E.A.; Maciel, M.I.S.; Lima, V.L.A.G.; Leal, F.L.L.; Caetano, A.C.S.; Nascimento, R.J. Antioxidant capacity of vegetables commonly consumed. Food Sci. Technol. 2006, 26, 639–644. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).