Biotechnological Valorisation of Oilseed Cakes in the Formulation of Vegan Yoghurt-like Fermented Beverages
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Comparative Samples
2.2. Materials Collection and Samples Preparation
2.3. Methods
2.3.1. Fermentation and Preparation of Yoghurt-like Samples
2.3.2. Proximate Composition Analysis
2.3.3. Titrable Acidity
2.3.4. Density
2.3.5. Viscosity
2.3.6. Syneresis
2.3.7. Preliminary Sensory Evaluation by the Check-All-That-Apply (CATA) Method
2.3.8. Total Polyphenols
2.3.9. DPPH Radical Scavenging Activity
2.3.10. ABTS Radical Cation Scavenging Activity
2.3.11. Microbiological Analyses of Yoghurt-like Beverages
Sample Preparation, Dilutions and Plate Counts
Total Viable Counts
Coliforms
Yeasts and Moulds
2.3.12. Statistical Analysis
3. Results
3.1. Physicochemical Characteristics and Composition of Oilseed Cakes
3.2. Proximate Composition of Yoghurt-like Beverages
3.3. Physicochemical Characteristics of Yoghurt-like Beverages
3.4. Preliminary Sensory Evaluation of Yoghurt-like Beverages
3.5. Bioactive Profile of Yoghurt-like Beverages
3.6. Microbiological Parameters of Yoghurt-like Beverages
4. Conclusions
5. Patent
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, M.; Tilman, D. Comparative Analysis of Environmental Impacts of Agricultural Production Systems, Agricultural Input Efficiency, and Food Choice. Environ. Res. Lett. 2017, 12, 064016. [Google Scholar] [CrossRef]
- Chia, A.; Shou, Y.; Wong, N.M.Y.; Cameron-Smith, D.; Sim, X.; Van Dam, R.M.; Chong, M.F.-F. Complexity of Consumer Acceptance to Alternative Protein Foods in a Multiethnic Asian Population: A Comparison of Plant-Based Meat Alternatives, Cultured Meat, and Insect-Based Products. Food Qual. Prefer. 2024, 114, 105102. [Google Scholar] [CrossRef]
- Capcanari, T.N.; Covaliov, E.F.; Negoița, C.L. Hemp (Cannabis sativa L.) Seeds Nutritional Aspects and Food Production Perspectives: A Review. Food Syst. 2024, 7, 52–58. [Google Scholar] [CrossRef]
- Capper, J.L. Opportunities and Challenges in Animal Protein Industry Sustainability: The Battle Between Science and Consumer Perception. Anim. Front. 2020, 10, 7–13. [Google Scholar] [CrossRef]
- Boeck, T.; Sahin, A.W.; Zannini, E.; Arendt, E.K. Nutritional Properties and Health Aspects of Pulses and Their Use in Plant-based Yogurt Alternatives. Comp. Rev. Food Sci. Food Safe 2021, 20, 3858–3880. [Google Scholar] [CrossRef]
- Cardello, A.V.; Llobell, F.; Giacalone, D.; Chheang, S.L.; Jaeger, S.R. Consumer Preference Segments for Plant-Based Foods: The Role of Product Category. Foods 2022, 11, 3059. [Google Scholar] [CrossRef]
- Hadidi, M.; Aghababaei, F.; Gonzalez-Serrano, D.J.; Goksen, G.; Trif, M.; McClements, D.J.; Moreno, A. Plant-Based Proteins from Agro-Industrial Waste and by-Products: Towards a More Circular Economy. Int. J. Biol. Macromol. 2024, 261, 129576. [Google Scholar] [CrossRef] [PubMed]
- Khanna, M.; Zilberman, D.; Hochman, G.; Basso, B. An Economic Perspective of the Circular Bioeconomy in the Food and Agricultural Sector. Commun. Earth Environ. 2024, 5, 507. [Google Scholar] [CrossRef]
- Pal, P.; Singh, A.K.; Srivastava, R.K.; Rathore, S.S.; Sahoo, U.K.; Subudhi, S.; Sarangi, P.K.; Prus, P. Circular Bioeconomy in Action: Transforming Food Wastes into Renewable Food Resources. Foods 2024, 13, 3007. [Google Scholar] [CrossRef]
- Kårlund, A.; Gómez-Gallego, C.; Korhonen, J.; Palo-oja, O.-M.; El-Nezami, H.; Kolehmainen, M. Harnessing Microbes for Sustainable Development: Food Fermentation as a Tool for Improving the Nutritional Quality of Alternative Protein Sources. Nutrients 2020, 12, 1020. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, Y.; Ma, T.; Liang, Q.; Sun, J.; Wu, X.; Song, Y.; Nie, H.; Huang, J.; Mu, G. Fermented Dairy Products as Precision Modulators of Gut Microbiota and Host Health: Mechanistic Insights, Clinical Evidence, and Future Directions. Foods 2025, 14, 1946. [Google Scholar] [CrossRef]
- Jang, H.J.; Lee, N.-K.; Paik, H.-D. Overview of Dairy-Based Products with Probiotics: Fermented or Non-Fermented Milk Drink. Food Sci. Anim. Resour. 2024, 44, 255–268. [Google Scholar] [CrossRef]
- Plamada, D.; Teleky, B.-E.; Nemes, S.A.; Mitrea, L.; Szabo, K.; Călinoiu, L.-F.; Pascuta, M.S.; Varvara, R.-A.; Ciont, C.; Martău, G.A.; et al. Plant-Based Dairy Alternatives—A Future Direction to the Milky Way. Foods 2023, 12, 1883. [Google Scholar] [CrossRef]
- Silva, A.R.A.; Silva, M.M.N.; Ribeiro, B.D. Health Issues and Technological Aspects of Plant-Based Alternative Milk. Food Res. Int. 2020, 131, 108972. [Google Scholar] [CrossRef]
- Geburt, K.; Albrecht, E.H.; Pointke, M.; Pawelzik, E.; Gerken, M.; Traulsen, I. A Comparative Analysis of Plant-Based Milk Alternatives Part 2: Environmental Impacts. Sustainability 2022, 14, 8424. [Google Scholar] [CrossRef]
- Radu, O.; Covaliov, E.; Capcanari, T. Technological Properties and Functional Food Potential of Oilseed Cakes. Ukr. Food J. 2024, 13, 287–302. [Google Scholar] [CrossRef]
- Kumar, M.; Kumari, N.; Prakash, S.; Sharma, N.; Rajat; Radha; Sharma, K.; Chandran, D.; Eswaran, S.; Panesar, P.S. Oilseed Meal as a Source of Protein: Introductory Remarks. In Oilseed Meal as a Sustainable Contributor to Plant-Based Protein; Kumar, M., Punia Bangar, S., Panesar, P.S., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–29. ISBN 978-3-031-47879-6. [Google Scholar]
- Capcanari, T.; Covaliov, E.; Negoița, C. Harnessing Hemp (Cannabis sativa L.) Seed Cake Proteins: From Concentrate Production to Enhanced Choux Pastry Quality. Foods 2025, 14, 567. [Google Scholar] [CrossRef] [PubMed]
- Nevara, G.A.; Giwa Ibrahim, S.; Syed Muhammad, S.K.; Zawawi, N.; Mustapha, N.A.; Karim, R. Oilseed Meals into Foods: An Approach for the Valorization of Oilseed by-Products. Crit. Rev. Food Sci. Nutr. 2023, 63, 6330–6343. [Google Scholar] [CrossRef] [PubMed]
- Łopusiewicz, Ł.; Kwiatkowski, P.; Drozłowska, E. Production and Characterization of Yogurt-Like Fermented Beverage Based on Camelina (Camelina sativa L.) Seed Press Cake. Appl. Sci. 2022, 12, 1085. [Google Scholar] [CrossRef]
- Deziderio, M.A.; De Souza, H.F.; Kamimura, E.S.; Petrus, R.R. Plant-Based Fermented Beverages: Development and Characterization. Foods 2023, 12, 4128. [Google Scholar] [CrossRef]
- Capcanari, T.; Covaliov, E.; Negoița, C.; Siminiuc, R.; Chirsanova, A.; Reșitca, V.; Țurcanu, D. Hemp Seed Cake Flour as a Source of Proteins, Minerals and Polyphenols and Its Impact on the Nutritional, Sensorial and Technological Quality of Bread. Foods 2023, 12, 4327. [Google Scholar] [CrossRef]
- Kadam, D.; Lele, S.S. Value Addition of Oilseed Meal: A Focus on Bioactive Peptides. Food Meas. 2018, 12, 449–458. [Google Scholar] [CrossRef]
- Vichare, S.A.; Morya, S. Exploring Waste Utilization Potential: Nutritional, Functional and Medicinal Properties of Oilseed Cakes. Front. Food. Sci. Technol. 2024, 4, 1441029. [Google Scholar] [CrossRef]
- Sarkar, N.; Chakraborty, D.; Dutta, R.; Agrahari, P.; Sundaram, D.; Singh, A.; Jacob, S. A Comprehensive Review on Oilseed Cakes and Their Potential as a Feedstock for Integrated Biorefinery. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 376. [Google Scholar] [CrossRef]
- Zaier, H.; Maktouf, S.; Roussos, S.; Rhouma, A. Filamentous Fungi Isolated from Tunisian Olive Mill Wastes: Use of Solid-State Fermentation for Enzyme Production. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12125. [Google Scholar] [CrossRef]
- Masiá, C.; Geppel, A.; Jensen, P.E.; Buldo, P. Effect of Lactobacillus Rhamnosus on Physicochemical Properties of Fermented Plant-Based Raw Materials. Foods 2021, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Abu-Ghannam, N. Probiotic Fermentation of Plant Based Products: Possibilities and Opportunities. Crit. Rev. Food Sci. Nutr. 2012, 52, 183–199. [Google Scholar] [CrossRef]
- Boistean, A.; Radu, O.; Covaliov, E.; Capcanari, T. Process for Obtaining Plant-Based Drinking Yoghurt. Short-term Patent No. 1821, issued by the State Agency on Intellectual Property of the Republic of Moldova, 15 July 2024. [Google Scholar]
- ISO 6731:2010/IDF 21:2010; Milk, Cream and Evaporated Milk—Determination of Total Solids Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2010.
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; Method 923.03—Ash of Flour; AOAC International: Rockville, MD, USA, 2023. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Method 2001.11—Crude Protein in Foods by Kjeldahl Method; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- ISO 1211:2010/IDF 1:2010; Milk—Determination of Fat Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2010.
- AOAC. Official Methods of Analysis of AOAC International, 15th ed.; Method 985.29—Total Dietary Fiber in Foods: Enzymatic–Gravimetric Method; AOAC International: Arlington, VA, USA, 1990. [Google Scholar]
- AOAC. Official Method 942.15Acidity (Titratable) of Fruit Products. In Official Methods of Analysis of AOAC INTERNATIONAL; Latimer, G.W., Ed.; Oxford University Press: New York, NY, USA, 2023; ISBN 978-0-19-761013-8. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 19th ed.; Method 925.22—Specific Gravity of Milk; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Varnaitė, L.; Keršienė, M.; Šipailienė, A.; Kazernavičiūtė, R.; Venskutonis, P.R.; Leskauskaitė, D. Fiber-Rich Cranberry Pomace as Food Ingredient with Functional Activity for Yogurt Production. Foods 2022, 11, 758. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Measurement of Total Phenolics and Tannins Using Folin-Ciocalteu Method. In Quantification of Tannins in Tree and Shrub Foliage; Springer: Dordrecht, The Netherlands, 2003; pp. 49–51. ISBN 978-90-481-6428-8. [Google Scholar]
- Lin, J.; Zhou, W. Role of Quercetin in the Physicochemical Properties, Antioxidant and Antiglycation Activities of Bread. J. Funct. Foods 2018, 40, 299–306. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Alcolea, J.F.; Acosta, M. Estimation of Free Radical-quenching Activity of Leaf Pigment Extracts. Phytochem. Anal. 2001, 12, 138–143. [Google Scholar] [CrossRef]
- ISO 6887-1:2017; Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination—Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilutions. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 7218:2007; Microbiology of Food and Animal Feeding Stuffs—General Requirements and Guidance for Microbiological Examinations. International Organization for Standardization: Geneva, Switzerland, 2007.
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 4832:2006; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coliforms—Colony-Count Technique. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 21527-1:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. International Organization for Standardization: Geneva, Switzerland, 2008.
- Grahovac, N.; Aleksić, M.; Trajkovska, B.; Marjanović Jeromela, A.; Nakov, G. Extraction and Valorization of Oilseed Cakes for Value-Added Food Components—A Review for a Sustainable Foodstuff Production in a Case Process Approach. Foods 2025, 14, 2244. [Google Scholar] [CrossRef]
- Polyzos, N.; Fernandes, Â.; Calhelha, R.C.; Petrović, J.; Soković, M.; Ferreira, I.C.F.R.; Barros, L.; Petropoulos, S.A. Biochemical Composition of Pumpkin Seeds and Seed By-Products. Plants 2024, 13, 2395. [Google Scholar] [CrossRef] [PubMed]
- Rakita, S.; Kokić, B.; Manoni, M.; Mazzoleni, S.; Lin, P.; Luciano, A.; Ottoboni, M.; Cheli, F.; Pinotti, L. Cold-Pressed Oilseed Cakes as Alternative and Sustainable Feed Ingredients: A Review. Foods 2023, 12, 432. [Google Scholar] [CrossRef]
- Cirkovic Velickovic, T.D.; Stanic-Vucinic, D.J. The Role of Dietary Phenolic Compounds in Protein Digestion and Processing Technologies to Improve Their Antinutritive Properties. Comp. Rev. Food Sci. Food Safe 2018, 17, 82–103. [Google Scholar] [CrossRef] [PubMed]
- Schefer, S.; Oest, M.; Rohn, S. Interactions between Phenolic Acids, Proteins, and Carbohydrates—Influence on Dough and Bread Properties. Foods 2021, 10, 2798. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Xu, Y.; Shi, C.; Liu, Y.; Bi, S. Formation Mechanism and Functional Properties of Walnut Protein Isolate and Soy Protein Isolate Nanoparticles Using the pH-Cycle Technology. Front. Nutr. 2023, 10, 1135048. [Google Scholar] [CrossRef]
- Lv, S.; Taha, A.; Hu, H.; Lu, Q.; Pan, S. Effects of Ultrasonic-Assisted Extraction on the Physicochemical Properties of Different Walnut Proteins. Molecules 2019, 24, 4260. [Google Scholar] [CrossRef]
- Kaur, R.; Ghoshal, G. Sunflower Protein Isolates-Composition, Extraction and Functional Properties. Adv. Colloid. Interface Sci. 2022, 306, 102725. [Google Scholar] [CrossRef]
- Zheng, R.; Chen, Y.; Wang, Y.; Rogers, M.A.; Cao, Y.; Lan, Y. Microstructure and Physical Properties of Novel Bigel-Based Foamed Emulsions. Food Hydrocoll. 2023, 134, 108097. [Google Scholar] [CrossRef]
- Manufacturing Yogurt and Fermented Milks, 1st ed.; Chandan, R.C., Kilara, A., Eds.; Wiley: Hoboken, NJ, USA, 2013; ISBN 978-1-119-96708-8. [Google Scholar]
- De Santis, D.; Giacinti, G.; Chemello, G.; Frangipane, M.T. Improvement of the Sensory Characteristics of Goat Milk Yogurt. J. Food Sci. 2019, 84, 2289–2296. [Google Scholar] [CrossRef]
- Bulut, M.; Adal, E.; Aktar, T. Plant Protein Enrichment Effect on the Physical, Chemical, Microbiological, and Sensory Characteristics of Yogurt. Food Process. Preserv. 2022, 46, e16865. [Google Scholar] [CrossRef]
- Ribeiro, A.C.P.; Magnani, M.; Baú, T.R.; Esmerino, E.A.; Cruz, A.G.; Pimentel, T.C. Update on emerging sensory methodologies applied to investigating dairy products. Curr. Opin. Food Sci. 2024, 56, 101135. [Google Scholar] [CrossRef]
- Gupta, M.K.; Torrico, D.D.; Ong, L.; Gras, S.L.; Dunshea, F.R.; Cottrell, J.J. Plant and Dairy-Based Yogurts: A Comparison of Consumer Sensory Acceptability Linked to Textural Analysis. Foods 2022, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Grasso, N.; Alonso-Miravalles, L.; O’Mahony, J.A. Composition, Physicochemical and Sensorial Properties of Commercial Plant-Based Yogurts. Foods 2020, 9, 252. [Google Scholar] [CrossRef]
- Bayram, O.Y.; Kinik, O.; Büyükkileci, C. Functional and Phenolic Characterization of Medicinal Plant-Enriched Strained Yogurt: Bioactivity and Storage Stability. Food Meas. 2025, 19, 7557–7570. [Google Scholar] [CrossRef]
- Al-Quwaie, D.A.; Allohibi, A.; Aljadani, M.; Alghamdi, A.M.; Alharbi, A.A.; Baty, R.S.; Qahl, S.H.; Saleh, O.; Shakak, A.O.; Alqahtani, F.S.; et al. Characterization of Portulaca Oleracea Whole Plant: Evaluating Antioxidant, Anticancer, Antibacterial, and Antiviral Activities and Application as Quality Enhancer in Yogurt. Molecules 2023, 28, 5859. [Google Scholar] [CrossRef] [PubMed]
- Şanlıdere Aloğlu, H.; Öner, Z. Determination of Antioxidant Activity of Bioactive Peptide Fractions Obtained from Yogurt. J. Dairy. Sci. 2011, 94, 5305–5314. [Google Scholar] [CrossRef]
- Brück, W.M.; Díaz Escobar, V.D.; Droz-dit-Busset, L.; Baudin, M.; Nicolet, N.; Andlauer, W. Fermentative Liberation of Ellagic Acid from Walnut Press Cake Ellagitannins. Foods 2022, 11, 3102. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A Comparative Review on the Extraction, Antioxidant Content and Antioxidant Potential of Different Parts of Walnut (Juglans regia L.) Fruit and Tree. Molecules 2019, 24, 2133. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Wei, X.; Xu, C.; Cavender, G.; Lin, W.; Sun, S. Invited Review: Advances in Yogurt Development—Microbiological Safety, Quality, Functionality, Sensory Evaluation, and Consumer Perceptions across Different Dairy and Plant-Based Alternative Sources. J. Dairy. Sci. 2025, 108, 33–58. [Google Scholar] [CrossRef]
- Zamfir, M.; Angelescu, I.-R.; Voaides, C.; Cornea, C.-P.; Boiu-Sicuia, O.; Grosu-Tudor, S.-S. Non-Dairy Fermented Beverages Produced with Functional Lactic Acid Bacteria. Microorganisms 2022, 10, 2314. [Google Scholar] [CrossRef]
- El-Sayed, A.S.; Ibrahim, H.; Farag, M.A. Detection of Potential Microbial Contaminants and Their Toxins in Fermented Dairy Products: A Comprehensive Review. Food Anal. Methods 2022, 15, 1880–1898. [Google Scholar] [CrossRef]
- Zhi, N.-N.; Zong, K.; Thakur, K.; Qu, J.; Shi, J.-J.; Yang, J.-L.; Yao, J.; Wei, Z.-J. Development of a Dynamic Prediction Model for Shelf-Life Evaluation of Yogurt by Using Physicochemical, Microbiological and Sensory Parameters. CyTA—J. Food 2018, 16, 42–49. [Google Scholar] [CrossRef]
- Asante, R.O.; Akese, S.; Mensah, T. Physicochemical, Microbial, and Nutritional Evaluation of Tiger Nut Yogurt as a Plant-Based Alternative to Conventional Dairy Yogurt. Food Sci. Nutr. 2025, 13, e70897. [Google Scholar] [CrossRef] [PubMed]
- European Union. European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Off. J. Eur. Union 2005, 338, 1–26. Available online: https://eur-lex.europa.eu/eli/reg/2005/2073/oj/eng (accessed on 14 November 2025).
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: London, ON, Canada, 2002. [Google Scholar]
- CXS 243-2003; Standard for Fermented Milks; Adopted in 2003; Revised in 2008, 2010, 2018; Amended in 2022 and 2024. FAO/WHO: Rome, Italy, 2024.
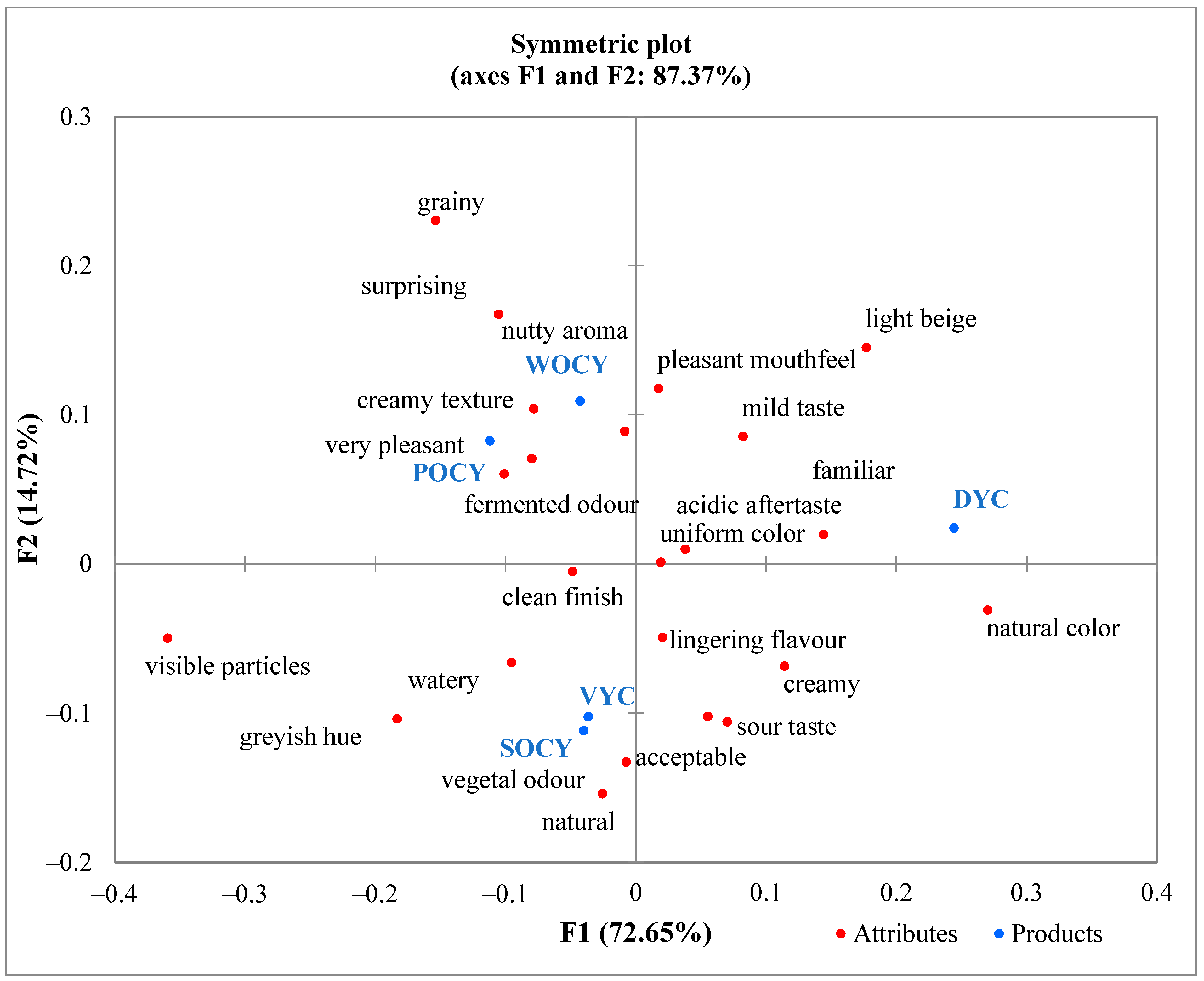

| Attribute Category | CATA Attributes |
|---|---|
| Visual Aspect | homogeneous appearance, visible particles, creamy texture, dull appearance, phase separation, sediment formation, unappealing look |
| Colour | light beige, yellowish tone, greyish hue, brownish tint, natural colour, artificial colour, uniform colour, non-uniform colour |
| Odour | nutty aroma, vegetal odour, earthy odour, fermented odour, sour odour, pleasant odour, unpleasant odour |
| Flavour | mild taste, bitter taste, sour taste, sweet taste, unpleasant flavour |
| Aftertaste | clean finish, nutty aftertaste, bitter aftertaste, acidic aftertaste, lingering flavour, unpleasant residual taste |
| Mouthfeel | smooth, creamy, viscous, watery, thick, grainy, pleasant mouthfeel, unpleasant mouthfeel |
| Overall Acceptability | very pleasant, acceptable, neutral, slightly unpleasant, unpleasant |
| Emotional Response | comforting, natural, surprising, familiar, strange, unappealing |
| Parameter | POC | WOC | SOC | Reference Values (Literature Range) |
|---|---|---|---|---|
| Moisture (%) | 7.2 ± 0.2 a | 8.0 ± 0.3 b | 8.1 ± 0.3 b | 6–9 |
| Ash (% d.m.) | 1.42 ± 0.10 a | 5.54 ± 0.15 bc | 5.0 ± 0.20 b | 4.5–6.0 |
| Acidity (degrees) | 6.60 ± 0.25 a | 13.5 ± 0.4 c | 12.5 ± 0.3 b | 12–14 |
| Crude protein (% d.m.) | 59.8 ± 1.8 c | 49.9 ± 1.5 b | 44.0 ± 1.3 a | 43–52 |
| Crude fat (% factual) | 14.6 ± 0.7 c | 7.9 ± 0.5 a | 9.0 ± 0.6 b | 8–12 |
| Crude fat (% d.m.) | 15.7 ± 0.8 c | 8.6 ± 0.6 a | 9.5 ± 0.7 b | 8–12 |
| Fibre (% d.m.) | 10.5 ± 0.4 a | 12.1 ± 0.5 b | 14.2 ± 0.6 c | 10–15 |
| Carbohydrates (NFE, % d.m.) | 12.7 ± 0.5 a | 23.9 ± 0.6 b | 27.2 ± 0.7 c | 18–25 |
| Parameter | POCY | WOCY | SOCY | VYC * | DYC * |
|---|---|---|---|---|---|
| Moisture (%) | 88.5 ± 0.3 b | 88.0 ± 0.2 b | 87.2 ± 0.4 a | 89.35 | 86.6 |
| Protein (%) | 4.6 ± 0.1 c | 3.7 ± 0.2 a | 4.2 ± 0.1 b | 2.1 | 3.4 |
| Fat (%) | 2.3 ± 0.1 c | 1.4 ± 0.1 b | 1.2 ± 0.1 a | 8.7 | 2.7 |
| Carbohydrates (%) | 3.3 ± 0.2 b | 3.9 ± 0.2 c | 4.8 ± 0.3 e | 1.9 | 4.6 |
| Fibre (%) | 1.8 ± 0.1 bc | 1.5 ± 0.15 a | 1.9 ± 0.1 c | 1.7 | – |
| Ash (%) | 0.21 ± 0.01 a | 0.73 ± 0.02 c | 0.80 ± 0.02 d | 0.58 | 0.75 |
| Parameter | POCY | WOCY | SOCY | VYC | DYC |
|---|---|---|---|---|---|
| Acidity (% lactic acid) | 0.87 ± 0.01 a | 0.98 ± 0.02 b | 0.77 ± 0.03 a | 0.81 ± 0.02 a | 0.83 ± 0.02 a |
| Density (g·cm−3) | 1.033 ± 0.003 a | 1.019 ± 0.002 a | 1.012 ± 0.002 a | 0.981 ± 0.001 a | 0.985 ± 0.004 a |
| Viscosity (Pa·s, 20 °C) | 0.91 ± 0.02 b | 0.97 ± 0.01 bc | 0.87 ± 0.03 b | 0.63 ± 0.06 a | 1.30 ± 0.02 c |
| Syneresis, 120 h storage (%) | 14.0 ± 1.0 b | 16.5 ± 0.5 c | 18.0 ± 1.0 d | 21.5 ± 0.5 e | 11.0 ± 1.0 a |
| Parameter | POCY | WOCY | SOCY | VYC | DYC |
|---|---|---|---|---|---|
| Total polyphenol content (mg GAE kg−1) | 564.85 ± 2.78 c | 1108.97 ± 3.87 e | 856.43 ± 4.42 d | 238.82 ± 3.14 b | 96.10 ± 2.80 a |
| DPPH (mg Trolox kg−1) | 278.49 ± 4.33 c | 412.54 ± 5.38 e | 338.49 ± 4.54 d | 125.05± 1.75 b | 48.72 ± 2.24 a |
| ABTS, mg TE g−1 DW | 37.6 ± 0.3 c | 51.5 ± 0.6 e | 44.9 ± 0.3 d | 25.0 ± 0.4 b | 12.3 ± 0.8 a |
| Parameter | POCY | WOCY | SOCY | VYC | DYC |
|---|---|---|---|---|---|
| Total counts after fermentation (CFU mL−1) | 5.4 × 105 | 4.9 × 105 | 4.1 × 105 | 4.6 × 105 | 3.6 × 105 |
| Total counts after 14 days of storage (CFU mL−1) | 9.7 × 106 | 8.6 × 107 | 8.3 × 107 | 7.9 × 107 | 1.2 × 107 |
| Coliforms | NF | NF | NF | NF | NF |
| Yeasts and moulds | NF | NF | NF | NF | NF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, O.; Capcanari, T.; Boiștean, A.; Covaliov, E. Biotechnological Valorisation of Oilseed Cakes in the Formulation of Vegan Yoghurt-like Fermented Beverages. Beverages 2025, 11, 164. https://doi.org/10.3390/beverages11060164
Radu O, Capcanari T, Boiștean A, Covaliov E. Biotechnological Valorisation of Oilseed Cakes in the Formulation of Vegan Yoghurt-like Fermented Beverages. Beverages. 2025; 11(6):164. https://doi.org/10.3390/beverages11060164
Chicago/Turabian StyleRadu, Oxana, Tatiana Capcanari, Alina Boiștean, and Eugenia Covaliov. 2025. "Biotechnological Valorisation of Oilseed Cakes in the Formulation of Vegan Yoghurt-like Fermented Beverages" Beverages 11, no. 6: 164. https://doi.org/10.3390/beverages11060164
APA StyleRadu, O., Capcanari, T., Boiștean, A., & Covaliov, E. (2025). Biotechnological Valorisation of Oilseed Cakes in the Formulation of Vegan Yoghurt-like Fermented Beverages. Beverages, 11(6), 164. https://doi.org/10.3390/beverages11060164








