Photodegradation of Retsina Wine: Does Pine Resin Protect Against Light-Induced Changes?
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Preparation and Extraction
2.3. GC-MS/MS Analysis
2.4. Colorimetric Analysis
2.5. Statistical Analysis
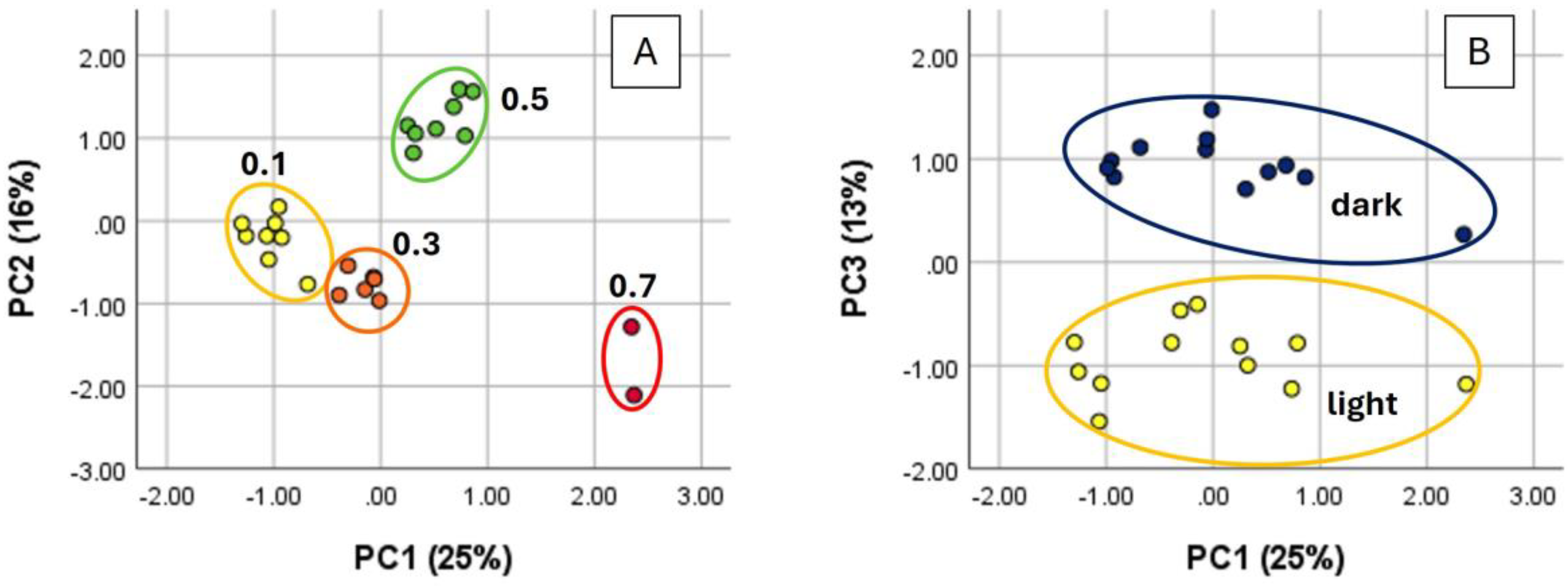
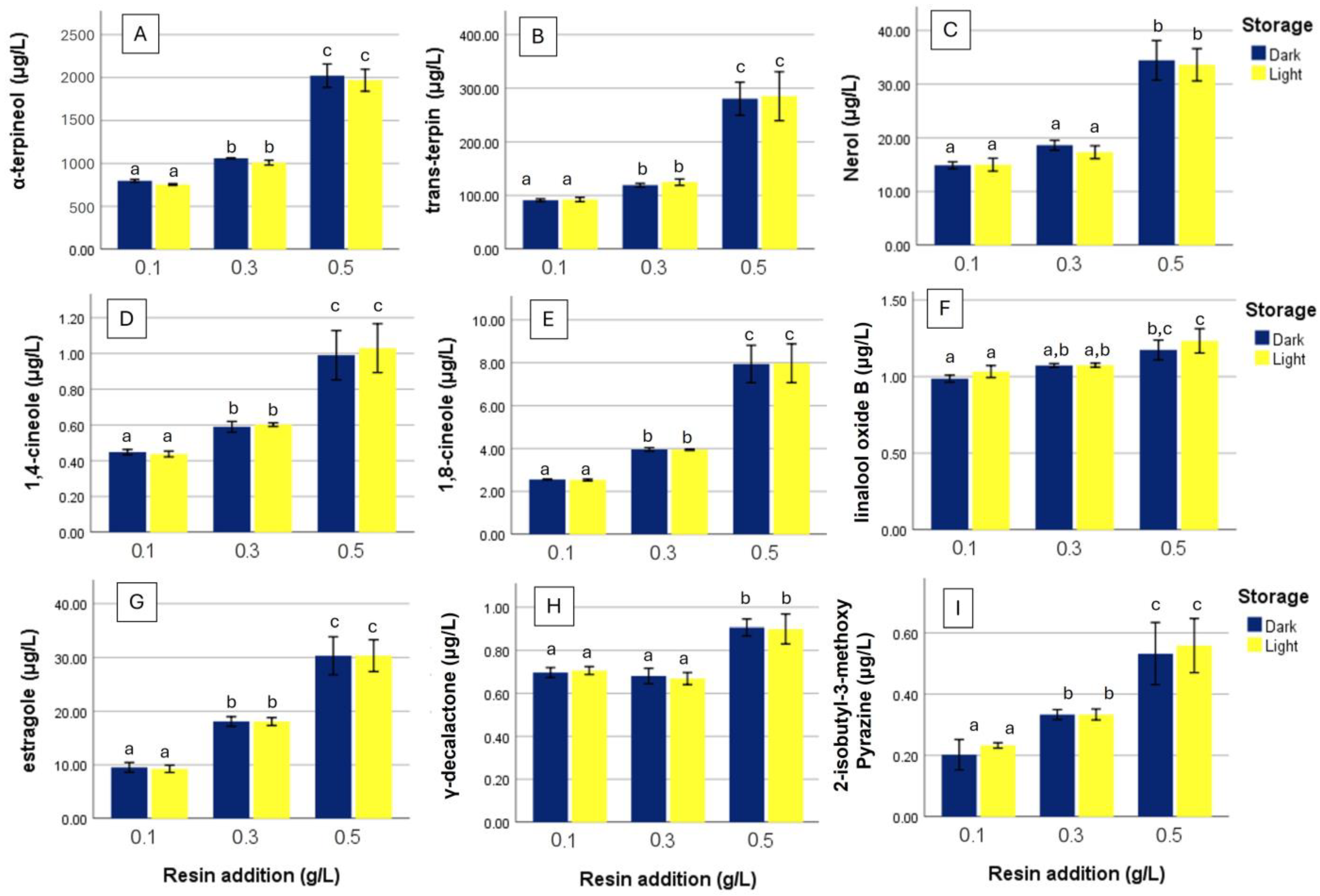
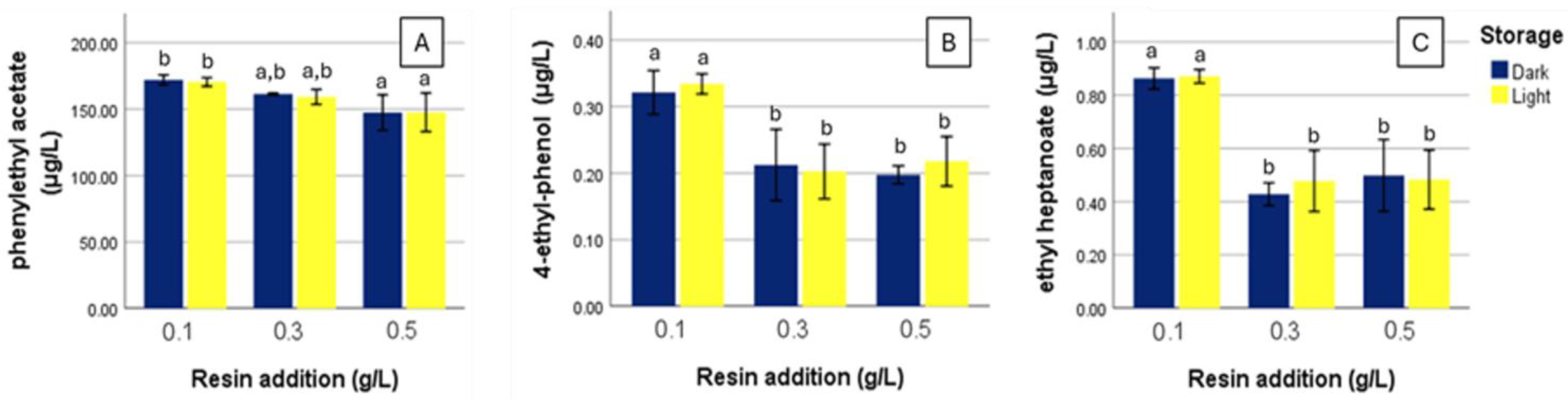
3. Results
4. Discussion
4.1. Light-Induced Compositional Changes
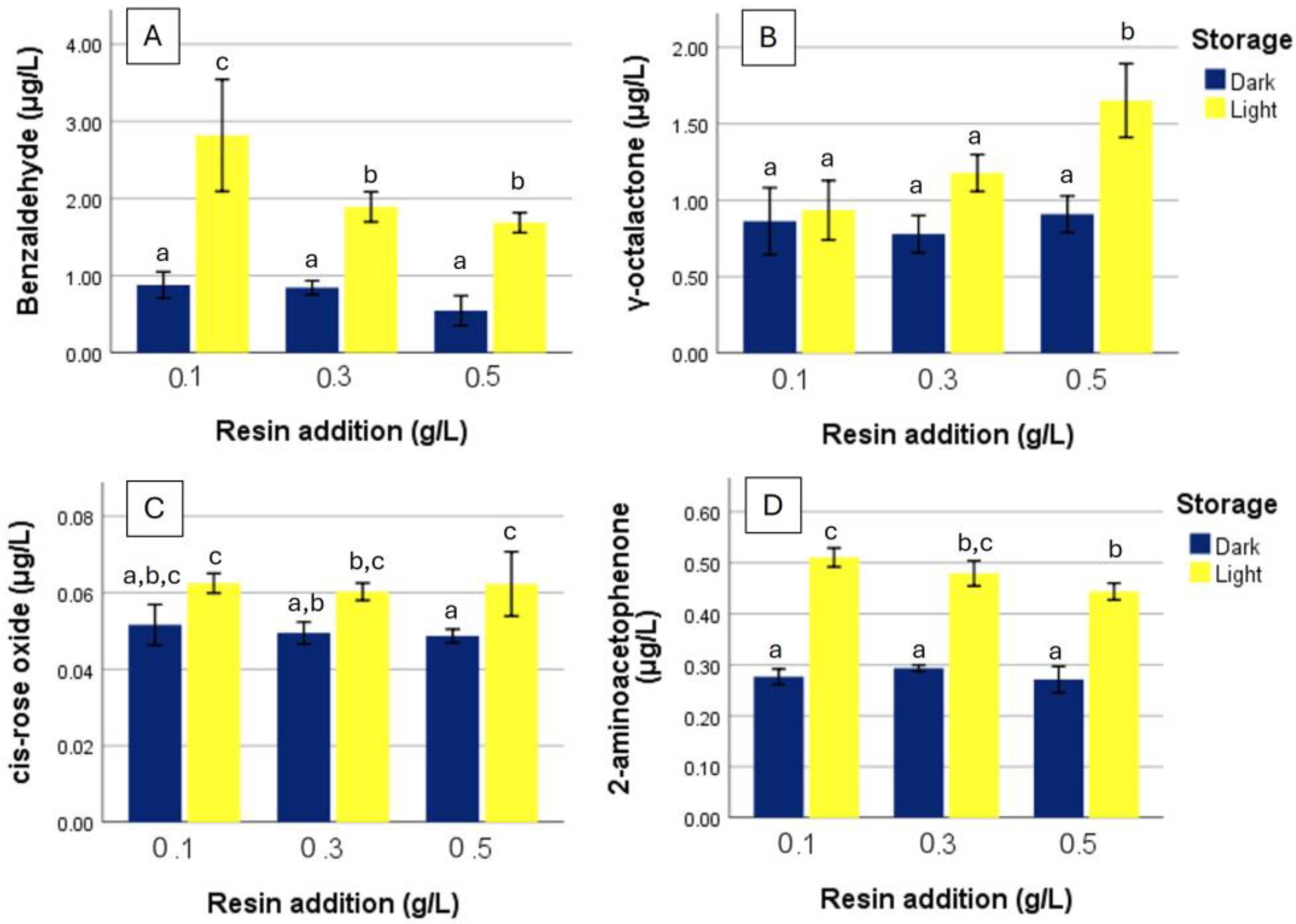
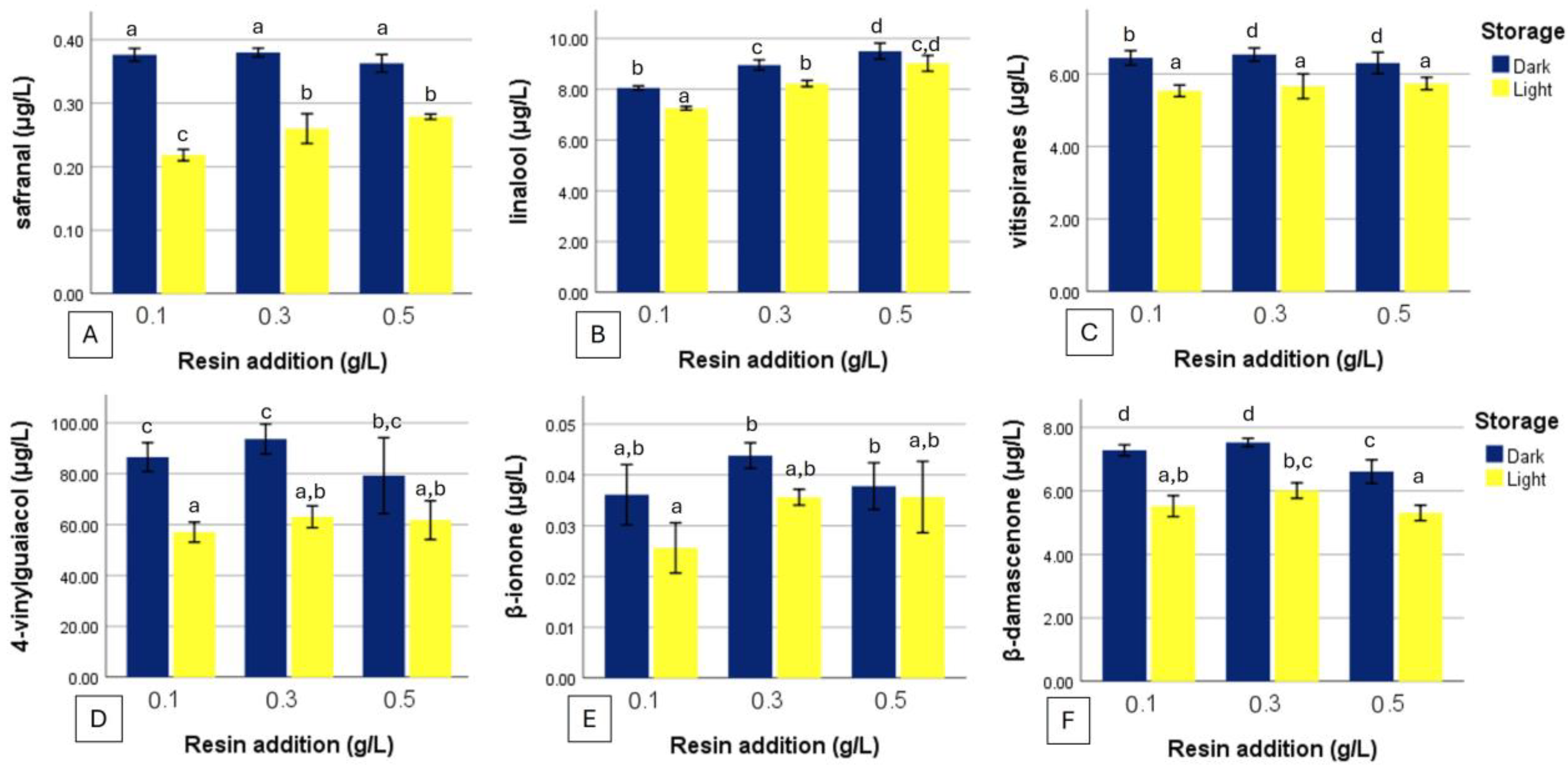
4.2. Degradation of Sensitive Aroma Compounds Under Light Exposure
4.3. Compounds Enhanced by Resin Addition
4.4. Compounds Reduced by Resin Addition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mc Govern, P.E.; Glusker, D.L.; Exner, L.J.; Mc Govern, P.E. Neolithic Resinated Wine. Nature 1996, 381, 480–481. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, P.; Zeng, X.A.; Fang, Z. The Art of Flavored Wine: Tradition and Future. Trends Food Sci. Technol. 2021, 116, 130–145. [Google Scholar] [CrossRef]
- Alonso González, P.; Parga-Dans, E. Vino de Tea (pine heartwood wine) from La Palma (Spain): Ethnographic and physic-chemical characterization. J. Ethn. Foods 2020, 7, 14. [Google Scholar] [CrossRef]
- Assimopoulou, A.N.; Zlatanos, S.N.; Papageorgiou, V.P. Antioxidant Activity of Natural Resins and Bioactive Triterpenes in Oil Substrates. Food Chem. 2005, 92, 721–727. [Google Scholar] [CrossRef]
- Roussis, V.; Tsoukatou, M.; Petrakis, P.V.; Chinou, I. Volatile constituents of needles of five Pinus species grown in Greece. Phytochemistry 1995, 39, 357–361. [Google Scholar] [CrossRef]
- Proestos, C.; Chorianopoulos, N.; Nychas, G.-J.E.; Komaitis, M. HPLC analysis of phenolic substances in Greek wines. Food Control. 2005, 16, 319–323. [Google Scholar] [CrossRef]
- EU Regulation (EU) No 251/2014 of the European Parliament and of the Council on the Definition, Description, Presentation, Labelling and the Protection of Geographical Indications of Aromatised Wine Products and Repealing Council Regulation (EEC) No 1601/91. In Official Journal of the European Union; Publications Office of the European Union: Luxembourg, 2014.
- EU Regulation (EU) No 1308/2013 of the European Parliament and of the Council on Establishing a Common Organisation of the Markets in Agricultural Products and Repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32013R1308 (accessed on 16 July 2025).
- EU EAmbrosia—Ρετσίνα Αττικής. Available online: https://ec.europa.eu/agriculture/eambrosia/geographical-indications-register/details/EUGI00000007205 (accessed on 16 July 2025).
- Μπέτζιος. Βασίλειος Συμβολή Εις Την Μελέτην Του Ρητινίτου Οίνου: Διάκρισις Ρητινίτου Από Αρητινώτου Οίνου. Ph.D. Thesis, Agricultural University of Athens, Attica, Greece, 1978. [CrossRef]
- Nakas, A.; Virgiliou, C.; Samara, D.; Kechri, E.; Assimopoulou, A.N. Exploring the Effect of Pinus Halepensis Resin Quality on the Vinification of Retsina by Untargeted Profile Analysis. Open Explor. 2024, 2, 497–524. [Google Scholar] [CrossRef]
- Natskoulis, P.I.; Miliordos, D.E.; Koutsouris, A.N.; Tarantilis, P.A.; Pappas, C.S.; Kallithraka, S.; Kotseridis, Y.; Metafa, M. Optimisation of Retsina Wine Quality: Effects of Resin Concentration, Yeast Strain, and Oak Chip Type. Foods 2024, 13, 3376. [Google Scholar] [CrossRef] [PubMed]
- Hamzaoui, E.; Zallez, O.B.Y.; Buñay, J.; Leremboure, M.; Argui, H.; Baron, S.; Said, H.; Lobaccaro, J.M.A.; Akriche, S. Comparative Study of Essential Oils from Tunisian Pinus Halepensis Mill. by Hydrodistillation and Microwave-Assisted Processes: Chemical Composition and Antioxidant and Cytotoxic Potential against Prostate and Cervical Cancer Cells. ACS Omega 2024, 9, 34128–34139. [Google Scholar] [CrossRef]
- Alonso González, P.; Parga Dans, E.; Ballester, J. Volatile composition of light red wines aged in Canary pine barrels from La Palma (Canary Islands, Spain). OENO One 2022, 56, 29–40. [Google Scholar] [CrossRef]
- Chatzistavridi, M.M.; Christofi, S.; Kallithraka, S. Fortification of White Wines with Antioxidants and Se: Impacts on Browning Development and Phenolic Content. Beverages 2024, 10, 31. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; Wiley Online Library: Hoboken, NJ, USA, 2016; pp. 179–193. [Google Scholar] [CrossRef]
- Furet, A.; Sicello, A.; Guillemat, B.; Absalon, C.; Langleron, E.; Bassani, D.M. Revisiting the mechanism responsible for the light-struck flavor in white wines and Champagnes. Food Chem. 2022, 372, 131281. [Google Scholar] [CrossRef]
- Fracassetti, D.; Ballabio, D.; Mastro, M.; Tirelli, A.; Jeffery, D.W. Response Surface Methodology Approach to Evaluate the Effect of Transition Metals and Oxygen on Photo-Degradation of Methionine in a Model Wine System Containing Riboflavin. J. Agric. Food Chem. 2022, 70, 16347–16357. [Google Scholar] [CrossRef]
- Fracassetti, D.; Limbo, S.; Messina, N.; Pellegrino, L.; Tirelli, A. Light-Struck Taste in White Wine: Protective Role of Glutathione, Sulfur Dioxide and Hydrolysable Tannins. Molecules 2021, 26, 5297. [Google Scholar] [CrossRef]
- Carlin, S.; Mattivi, F.; Durantini, V.; Dalledonne, S.; Arapitsas, P. Flint Glass Bottles Cause White Wine Aroma Identity Degradation. Proc. Natl. Acad. Sci. USA 2022, 119, e2121940119. [Google Scholar] [CrossRef]
- Fracassetti, D.; Di Canito, A.; Bodon, R.; Messina, N.; Vigentini, I.; Foschino, R.; Tirelli, A. Light-Struck Taste in White Wine: Reaction Mechanisms, Preventive Strategies and Future Perspectives to Preserve Wine Quality. Trends Food Sci. Technol. 2021, 112, 547–558. [Google Scholar] [CrossRef]
- Fracassetti, D.; Limbo, S.; Pellegrino, L.; Tirelli, A. Light-Induced Reactions of Methionine and Riboflavin in Model Wine: Effects of Hydrolysable Tannins and Sulfur Dioxide. Food Chem. 2019, 298, 124952. [Google Scholar] [CrossRef] [PubMed]
- Fracassetti, D.; Saligari, A.; Messina, N.; Bodon, R.; Mazzini, S.; Borgonovo, G.; Tirelli, A. Insight into the characterization of commercial oenological tannins. Food Chem. Adv. 2023, 2, 100205. [Google Scholar] [CrossRef]
- Carlin, S.; Lotti, C.; Correggi, L.; Mattivi, F.; Arapitsas, P.; Vrhovšek, U. Measurement of the Effect of Accelerated Aging on the Aromatic Compounds of Gewurztraminer and Teroldego Wines, Using a SPE-GC-MS/MS Protocol. Metabolites 2022, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- OIV Chromatic Characteristics (Type-I)|OIV. Available online: https://www.oiv.int/standards/compendium-of-international-methods-of-wine-and-must-analysis/annex-a-methods-of-analysis-of-wines-and-musts/section-2-physical-analysis/chromatic-characteristics-%28type-i%29 (accessed on 16 July 2025).
- Mattay, J.; Griesbeck Vch, A.G. Photochemical Key Steps in Organic Synthesis. In Photochemical Key Steps in Organic Synthesis; Wiley Online Library: Hoboken, NJ, USA, 1994. [Google Scholar] [CrossRef]
- Delaiti, S.; Nardin, T.; Roman, T.; Cappello, N.; Larcher, R.; Pedò, S. Exploring the relationship between field available water capacity (AWC) and atypical aging (ATA) development in base wines. J. Food Sci. 2024, 89, 9515–9528. [Google Scholar] [CrossRef] [PubMed]
- Arapitsas, P.; Carlin, S.; Mattivi, F.; Rapaccioli, A.; Vrhovsek, U.; Guella, G. Monoterpenoids Isomerization and Cyclization Processes in Gewürztraminer Wines: A Kinetic Investigation at Different PH and Temperatures. Food Res. Int. 2024, 196, 115017. [Google Scholar] [CrossRef]
- Wau, J.S.; Robertson, M.J.; Oelgemöller, M. Solar Photooxygenations for the Manufacturing of Fine Chemicals—Technologies and Applications. Molecules 2021, 26, 1685. [Google Scholar] [CrossRef]
- Dincalp, H.; Içli, S. Photosynthesis of Rose Oxide by Concentrated Sunlight in the Absence of Singlet Oxygen. J. Photochem. Photobiol. A Chem. 2001, 141, 147–151. [Google Scholar] [CrossRef]
- Cabua, M.C.; Velichko, V.; Pilia, L.; Moi, D.; Secci, F. Low-Impact Synthesis of γ-Lactones through Photoinduced Baeyer-Villiger Oxidation of Cyclic Ketones. Eur. J. Org. Chem. 2024, 27, e202400226. [Google Scholar] [CrossRef]
- Pineau, B.; Barbe, J.C.; Van Leeuwen, C.; Dubourdieu, D. Which Impact for β-Damascenone on Red Wines Aroma? J. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef]
- Tomasino, E.; Bolman, S. The Potential Effect of β-Ionone and β-Damascenone on Sensory Perception of Pinot Noir Wine Aroma. Molecules 2021, 26, 1288. [Google Scholar] [CrossRef] [PubMed]
- Marinaki, M.; Sampsonidis, I.; Lioupi, A.; Arapitsas, P.; Thomaidis, N.; Zinoviadou, K.; Theodoridis, G. Development of Two-Level Design of Experiments for the Optimization of a HS-SPME-GC-MS Method to Study Greek Monovarietal PDO and PGI Wines. Talanta 2023, 253, 123987. [Google Scholar] [CrossRef]
- Tzamourani, A.; Lytra, G.; Goulioti, E.; Gammacurta, M.; Marchal, A.; Evangelou, A.; Arapitsas, P.; Kotseridis, Y.; Paraskevopoulos, I.; Dimopoulou, M. Profound Analysis of the Impact of Indigenous Saccharomyces cerevisiae Strains on the Chemical and Sensorial Profile of Assyrtiko Wine. ACS Food Sci. Technol. 2025, 5, 2286–2295. [Google Scholar] [CrossRef]
- Luzzini, G.; Slaghenaufi, D.; Ugliano, M. Approaches to the classification of wine aroma ageing potential. Applications to the case of terpenoids in Valpolicella red wines. OENO One 2022, 56, 231–242. [Google Scholar] [CrossRef]
- Zeller, A.; Rychlik, M. Impact of Estragole and Other Odorants on the Flavour of Anise and Tarragon. Flavour Fragr. J. 2007, 22, 105–113. [Google Scholar] [CrossRef]
- Roufa, P.; Evangelou, A.; Beris, E.; Dourtoglou, T.; Carlin, S.; Lotti, C.; Vrhovšek, U.; Chatzilazarou, A.; Shehadeh, A. Volatile composition of Muscat of samos wine after pre- and postfermentation addition of the herbs Salvia officinalis, Melissa officinalis and Cannabis sativa. Int. J. Agric. Biosci. 2025, 14, 1–8. Available online: https://www.ijagbio.com/pdf-files/25-256.pdf (accessed on 16 July 2025).
- Mattivi, F.; Monetti, A.; Vrhovšek, U.; Tonon, D.; Andrés-Lacueva, C. High-Performance Liquid Chromatographic Determination of the Riboflavin Concentration in White Wines for Predicting Their Resistance to Light. J. Chromatogr. A 2000, 888, 121–127. [Google Scholar] [CrossRef] [PubMed]
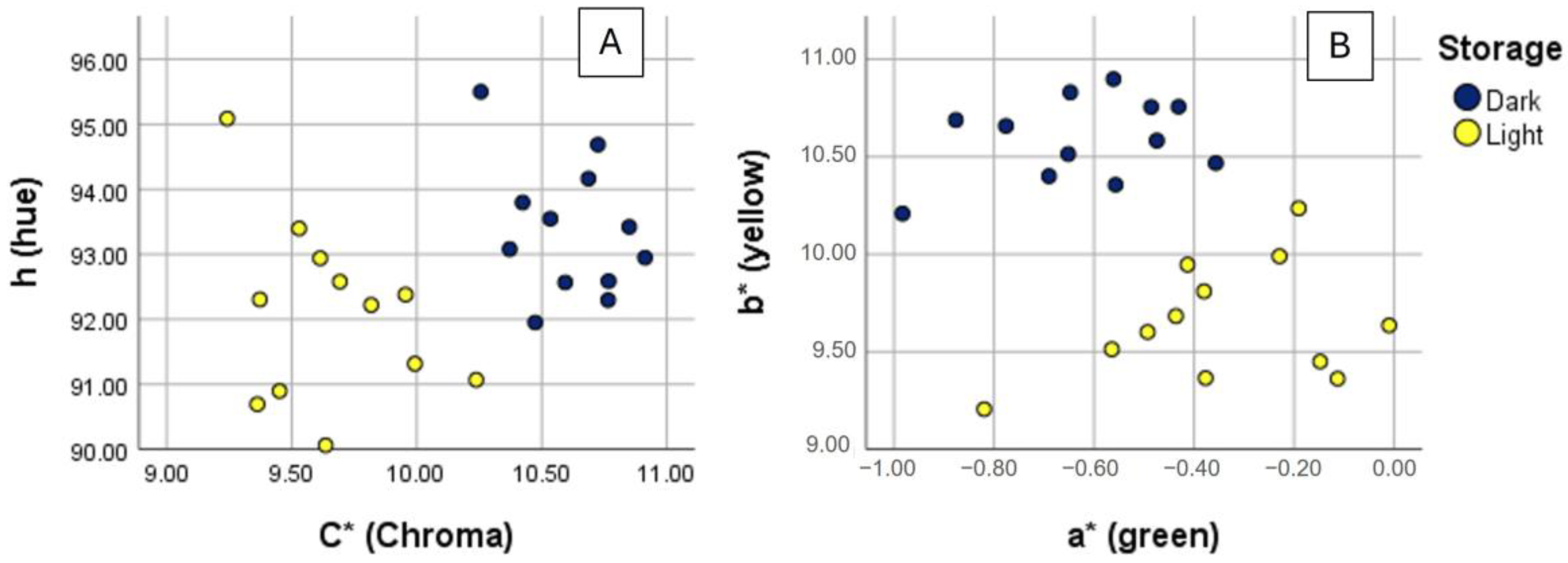
| Light Resin 0.1 g/L | Dark Resin 0.1 g/L | Light Resin 0.3 g/L | Dark Resin 0.3 g/L | Light Resin > 0.5 g/L | Dark Resin > 0.5 g/L | |
|---|---|---|---|---|---|---|
| Mean ± Std.dev 1 | ||||||
| L* | 96.45 ± 0.36 a | 96.68 ± 0.27 a,b | 96.76 ± 0.15 a,b | 96.88 ± 0.17 a,b | 97.06 ± 0.26 a,b | 97.03 ± 0.24 b |
| A* | −0.12 ± 0.077 c | −0.45 ± 0.084 b | −0.34 ± 0.1 b,c | −0.57 ± 0.08 a,b | −0.54 ± 0.17 a,b | −0.79 ± 0.14 a |
| B* | 9.67 ± 0.39 a | 10.54 ± 0.17 b | 9.92 ± 0.094 a | 10.72 ± 0.19 b | 9.47 ± 0.19 a | 10.56 ± 0.25 b |
| C* | 9.67 ± 0.39 a | 10.55 ± 0.17 b | 9.92 ± 0.092 a | 10.73 ± 0.19 b | 9.49 ± 0.18 a | 10.59 ± 0.24 b |
| h | 90.68 ± 0.44 a | 92.47 ± 0.48 b | 91.97 ± 0.57 a,b | 93.03 ± 0.48 b,c | 93.26 ± 1.10 b,c | 94.31 ± 0.81 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polymeros, G.; Carlin, S.; Reale, F.; Nikolou, E.; Nikolou, V.; Vrhovsek, U.; Arapitsas, P. Photodegradation of Retsina Wine: Does Pine Resin Protect Against Light-Induced Changes? Beverages 2025, 11, 139. https://doi.org/10.3390/beverages11050139
Polymeros G, Carlin S, Reale F, Nikolou E, Nikolou V, Vrhovsek U, Arapitsas P. Photodegradation of Retsina Wine: Does Pine Resin Protect Against Light-Induced Changes? Beverages. 2025; 11(5):139. https://doi.org/10.3390/beverages11050139
Chicago/Turabian StylePolymeros, George, Silvia Carlin, Francesco Reale, Evangelos Nikolou, Vasilios Nikolou, Urska Vrhovsek, and Panagiotis Arapitsas. 2025. "Photodegradation of Retsina Wine: Does Pine Resin Protect Against Light-Induced Changes?" Beverages 11, no. 5: 139. https://doi.org/10.3390/beverages11050139
APA StylePolymeros, G., Carlin, S., Reale, F., Nikolou, E., Nikolou, V., Vrhovsek, U., & Arapitsas, P. (2025). Photodegradation of Retsina Wine: Does Pine Resin Protect Against Light-Induced Changes? Beverages, 11(5), 139. https://doi.org/10.3390/beverages11050139









