Abstract
Infusions of the leaves of Ribes magellanicum (Grossulariaceae) are used as a digestive in southernmost South America. This work aimed to assess the composition and activity of infusions and MeOH:H2O 7:3 extracts of R. magellanicum leaves on enzymes related to metabolic syndrome (α-glucosidase, α-amylase, and pancreatic lipase), as well as their antioxidant capacity. Samples from a longitudinal gradient from central southern Chile to the islands in the Beagle Channel were investigated. Lyophilized infusions and extracts were used for all determinations, including inhibition of the selected enzymes, total phenolic (TP), total flavonoid (TF), total procyanidins (TPC), and antioxidant capacity (DPPH, FRAP, TEAC, and ORAC). The composition of the samples was assessed by HPLC-DAD. Some 99 compounds were tentatively identified by HPLC-MSn. The main phenolics were quantified using calibration curves with reference compounds. Relevant differences exist in the ratio of constituents in infusions compared to hydroalcoholic extracts. The samples were inactive towards α-amylase and pancreatic lipase at 100 and 50 µg/mL, respectively. Assay-guided isolation of α-glucosidase inhibitors led to fractions with high activity (IC50: 0.02–0.05 µg/mL). The strong inhibition of α-glucosidase and antioxidant capacity of the infusion and extracts of R. magellanicum leaves support its traditional use in southern Patagonia.
1. Introduction
Herbal infusions and functional beverages made of herbs, spices, and fermented plant products are increasingly popular as beverages [1,2,3]. Patagonian berries have been used as food and medicine since prehistoric times in Chile and Argentina. Ribes magellanicum Poir. (Grossulariaceae) is a widespread species in southern Patagonia, and the leaves were used to prepare an infusion with a pleasant taste and digestive properties. On 28 July 2025, the name of the plant was checked against both World Flora Online (https://www.worldfloraonline.org) and the MPNS database (http://mpns.kew.org). In traditional Mapuche medicine [4], the leaves of the Ribes species were reported to be used to prepare an infusion with refreshing and medicinal properties to relieve diarrhea and dysentery, as well as to treat bleeding, and were used for skin diseases. The R. magellanicum leaves were used to treat liver and intestinal diseases by the Huilliche and were tested for antimicrobial activity. Only the ethanol extract showed a very weak effect against Escherichia coli EDL 933 [5]. The use of Ribes leaf infusions as a herbal tea is common in the Northern Hemisphere, where Ribes rubrum and R. nigrum are cultivated for their berries. The leaves of black currant are used in North European folk medicine for their diaphoretic and diuretic properties, and to relieve rheumatic pain [6]. In Turkey, leaves from the Ribes species are used for wound healing [7]. Sun et al. [8] revised the ethnopharmacological data of the genus and summarized the information on uses and pharmacology. The composition of a R. nigrum leaf infusion and its hydroalcoholic and methanol extracts was reported [9]. Flavonoids, catechins, and oligomers, as well as organic acids, were identified. The main compounds in the infusion were quercetin 3-O-glucoside, kaempferol, and isorhamnetin glucoside and kaempferol acetyl hexoside, among others [9]. The compounds identified in the leaves can explain the anti-inflammatory and antioxidant activity found in the R. nigrum extract [10]. Herbal teas, consumed worldwide for their digestive and health-promoting properties, offer a pleasant taste and aroma. They serve as a form of intake of bioactive natural products, sometimes in mixtures. A main constituent is often combined with additional medicinal and aromatic plants.
The fruits of R. magellanicum have been investigated for their composition, antioxidant properties, inhibition of enzymes related to metabolic syndrome, anti-inflammatory effects, and activity on advanced glycation end-products. However, little is known about the (bio)activity and metabolite content in the leaf infusions. Plant infusions and herbal teas contain compounds of different biosynthetic origins that can reduce or prevent metabolic disorders. They act by inhibiting the enzymes pancreatic lipase, α-amylase, and α-glucosidase, which are responsible for the breakdown of lipids and carbohydrates, respectively [11,12].
The aim of this work was to assess the effect of both the infusion and the MeOH:H2O 7:3 (v/v) extract of R. magellanicum leaves towards α-glucosidase, α-amylase, and pancreatic lipase. Additionally, we aimed to compare the composition of the leaf infusion and the MeOH:H2O 7:3 (v/v) extracts across a gradient from southern-central Chile to Navarino Island, located south of Tierra del Fuego at the Beagle Channel.
2. Materials and Methods
2.1. Reagents and Chemicals
The HPLC-grade solvents, Folin–Ciocalteu reagent, potassium sodium tartrate, potassium persulfate, Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), and FeCl3·6H2O were purchased from Merck (Darmstadt, Germany). The enzymes α-amylase from porcine pancreas (A3176; EC 3.2.1.1), α-glucosidase from Saccharomyces cerevisiae (G5003; EC 3.2.1.20), lipase from porcine pancreas type II (L-3126; EC 3.1.1.3), the resin Amberlite® XAD-7, and the reagents AlCl3, dinitrosalicylic acid, NaHCO3, Na2CO3, sodium acetate, starch, quercetin, (+)-catechin, gallic acid, 4-nitrophenyl-α-D-glucopyranoside, p-nitrophenyl palmitate, acarbose, L-glutamine, 2′,7′-dichlorodihydrofluorescein diacetate, DPPH (2,2-diphenyl-1-picrylhydrazyl radical), 2,4,6-tri(2-pyridyl)1,3,5-triazine (TPTZ), AAPH (2,2′-azobis(2-methylpropionamidine) dihydrochloride, and 2,2ʹ-azobis-(2-amidinopropane) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Standard compounds, including kaempferol, kaempferol 3-O-β-glucoside, quercetin, quercetin 3-O-β-glucoside, rutin, catechin, trans-ferulic acid, and cyanidin 3-glucoside, were purchased from PhytoLab (Vestenbergsgreuth, Germany). Orlistat was obtained from Laboratorio Chile (Santiago, Chile). A Barnsted EasyPure water filter (Thermo Scientific, Marietta, OH, USA) was used to obtain ultrapure water.
2.2. Plant Material
Mature leaves from Ribes magellanicum were collected from Parque Nacional Conguillio, Región de la Araucanía (38°39′0″ S, 71°37′50″ W, 1168 mosl; 12 December 2022), Frutillar Alto, Región de Los Lagos (41°7′0″ S, 73°6′0″ W, 169 mosl; 26 December 2022), Lago Espolón, Región de Los Lagos, (43°5′38″ S, 72°1′22″ W; 5 February 2023), Cuesta Queulat, Región de Aysén (44°24′16″ S, 72°24′13″ W; 6 February 2023) Reserva Nacional Magallanes, Región de Magallanes y Antártica Chilena (53°9′0″ S, 70°55′0″ W, 54 mosl, 15 January 2022), Puerto Williams, Isla Navarino, Región de Magallanes y Antártica Chilena (52°31′60″ S, 72°7′0″ W, 1 mosl, 25 January 2022). The samples at Navarino Island were collected on 25 January 2022 from shrubs growing at the Upushuaia bay, at the seashore of the Beagle channel, as well as in the forest at Parque Omora and the degraded, overgrazed area of Puente Rio Guanaco and Camping CONAF [13]. The collection places are shown in Figure 1.
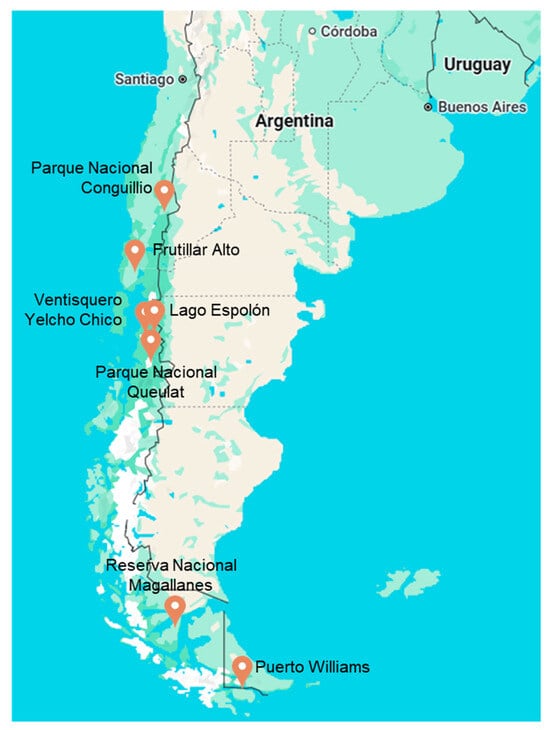
Figure 1.
Collection places of R. magellanicum leaves. This figure was created using Google Earth.
2.3. Sample Processing and Extraction
The leaves (5–20 g of each sampling place) were dried to determine the moisture content, and the dry material was powdered in a Sindelen LCM-18000GF blender (Santiago de Chile, Chile) using the flour mode. The powdered material (500 mg) was extracted three times with 50 mL of MeOH:H2O (7:3 v/v). The mixture was sonicated for 20 min in a Bransonic M5800-E sonicator (Branson Ultrasonic Corporation, Danbury, CT, USA) at 160 W, 40 KHz. Sonication was used to increase the extraction of the soluble. The combined solution was filtered and taken to dryness under reduced pressure (Heidolph Laborota 4001, Heidolph Instruments, Schwabach, Germany). The remaining solubles were resuspended in 15 mL of distilled water and lyophilized in a Labogene Coolsafe 55-15 Pro-230 lyophilizer (Lynge, Denmark) to afford the MeOH:H2O 7:3 extracts that were used for analyses. Infusions of dry leaves from Conguillío, Frutillar, and Reserva Nacional Magallanes were prepared using 2.5 g of powdered leaves, adding 100 mL of boiled water. The infusions were prepared to simulate the traditional preparation of the beverage. After cooling, the solution was filtered and lyophilized to afford the hot water-soluble fraction from the herbal tea.
2.4. Phenolic, Flavonoid, and Procyanidin Contents
The Folin–Ciocalteu method was used to determine the total phenolic content (TP) of the extracts and infusions [14], and the results are shown as mg equivalents of gallic acid (GAE)/100 g of extract/infusion. The AlCl3 method was used to assess the total flavonoid content [15,16], and the results are presented as mg catechin equivalents (CE)/100 g of extract. The 4-dimethylaminocinnamaldehyde (DMAC) method was selected to measure the procyanidin content (TPC) [15,16]. The TPC data are shown as mg catechin equivalents (CE)/100 g of extract.
2.5. Antioxidant Capacity
Four methods were used to assess the antioxidant capacity of the extracts as follows: Discoloration of the 2,2-diphenyl-1-picrylhydrazyl (DPPH) [14,15,16], ferric cation reduction (FRAP) [14,15,16], Trolox equivalent antioxidant capacity (TEAC) [14,15,16], and oxygen radical absorbance capacity (ORAC) [14,17]. The samples were analyzed in individual experiments. The stock solutions ranged from 5–300 μg/mL. The calibration curves for the FRAP, TEAC, and ORAC assays were carried out with Trolox. The positive controls were quercetin and catechin. The results are shown as SC50 (μg/mL) for DPPH, µmol TE/g extract for FRAP and ORAC, and as µmol TE/g extract for TEAC, respectively.
2.6. Enzyme Inhibition Assays
The inhibition of the key enzymes that are related to metabolic syndrome (α-amylase, α-glucosidase, and pancreatic lipase) by the leaf extracts was determined as described in [14].
2.6.1. α-Amylase
The inhibition of α-amylase by the samples was determined at 100 µg/mL. α-amylase was prepared in ice-cold water at 0.5 mg/mL and maintained at 4 °C. The samples were dissolved in 0.02 M sodium phosphate buffer, pH 6.9. The sample (100 µL) was mixed with sodium phosphate buffer containing a 0.5 mg/mL α-amylase solution (100 µL of 0.02 M), and the mixture was pre-incubated at 37 °C for 10 min. Starch was dissolved in phosphate buffer (100 mg of starch in 10 mL of 0.02 M phosphate buffer, pH 6.9), boiled in a water bath for 15 min, and was under occasional agitation. Then, 100 µL of a 1% starch solution in sodium phosphate buffer was added and mixed. The mixture was incubated again at 37 °C for 20 min, and then, 200 µL of the color reagent was added. The test tubes were boiled for 15 min, and then, 40 µL of the solution was mixed with 210 µL of water. The absorbance was measured in a TECAN Infinite M Nano+ universal microplate reader (Grödig, Austria) at 550 nm. Acarbose was used as a standard inhibitor. The results are shown as a percentage of inhibition as mean values ± SD.
2.6.2. α-Glucosidase
The samples were assessed at final concentrations of 0.1, 1, 10, and 100 µg/mL [14]. The sample (120 µL, dissolved in 0.1 M sodium phosphate buffer, pH 6.8) was mixed with 20 µL of the α-glucosidase solution (0.25 U/mL, in sodium phosphate buffer), and the mixture was pre-incubated at 37 °C for 15 min. The substrate, 5 mM p-nitrophenyl-α-D-glucopyranoside (in sodium phosphate buffer) (20 µL), was added, and the mixture was incubated again at 37 °C for 15 min. To stop the reaction, 80 µL of 0.2 M sodium carbonate was added, and the absorbance was measured at 415 nm in a universal microplate reader. Acarbose was used as a standard inhibitor. The results are shown as percentages of inhibition or IC50 (µg/mL) as mean values ± SD.
2.6.3. Lipase
The method is based on the enzymatic hydrolysis of p-nitrophenyl palmitate to p-nitrophenol. Lipase was dissolved in ultrapure water at 20 mg/mL. The enzyme solution was centrifuged at 13,000 rpm for 10 min at 4 °C. Only the supernatant was used for the reaction mixture. The inhibition of the porcine pancreatic lipase by the samples was assessed at a final concentration of 50 µg/mL [14]. The reaction mixture contained the extract (50 µL), enzyme solution (150 µL), the substrate (450 µL), and 100 mM Tris, pH 8.2 assay buffer (400 µL). The mixture was incubated at 37 °C for 2 h in a water bath. The absorbance was measured at 400 nm in a Genesys 10UV spectrophotometer (Thermo Spectronic, Rochester, NY, USA). Orlistat® was used as the reference compound. All determinations were performed in quadruplicate, and the results are presented as percentage mean values ± SD.
2.7. Assay-Guided Isolation of α-Glucosidase Inhibitors from R. Magellanicum Leaves
Some 10 g of dry leaves from the Navarino Island collection were extracted with MeOH:H2O 7:3 (3x) in a 10:1 solvent/dried plant ratio. After filtration, all the methanol from the hydroalcoholic solution was removed by distillation under reduced pressure by using a rotary evaporator (Heidolph Hei-VAP Advantage, Schwabach, Germany) and temperatures below 45 °C. After removal of the organic solvent, the aqueous solution was frozen at −20 °C for lyophilization, yielding 2.3 g of a soluble (23% w/w yield). The lyophilized extract was resuspended in MeOH:H2O 7:3, centrifuged to remove non-polar residues, and loaded into a Sephadex LH-20 column. The column length was 68 cm; the internal diameter was 3.5 cm, and it was loaded with a 29 cm Sephadex LH-20. The void volume was 92 mL. The composition of the extract and fractions was analyzed by TLC (silica gel 60 F254 plates, Merck, Darmstadt, Germany) using EtOAc:acetic acid:H2O 10.0:1.2:1.0 (v/v/v) as the mobile phase. The compounds were visualized under UV light and after spraying them with natural products reagent (diphenylboric acid-β-ethylamino ester) (NPR) [18]. Fractions were collected and pooled together according to the TLC patterns as follows: Fraction 1 (32 mL, 12 mg, discarded); fractions 2/4 (21.5 mL,122 mg); fractions 5/8 (39 mL, 664 mg), fraction 9 (11 mL, 122 mg); fractions 10/11 (54.5 mL, 256 mg); fractions 12/13 (36 mL, 74 mg); fraction 14 (13.5 mL, 20 mg), fractions 15/16 (29 mL, 43 mg); fractions 17/20 (68.5 mL, 68 mg); fractions 21/22 (44.5 mL, 16 mg); fractions 23/24 (75.5 mL, 49.6 mg); fraction 25 (60 mL, 17 mg); fractions 26/28 (13 mL; 34.9 mg); 29/32 (240 mL, 36.9 mg), 33 (90 mL, 82 mg). The fractions were assessed for α-glucosidase inhibition, and the composition was compared by TLC and HPLC-DAD.
2.8. Chromatographic Analyses
2.8.1. Thin Layer Chromatography (TLC) Analysis
The composition of the extracts was compared using TLC silica gel 60 F254 plates (Merck, Darmstadt, Germany). The mobile phase was EtOAc:Acetic acid:water 10.0:1.2:1.0 (v/v/v), and the compounds were detected under UV light before and after spraying with diphenylboric acid-β-ethylamino ester in methanol (natural products reagent, NPR) [18].
2.8.2. HPLC-DAD Profiles
The composition of the extracts was analyzed using Shimadzu HPLC equipment (Shimadzu Corporation, Kyoto, Japan) with a SPD-M20A UV diode array detector, a LC-20AT pump, a CTO-20 AC column oven, and LabSolution software. The analytical column was a reversed-phase Kinetex EVO C18 column (250 mm × 4.6 mm, 5 μm particle size, 100 Å pore diameter; Phenomenex Inc., Torrance, CA, USA) that was maintained at 30 °C. Each sample was dissolved in the mobile phase (1 mg/mL), filtered through a 0.22 µm PVDF syringe filter (Agela Technologies, Wilmington, DE, USA), and 20 µL was injected for analysis. The solvent system used for the HPLC analyses consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The linear gradient was: 0–5 min, 95% A; 5–25 min, 95–75% A; 25–50 min, 75–42% A; 50–60 min, 42–0% A; 60–65 min, 0–45% A; and 65–75 min, 45–95% A. The volume injected was 20 μL of a 1 mg/mL solution, and the flow rate was 0.8 mL/min. UV–visible spectra (220 to 600 nm) were recorded for the peak characterizations. The eluted compounds were monitored at 205, 280, and 360 nm. The HPLC traces were used to compare the occurrence of the main constituents and for quantification.
2.8.3. HPLC-DAD-MS/MS
The HPLC-DAD-MS/MS analyses were performed in a Thermo Fisher Scientific UHPLC system (Thermo Fisher Scientific, San Jose, CA, USA). The equipment included an Accela Open autosampler, 1250 quaternary UHPLC pump, and PDA detector. The PDA detector was interfaced with an Orbitrap Velos Pro mass spectrometer hybrid linear ion trap (LTQ). The liquid chromatography separation for DAD-ESI-MS/MS detection was carried out using a 2.1 × 150 mm, 2.7 μm CORTEX T3 column (Waters Corporation, Milford, MA, USA). The column was maintained at 45°, and the flow rate was 0.3 mL/min.
The solvent systems used for the separation of the samples were 0.1% formic acid in water (v/v) (A) and 0.1% formic acid in acetonitrile (v/v) (B). The sequence was as follows: 4–20% B from 0.0–40.0 min; 20–45% B from 40.0–55.0 min; 45–100% B from 55.0–56.0 min; 100% B from 56.0–60.0 min; 100–4% B from 60–60.5 min; and 4% B from 60.5–70.0 min. The PDA scanning was 200–650 nm.
The electrospray ionization (ESI) source operated in the negative and positive ionization modes, looking for ions from m/z 120 to 1200. The heated capillary temperature was 270 °C. The source voltage was 4.5 kV. The sheath gas used was nitrogen at 30 arbitrary units. During the chromatographic run, a data-dependent acquisition mode was applied for the m/z survey scan in the FT cell. A linear MS/MS ion trap investigation of the top five most abundant precursor ions was performed. The FT full-scan MS was attained at 60,000 mass resolving power (m/z 400). Helium was used as a target gas for collision-induced dissociation (CID). The isolation width was 2 Da, and the normalized collision energy was 30%. Precursor ions that were selected for CID were dynamically excluded for 30 s from further MS/MS analysis. The resolving power for the MS2 scans was 7500. The raw data were processed using Xcalibur software (Thermo Fisher Scientific, San Jose, CA, USA).
2.9. Infrared (IR) Analysis
Infrared (IR) spectra were obtained using a Jasco FT/IR-4X spectrophotometer (Agilent, Santa Clara, CA, USA) in the transmittance mode. Spectra (36 scans) were obtained after background subtraction.
2.10. Statistical Analyses
For the statistical analyses, the GraphPad Prism version 5.00 software for Windows was used. The determinations are reported as the arithmetic means ± SD. One-way analysis of variance (ANOVA) followed by Tukey’s test (p < 0.05) was used to detect significant differences in the measurements.
3. Results
Eleven samples from R. magellanicum leaves, collected from the Araucania (38° S) to the southern Magellan regions in Chile (55° S), were analyzed for their phenolic composition, antioxidant capacity, and inhibition of enzymes related to metabolic syndrome. A comparison was undertaken between the composition and (bio)activity of MeOH:H2O 7:3 extracts and infusions of three samples. This was performed to compare the components in infusions and extracts, as well as to disclose possible differences in the phenolic profiles, antioxidant capacity, and enzyme inhibition.
3.1. Phenolic, Flavonoid, and Procyanidin Contents and Antioxidant Capacity
The extraction yield range of the extracts was between 13.5 and 29.9%, with large variations observed according to the individual plants or populations. The w/w extraction yields of the infusions were 16%, 24.4%, and 25.7% for Conguillio, Frutillar, and Reserva Nacional Magallanes (pool), respectively. The total phenolic, flavonoid, and procyanidin contents of the extracts were 8.40–20.34 g GAE, 6.33–13.14 g CE, and 2.95–9.27 g CE for TPO, TF, and TPC, respectively. For the infusions, the values ranged between 6.20 and 18.46 g GAE, 4.70–10.51 g CE, and 4.16–6.67 g CE, respectively (Table 1). In the antioxidant capacity study, the IC50 values of the extracts against DPPH were in the range of 9.56–34.29 µg/mL, with the Frutillar sample exhibiting better activity. In the ORAC, the best effect was observed for the extracts from Conguillio, Frutillar, and one of the shrubs from Reserva Nacional Magallanes, with values of 1215.24, 1123.92, and 1043.38 µmol TE/g extract, respectively. The FRAP and TEAC values showed a similar trend, with lower values for the infusion compared with the corresponding lyophilized MeOH:H2O 7:3 extracts (Table 1).

Table 1.
Percent w/w extraction yield, total phenolic (TP), total flavonoid (TF), total procyanidin (TPC) content, and antioxidant activity (DPPH, FRAP, TEAC, ORAC) from Chilean Ribes magellanicum leaf MeOH:H2O 7:3 extracts and selected infusions.
3.2. Enzyme Inhibition Assays
The lyophilized MeOH:H2O 7:3 extracts and infusions from the R. magellanicum leaves were assessed for their ability to inhibit the enzymes α-glucosidase, α-amylase, and pancreatic lipase. All samples exhibited a strong inhibition of α-glucosidase, with IC50 values ranging from 0.02 to 0.19 µg/mL. The IC50 value of the reference compound, acarbose, was 118.17 µg/mL. The extracts showed greater activity towards α-glucosidase compared to the infusions (Table 2). All extracts and infusions were devoid of activity towards α-amylase at 100 µg/mL and lipase at 50 µg/mL.

Table 2.
Inhibition of the enzyme α-glucosidase by the lyophilized MeOH:H2O 7:3 extracts and infusions from R. magellanicum leaves.
3.3. Assay-Guided Isolation of the α-Glucosidase Inhibitors
After a comparison by TLC, the different pooled fractions from the Sephadex LH-20 leaf extract were assessed for α-glucosidase inhibition. Assay-guided isolation of the leaf extract showed the highest enzyme inhibition effect for the fraction pools 5–8, 29–32, and 33, with IC50 values of 0.05, 0.02, and 0.04 µg/mL, respectively (Table 3). The composition of the different fractions varies as Sephadex LH-20 permeation separates the constituents according to molecular size. The highest activity was observed for the fraction pool 29/32, which exhibited a broad group of signals with UV spectra (280 nm) that were compatible with procyanidins in HPLC. The FT-IR analysis of the fractions 29/32 showed characteristic signals of procyanidins, with a broad OH band at 3393 cm−1, 2920 (C–H stretching), 1607 (aromatic ring stretching), 1522 and 779 (procyanidin units with cis configuration), 1285 (ether C–O stretching from the pyran ring), and 822 (out-of-plane C–H deformations). The IR spectra are consistent with condensed tannins [19,20,21,22].

Table 3.
α-Glucosidase inhibition by Sephadex LH-20 fractions from the leaf extract of R. magellanicum.
3.4. HPLC–MS/MS Analyses
Ninety-nine compounds were tentatively identified in the leaf extracts, including phenylpropanoids, proanthocyanidins, anthocyanins, flavonoids, and simple phenolics, among others. The proposed identification is based on the fragmentation patterns, molecular formula, literature, and database analyses, including https://www.foodb.ca (accessed on 28 July 2025). The tentative identification of the Ribes leaf phenolics is summarized in Table 4, and the occurrence of the compounds in different extracts and active fractions is detailed in Table 5. Representative HPLC–MS/MS traces are presented in Figure 2.

Table 4.
Tentative identification of the main compounds from Ribes magellanicum leaves MeOH:H2O 7:3 extracts and infusion (I). RNM: Reserva Nacional Magallanes; U: Upushuaia MeOH:H2O 7:3 extract.

Table 5.
Content of the main compounds from the leaves of Ribes magellanicum from Chilean Patagonia. Data are expressed as mean values ± SD as mg of compound per 100 g of dry leaves.
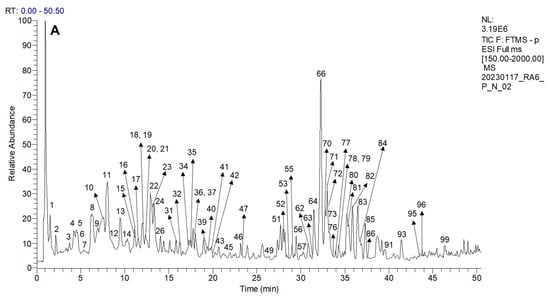
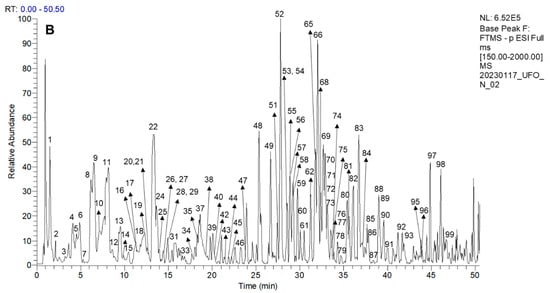
Figure 2.
Representative HPLC–MS/MS traces of the R. magellanicum MeOH:H2O 7:3 v/v leaf extracts. The chromatograms in negative ion mode are from the (A) Reserva Nacional Mgallanes (RNM) and (B) Upushuaia (U) collections.
3.4.1. Proanthocyanidins
Sixteen compounds were identified as proanthocyanidins, including the dimers 4, 10, 12, 17, 19, and 39, the trimers 5, 9, 14, 27, and 47, and the tetramers 7 and 29. The compounds were the dimers, trimers, and tetramers of the monomeric catechin 22, (epi)catechin 40, gallocatechin 8, and (epi)gallocatechin 18. The identity of catechin and (epi)catechin was confirmed by the co-injection and Rt of standards. However, the placement of the different monomers in the dimer, trimer, and tetramers remains to be established [26].
The compounds 19 and 39, with the molecular formula C30H25O12 for the [M-H]+ ion at m/z 577, show the base peak at m/z 425 and the fragment at m/z 289, supporting a B-type (epi)catechin-(epi)catechin dimer [29]. Compounds 10 and 17, with the [M-H]+ ion at m/z 593, fragments to m/z 425 and 289, are in agreement with (epi)catechin-(epi)gallocatechin dimers, and differ in the Rt, and were assigned as isomers 1 and 2, respectively. Compounds 4 and 12, with m/z 609 and fragmenting to m/z 441 and 305, were identified as (epi)gallocatechin-(epi)gallocatechin dimers 1 and 2, respectively.
Compound 9, with the m/z at 897 and fragmenting to m/z 771, 711, and 593, agrees with the (epi)gallocatechin-(epi)gallocatechin-(epi)catechin trimer. The mass spectrum of compounds 14 and 47 shows a molecular formula C45H37O19 for [M-H]+ ion and fragments to m/z 711 and 593 as well as m/z 755 and 695, respectively, and were assigned as (epi)gallocatechin-(epi)catechin-(epi)catechin trimers 1 and 2.
Compounds 5 and 7 were identified as the (epi)gallocatechin trimer and tetramer, respectively, by the molecular formula and fragmentation, supporting three and four (epi)gallocatechin units, respectively. The assignment was supported by the literature [26,29,30]. Compound 29, with the molecular formula C60H49O25, shows a [M-H]+ ion at m/z 1169 and fragments to m/z 881 and 423, which is compatible with (epi)gallocatechin-(epi)catechin-(epi)catechin-(epi)catechin tetramer. Compound 27 was assigned as (epi)catechin trimer by the UV spectrum, molecular formula, and fragmentation, in accordance with the literature [26].
3.4.2. Flavonoids
Some 40 flavonoids were tentatively identified in the R. magellanicum fruits, including 11 kaempferol, 19 quercetin, three myricetin, two flavanone, two flavanonol, two flavones, and a dihydrochalcone derivative.
The kaempferol derivatives were identified in the samples by the sugar and acyl losses, leading to the kaempferol base peak at m/z 285. The compounds tentatively assigned in the samples include 46 and 54, which showed the pseudomolecular ion at m/z 755, losing one hexose moiety (162 amu) to yield a fragment at m/z 593, which is compatible with kaempferol rutinoside. Therefore, both were assigned as kaempferol hexoside rutinoside 1 and 2. Peak 65 was tentatively identified as pentoside rutinoside due to the observed neutral losses of one pentose (132 amu) and one rutinose (308 amu). Compounds 78 and 83 exhibited a neutral loss of rutinose (308 amu) and were tentatively assigned as kaempferol rutinoside 1 and 2. The compounds 75, 81, 85, 86, 91, and 93 displayed neutral losses of hexoside pentoside (294 amu), hexoside (162 amu), hexoside (162 amu), glucuronide (176 amu), acetylhexoside (204 amu), and acetylhexoside (204 amu), respectively.
Nineteen compounds were assigned as quercetin and its glycosides based on the neutral loss of sugars and acyl substituents, leading to the base peak of quercetin at m/z 301.
Compounds 36 and 49 showed the loss of two hexoses, while 48 lost one hexose and rutinose, and 52 lost a pentose and rutinose. The diglycosides showed neutral losses of hexose (162 amu) and pentose (132 amu) 56, pentose (132 amu) and glucuronic acid (176 amu) 58, hexose (162 amu) and rhamnose (146 amu) 61, rutinose (308 amu) 66, and two pentoses (264 amu) 69. The monoglycosides included the glucuronide 70, the hexoside 71, and the pentoside 80, which exhibited neutral losses of 176, 162, and 132 amu, respectively. Acyl glycosides included the malonyl dihexoside 59, acetyl hexoside pentoside 68, the acetyl hexosides 79 and 82, coumaroyl hexoside pentoside 97, and coumaroyl hexoside 98, which displayed losses of 410, 336, 204, 440, and 308 amu, respectively. Free quercetin 99 was confirmed by the spectrometric evidence as well as by co-injection of a standard [14]. Three myricetin glycosides were identified, including the pentoside 63, the hexoside 76, and the rutinoside 51, according to the neutral loss of pentose (132 amu), hexose (162 amu), and rutinose (308 amu), leading to the base peak of myricetin at m/z 317.
Two flavanones were identified in the samples, including naringenin C-dihexoside 35 and eriodictyol hexoside 41. Compound 35 showed the characteristic losses of 120 and 90 amu for C-glycosyflavanones, and a base peak at m/z 355 [31] while the hexoside 41 is compatible with eriodictyol hexoside [31]. Taxifolin glucuronide was assigned based on the neutral loss of glucuronic acid and the base peak at m/z 303, in agreement with compound 43.
The C-dihexoside from the dihydrochalcone phloretin (compound 77) was tentatively identified in the extracts based on the characteristic losses of 120, 90, and 30 amu fragments, leading to the m/z peak at 357, which is in agreement with the literature [31]. Phloretin shows beneficial effects on diabetes [32]. Apigenin hexoside 87 was identified by the neutral loss of hexose, leading to the base peak of deprotonated apigenin at m/z 269. Luteolin hexoside 73 was identified by the neutral loss of hexose, leading to the m/z at 285 and UV spectrum with maxima at 345 nm, supporting the assignment. Flavonoids are widespread in Ribes fruits [29,30].
3.4.3. Phenylpropanoids
The compounds 11, 16, and 24 showed the molecular formula and fragmentation characteristic of caffeoylquinic acids [24] and were assigned as 3-caffeoylquinic acid, 4-caffeoylquinic acid, and 5-caffeoylquinic acid, respectively [24]. The identities of compounds 11 and 24 were confirmed by comparison with a reference standard. The related compounds 15, 30, and 38 showed the molecular formula C16H17O8 for [M-H]+, which is in agreement with coumaroylquinic acids. The compounds 15, 30, and 38 were assigned as 3-, 4-, and 5-coumaroylquinic acid, respectively. Three feruloylquinic acids (compounds 25, 33, and 45) were identified by the neutral loss of 174 amu, leading to the base peak at m/z 193 (compound 25) and fragments at 193 and 161 amu (compound 33) and 191 amu (compound 45). They were identified as 3-feruloylquinic acid, feruloylquinic acid, and 5-feruloylquinic acid, respectively [24].
Several hexosides from caffeic acid (compounds 13 and 21), coumaric acid (20, 23, and 26), ferulic acid (31), and sinapic acid (28) occurred in the extracts and were identified by the neutral loss of hexose (162 amu), leading to the corresponding base peak for the phenylpropanoid moiety. Caffeic acid acetylhexoside (compound 42) and caffeoylshikimic acid (compound 44) were identified by the molecular formula and base peak from the phenolic moiety. Caffeoylquinic acids were described from the fruits of several Patagonian Ribes species, including R. magellanicum [15,16,29,30].
3.4.4. Flavonolignans
The compounds 57 and 64 showed a [M-H]+ ion at m/z 467 and a base peak at m/z 357, in agreement with an Apocynin E-related flavonolignan, as reported from Trichilia catigua bark [28]. The compounds 84 and 92 showed a molecular formula C24H19O9 for the [M-H]+ ion at 451 amu and a base peak at m/z 341, suggesting the occurrence of flavonolignans related to Cinchonain I. The flavonolignans 84 and 92 were tentatively identified as Cinchonain I isomer 1 and 2, in agreement with [28]. Compound 53, with the molecular formula C39H31O15 for the [M-H]+ ion, showed a base peak at m/z 587, which was in agreement with a Cinchonain II isomer. Cinchonain II occurs in Anemopaegna arvense and Trichilia catigua, among others. Compound 74, with the m/z at 483 and the molecular formula C25H23O10 for the [M-H]+ ion, agrees with the data reported for Loropetaliside A [27], isolated from Loropetalum chinense.
3.4.5. Other Compounds
Protocatechuic acid hexoside (compound 6), two hexosides from gallic acid (compounds 2 and 3), and vanillic acid hexoside (34) were identified by the neutral loss of the hexose, leading to the base peak of the corresponding phenolic, which was in agreement with the literature and molecular formula [9]. Citric acid (compound 1) was detected in the samples, in agreement with [23]. The mass spectrum of compound 32, measured as the formic acid adduct, showed the [M-H]+ at m/z 385 and the fragments arising from the loss of hexose and water at m/z 223 and 205, respectively. The hexoside was assigned as roseoside, described from R. nigrum leaves [25]. The closely related compound 37 differs from 32 by a double bond equivalent and shows a m/z ion at 433, a molecular formula C20H33O10, and a base peak at m/z 387, compatible with the deprotonated molecule. The compound fragmented to low-intensity ions at m/z 225 and 207, supporting a cyclohexanone hexoside. The compound was tentatively identified as dihydroroseoside, isolated from Betula platyphylla var. japonica leaves [33].
Compound 88 showed a molecular formula C18H29O8 for the [M-H]+ ion at m/z 373 and lost a hexose followed by water, leading to the base peak at m/z 193. The aglycone requires a molecular formula C12H20O3 that is compatible with cucurbic acid/jasmonic acid isomer or ionone derivative [34]. However, the unambiguous assignment requires isolation and full characterization by spectroscopic and spectrometric means. The derivative 88 was tentatively assigned as ionone/cucurbic acid hexoside. Cucurbic acid and jasmonic acid are related signaling compounds that are important in the response to biotic and abiotic stress in plants [34]. Beta ionone is a powerful inhibitor of breast cancer cells’ proliferation [35].
Compound 89, with the molecular formula C23H26O10 for the [M-H]+ ion and m/z 461, showed the loss of a caffeoyl moiety and a neutral fragment compatible with the hydroxy phenethyl hexoside, being assigned as hydroxyphenethyl caffeoyl hexoside. The compound is related to the bitter phenylpropanoid glucoside that is isolated from Prunus grayana [36].
3.4.6. Unknown Compounds
Compound 62 showed the loss of hexose from the m/z 521 and fragments to 359 and 329 amu. The product could not be identified and is assigned as an unknown glycoside. Compound 90, with the molecular formula C24H35O14 for the [M-H]+ ion, showed a neutral loss of 180 and 118 amu, leading to the base peak at m/z 429 and ions at m/z 367 and 249. The compound is related to other products occurring in the active fractions of the leaf extract and needs to be isolated for full identification. A compound with the same molecular formula was described from Kunzea ambigua [37], but the fragmentation of the R. magellanicum compound rules out the structure.
Compounds 50 and 72, differing in the Rt, showed a m/z ion at 709 and lost a hexose linked to an aromatic moiety, leading to the base peak at m/z 547. The identity of the compounds could not be established and is reported as hexosides. The products 55 and 67, with m/z 611 and 449, respectively, lose 180 amu, which is assigned to an aliphatic-linked hexose, and base peaks at m/z 431 and 269, respectively. The three compounds eluting at the end of the run (94, 95, and 96) showed m/z ions at 519, 431, and 547 amu and could not be identified.
3.5. HPLC-DAD Fingerprints and Main Phenolics Quantification
The occurrence of the compounds 1–99 in selected samples and fractions is summarized in Table S1. The content of the main compounds was determined in the samples using calibration curves built with standard compounds (Table 5). Representative HPLC chromatograms for the different samples are shown in Figure 3. The chromatograms are presented at 330 and 360 nm to visualize the phenylpropanoids, such as chlorogenic acids and coumaroylquinic acids (UV maxima at 320–330 nm) and flavonol glycosides (UV maxima at 345–360 nm). The main compound in the extracts of most samples is quercetin-3-O-rutinoside (rutin) 66, except for the northern population at Conguillio National Park, where it was not detected. In the infusions of the Frutillar and RNM populations, 66 and quercetin dihexoside 49 are the main phenolics. In the Conguillio sample, quercetin 3-O-glucoside 71 and kaempferol acetyl hexoside 91 were the main phenolics, and were in higher content in the extract than in the infusion. Compounds 71 and 66 were the main constituents in the Lago Espolon and Quelulat extracts. Regarding caffeoyl and coumaroyl acid derivatives, 3-caffeoyquinic acid 11, 5-caffeoylquinic acid 24, and feruloylquinic acid 25 occur in all samples.
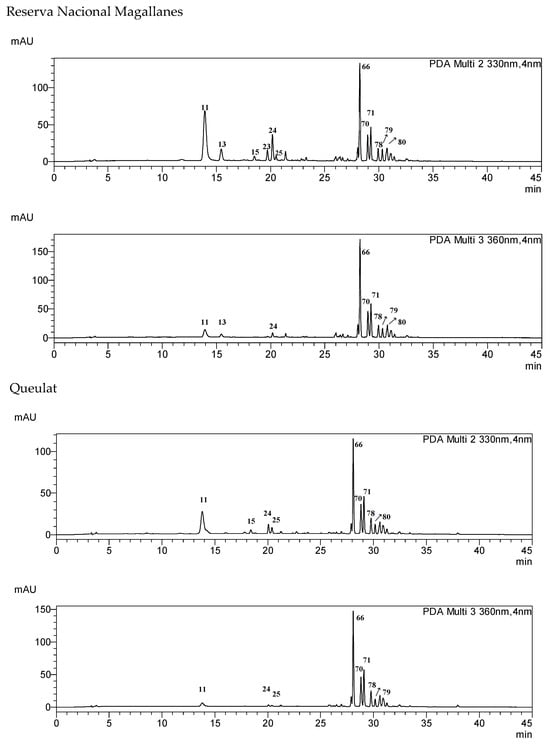
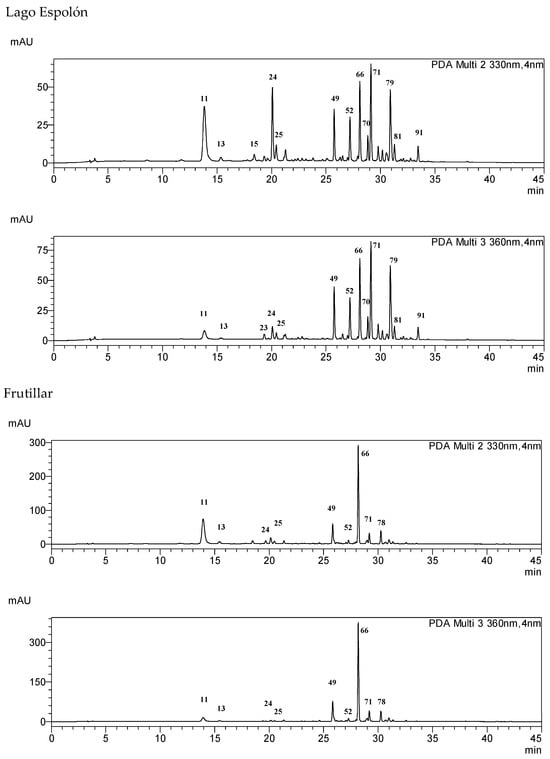
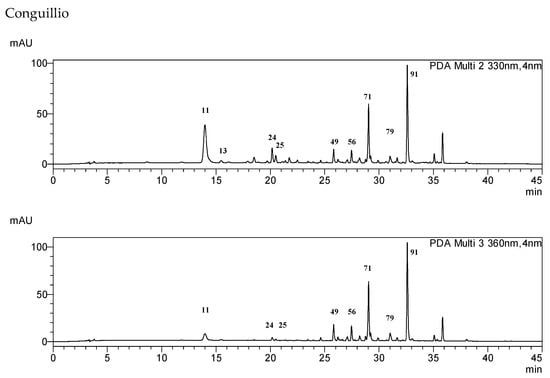
Figure 3.
HPLC-DAD fingerprints of the MeOH:H2O 7:3 leaf extract from Ribes magellanicum—detection: 330 and 360 nm.
4. Discussion
The aerial parts of berry bushes attract attention as they are a sustainable source of leaves for medicinal or nutraceutical purposes all year round, compared with the time during which the edible fruits are available. The information on bioactives from Ribes nigrum, Rubus idaeus, and Aronia melanocarpa leaf extracts was summarized and related to the secondary metabolites content and composition [38]. Several studies have shown the health-promoting properties of R. nigrum leaf extracts, including anti-inflammatory [10,39] and antioxidant effects [10]. The proanthocyanidins from R. nigrum inhibit the carrageenan-induced acute inflammation in rats [40]. The composition of infusion, hydroalcoholic, and methanol extracts from R. nigrum leaves was compared, affording valuable information on the metabolites from blackcurrant leaves [9]. In the R. nigrum infusion, quercetin 3-O glucoside, glucosides from isorhamnetin and kaempferol, as well as kaempferol acetyl hexoside, were identified. In R. magellanicum leaves (Table 5), the main compound in most samples was quercetin-3-O-rutinoside (rutin), followed by quercetin dihexoside. Kaempferol acetyl hexoside also occurs in our samples, but the content is lower in infusions than in the hydroalcoholic extracts. Overall, the main compounds are the same or structurally related to the constituents of R. nigrum leaves. The phenypropanoids 3-caffeoyquinic acid, 5-caffeoylquinic acid, and feruloylquinic acid are present in all R. magellanicum samples. Rutin, the 3-O-rutinoside of the flavonol quercetin, is a well-known bioflavonoid with extensive studies on its activities, metabolism, and health-beneficial effects [41]. Rutin modulates the molecular targets participating in inflammatory processes, cell cycle mediators, and drug transport, among others [42]. The flavonol quercetin stimulates glucose uptake in cells, improves insulin resistance, and regulates signaling pathways of glucose metabolism [43]. It also inhibits the enzyme α-glucosidase [44]. Quercetin and some of its derivatives have been approved for human use by the FDA [45] and can be used in functional foods and supplements [42]. According to [46], quercetin can be regarded as a potential preventer of neurodegenerative disorders. Kaempferol shows several effects on different biological targets, including inflammation. It occurs in R. magellanicum leaves, mainly as hexoside and acetyl hexoside. A review [47] summarizes the work and activities on the compound. Phenolic acids, including chlorogenic acid, caffeic acid, and ferulic acid, are α-glucosidase inhibitors [48].
Phytochemicals have a wide variety of functions in plants and trigger adaptive responses in cell stress pathways [49]. At the doses consumed in food and beverages, plant phytochemicals are non-toxic and might elicit mild cell stress responses. A large proportion of the compounds identified in R. magellanicum leaves are phenolics with well-known antioxidant properties. They occur in different concentrations and ratios and show a biphasic response (antioxidant/pro-oxidant), depending on the dose and concentration. These biphasic effects are known as hormetic and have been reported in several antioxidant/radical scavengers, including ferulic acid, resveratrol, curcumin, or linoleic acid [50]. The dose-dependent effect can explain, at least in part, the properties of herbal teas and plant foods in inflammatory processes or in enzyme inhibition activity. The effect of compounds with hormetic properties might be generated by activating transcriptional factors that regulate the cell response to oxidative stress, preventing inflammation. Low-toxicity phenylpropanoids, such as caffeic and ferulic acid [49], improve neuroprotection by their anti-inflammatory and antioxidant capacity at low doses and induce neurite growth and neuronal differentiation at high concentrations [50]. Catechin and epicatechin derivatives, including (epi) gallocatechin, their dimers, trimers, and tetramers, are antioxidant/free radical scavengers and occur in widely consumed infusions, including tea [49]. After intake, the compounds contained in foods and infusions undergo a series of modifications following gastric and intestinal digestion as well as biotransformation by the gut microbiota. The results are metabolites, including simple phenolics, derived from the starting compounds. In a study on gallic, protocatechuic, and vanillic acid on the nematode Caenorhabdites elegans [51], the phenolic acids increased heat-stress resistance, chemotaxis, and lifespan at micromolar concentrations, supporting a hormetic mechanism of action.
The relationship between diet, health, and bioactive compounds can be better understood from the hormetic perspective. Some reviews on the subject include the work of Mattson [52] on the effect on longevity and health, the impact of the concept of hormesis on health and biomedical research [53], and the perspectives of diet, exercise, and caloric restriction on the nutritional and bioenergetic cell state [54]. The sirtuin proteins regulate a wide range of cellular mechanisms that are activated by food constituents and can explain, at least in part, the health-beneficial effects of high vegetable and fruit diets (including herbal infusions), such as the Mediterranean diet [54].
Our findings in R. magellanicum leaves support its traditional use as a digestive beverage and encourage further development into a Patagonian herbal tea. As we studied leaf infusions (teas) and extracts, the compounds dissolved in hot water and solvent mixture can be compared (Table 5). The composition and α-glucosidase inhibition by hot and cold extracts from Hibiscus sabdariffa (roselle) were investigated [55] using quantitative 1H NMR to relate the composition with α-glucosidase inhibition. The α-glucosidase inhibition and catechin derivative composition of green tea under different extraction conditions were reported [56]. For catechins, the MeOH:H2O extraction was more effective than water infusion. The authors assessed the green tea extracts and their catechins on human α-glucosidase in differentiated Caco-2 cells. However, in the R. magellanicum leaves, flavonoids and phenylpropanoids are the main constituents both from infusions and extracts. Additional studies are needed to assess the effect of R. magellanicum leaf extracts and infusion on the glycaemia of animals and human users. Fruit extracts from Ribes stenocarpum showed hypoglycemic effects in alloxan-diabetic mice and normoglycemic animals [57].
5. Conclusions
Complex mixtures of phenolics occur in the infusions of R. magellanicum leaves. Caffeic and coumaric acids were identified as their isomeric quinic acids or as monoglycosides, eluting in the range of Rt 13–22 min (Figure 3). The main flavonoids were glycosides of the flavonols quercetin and kaempferol, and elute at Rt 25–34 min (Figure 3). Closer chemical profiles were observed for the southern continental Chile sample from Frutillar and that from the Navarino Island (Reserva Nacional Magallanes, RNM). However, the content of caffeic and coumaric acid derivatives is higher in RNM, and flavonoids were higher in the Frutillar population. Hot water was a good solvent for most of the phenolics, but the extraction yield, in a broad sense, was lower. The comparison allows a first approach to what should be expected as the actual intake of metabolites from the traditional beverage. The present work sets the basis for quantitative studies on the composition and enzyme-inhibitory properties of this Patagonian beverage. The chemical profiles of the samples from different locations in a latitudinal gradient from 38°39′ S to 52°31′ S can serve as a reference for chemical diversity studies on this native berry.
The present work supports the use of infusions of R. magellanicum leaves as a digestive by the ancient American cultures in southern South America. The strong activity against α-glucosidase, the chemical composition of infusions and extracts, suggests the potential of this traditional plant resource as an herbal tea with health-promoting effects. These findings encourage further work on the plant as well as the participation of local communities in the development of the species as a Patagonian herbal tea.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/beverages11050138/s1, Table S1: Occurrence of the compounds 1–99 in Ribes magellanicum leaves MeOH:H2O 7:3 extracts from Reserva Nacional Magallanes (RNM) and Upushuaia, infusion from RNM leaves, and the most active α-glucosidase inhibiting fractions from the leaf extract (RNM 14, 15/16 and 29/32)
Author Contributions
A.B.-E.: Formal analysis, Methodology, Conceptualization, and Writing—review and editing. C.T.: Formal analysis, Methodology, Conceptualization, and Writing—review and editing. C.R.: Methodology. S.M.: Methodology. D.G.: Formal analysis and Methodology. R.R.: Funding, Formal analysis, Methodology, and Writing—review and editing. V.S.: Formal analysis, Methodology, and Writing—review and editing. G.S.-H.: Formal analysis, Methodology, Conceptualization, Supervision, Writing—review and editing, and Funding. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Cape Horn International Center for Global Change Studies and Biocultural Conservation” (CHIC, FB210018) and FONDECYT 1210076.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available on request.
Acknowledgments
This research received funding from “Cape Horn International Center for Global Change Studies and Biocultural Conservation” (CHIC, FB210018) and FONDECYT 1210076. We are grateful to Paula Caballero (UMAG, Chile) for her help in collecting the samples.
Conflicts of Interest
The authors declare that they have no known competing financial interests.
References
- Puspitasari, Y.E.; Tuenter, E.; Breynaert, A.; Foubert, K.; Herawati, H.; Hariati, A.M.; Aulanni’am, A.; De Bruyne, T.; Hermans, N. α-Glucosidase inhibitory activity of tea and Kombucha from Rhizophora mucronata leaves. Beverages 2024, 10, 22. [Google Scholar] [CrossRef]
- Geraris Kartelias, I.; Panagiotakopoulos, I.; Nasopoulou, C.; Karantonis, H.C. Evaluating the effect of adding selected herbs, spices, and fruits to fermented Olympus Mountain tea (Sideritis scardica) Kombucha sweetened with Thyme honey: Assessment of physicochemical and functional properties. Beverages 2024, 10, 9. [Google Scholar] [CrossRef]
- Vasić, D.; Katanić Stanković, J.S.; Urošević, T.; Kozarski, M.; Naumovski, N.; Khan, H.; Popović-Djordjević, J. Insight into bioactive compounds, antioxidant and anti-diabetic properties of rosehip (Rosa canina L.)-based tisanes with addition of hibiscus flowers (Hibiscus sabdariffa L.) and saffron (Crocus sativus L.). Beverages 2024, 10, 1. [Google Scholar] [CrossRef]
- de Mösbach, W. Botánica Indígena de Chile; Aldunate, C., Villagrán, C., Eds.; Museo Chileno de Arte Precolombino: Santiago, Chile, 1992; 140p, Códigos BN: MC0027380. [Google Scholar]
- Mølgaard, P.; Holler, J.G.; Asar, B.; Liberna, I.; Rosenbæk, L.B.; Jebjerg, C.P.; Jørgensen, L.; Lauritzen, J.; Guzman, A.; Adsersen, A.; et al. Antimicrobial evaluation of Huilliche plant medicine used to treat wounds. J. Ethnopharmacol. 2011, 138, 219–227. [Google Scholar] [CrossRef]
- Raudsepp, P.; Kaldmäe, H.; Kikas, A.; Libek, A.-V.; Püssa, T. Nutritional quality of berries and bioactive compounds in the leaves of black currant (Ribes nigrum L.) cultivars evaluated in Estonia. J. Berry Res. 2010, 1, 53–59. [Google Scholar] [CrossRef]
- Kendir, G.; Süntar, I.; Çeribaşı, A.O.; Köroğlu, A. Activity evaluation on Ribes species, traditionally used to speed up healing of wounds: With special focus on Ribes nigrum. J. Ethnopharmacol. 2019, 237, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, N.; Xu, W.; Zhou, H. Genus Ribes Linn. (Grossulariaceae): A comprehensive review of traditional uses, phytochemistry, pharmacology and clinical applications. J. Ethnopharmacol. 2021, 276, 114166. [Google Scholar] [CrossRef]
- D’Urso, G.; Montoro, P.; Piacente, S. Detection and comparison of phenolic compounds in different extracts of black currant leaves by liquid chromatography coupled with high-resolution ESI-LTQ-Orbitrap MS and high-sensitivity ESI-Qtrap MS. J. Pharm. Biomed. Anal. 2020, 179, 112926. [Google Scholar] [CrossRef] [PubMed]
- Tabart, J.; Franck, T.; Kevers, C.; Pincemail, J.; Serteyn, D.; Defraigne, J.-O.; Dornmes, J. Antioxidant and anti-inflammatory activities of Ribes nigrum extracts. Food Chem. 2012, 131, 1116–1122. [Google Scholar] [CrossRef]
- Muller, C.J.F.; Joubert, E.; de Beer, D.; Sanderson, M.; Malherbe, C.J.; Fey, S.J.; Louw, J. Acute assessment of an aspalathin-enriched green rooibos (Aspalathus linearis) extract with hypoglycemic potential. Phytomedicine 2012, 20, 32–39. [Google Scholar] [CrossRef]
- Lim, J.; Ferruzzi, M.G.; Hamaker, B.R. Structural requirements of flavonoids for the selective inhibition of α-amylase versus α-glucosidase. Food Chem. 2022, 370, 130981. [Google Scholar] [CrossRef]
- Rozzi, R.; Massardo, F.; Anderson, C.B.; Heidinger, K.; Silander Jr, J.A. Ten principles for biocultural conservation at the southern tip of the Americas: The approach of the Omora Ethnobotanical Park. Ecol. Soc. 2006, 11, 1. Available online: http://www.ecologyandsociety.org/vol11/iss1/art43/ (accessed on 28 July 2025). [CrossRef]
- Nina, N.; Theoduloz, C.; Paillán, H.; Jiménez-Aspee, F.; Márquez, K.; Schuster, K.; Becker, L.; Oellig, C.; Frank, J.; Schmeda-Hirschmann, G. Chemical profile and bioactivity of Chilean bean landraces (Phaseolus vulgaris L.). J. Funct. Foods 2023, 104, 105513. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Theoduloz, C.; Vieira, M.N.; Rodríguez-Werner, M.A.; Schmalfuss, E.; Winterhalter, P.; Schmeda-Hirschmann, G. Phenolics from the Patagonian currants Ribes spp.: Isolation, characterization and cytoprotective effect in human AGS cells. J. Funct. Foods 2016, 26, 11–26. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Valdés, S.T.; Schulz, A.; Ladio, A.; Theoduloz, C.; Schmeda-Hirschmann, G. Antioxidant activity and phenolic profiles of the wild currant Ribes magellanicum from Chilean and Argentinean Patagonia. Food Sci. Nutr. 2016, 4, 595–610. [Google Scholar] [CrossRef]
- Theoduloz, C.; Burgos-Edwards, A.; Schmeda-Hirschmann, G.; Jimenez-Aspee, F. Effect of polyphenols from wild Chilean currants (Ribes spp.) on the activity of intracellular antioxidant enzymes in human gastric AGS cells. Food Biosci. 2018, 24, 80–88. [Google Scholar] [CrossRef]
- Wagner, H.; Bladt, S. Plant drug analysis. In A Thin Layer Chromatography Atlas; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Laghi, L.; Parpinello, G.P.; Del Rio, D.; Calani, L.; Mattioli, A.U.; Versari, A. Fingerprint of enological tannins by multiple techniques approach. Food Chem. 2010, 121, 783–788. [Google Scholar] [CrossRef]
- Ping, L.; Pizzi, A.; Guo, Z.D.; Brosse, N. Condensed tannins from grape pomace: Characterization by FTIR and MALDI TOF and production of environmentally friendly wood adhesive. Ind. Crops Prod. 2012, 40, 13–20. [Google Scholar] [CrossRef]
- Diwani, N.; Fakhfakh, J.; Athmouni, K.; Belhaj, D.; El Feki, A.; Allouche, N.; Ayadi, H.; Bouaziz-Ketata, H. Optimization, extraction, structure analysis and antioxidant properties of flavan-3-ol polymers: Proanthocyanidins isolated from Periploca angustifolia using surface response methodology. Ind. Crops Prod. 2020, 144, 112040. [Google Scholar] [CrossRef]
- Esquivel-Alvarado, D.; Muñoz-Arrieta, R.; Alfaro-Viquez, E.; Madrigal-Carballo, S.; Krueger, C.G.; Reed, J.D. Composition of anthocyanins and proanthocyanidins in three tropical Vaccinium species from Costa Rica. J. Agric. Food Chem. 2020, 68, 2872–2879. [Google Scholar] [CrossRef]
- Fernández-Fernández, R.; López-Martínez, J.C.; Romero-González, R.; Martínez-Vidal, J.L.; Alarcón Flores, M.I.; Garrido, F.A. Simple LC–MS determination of citric and malic acids in fruits and vegetables. Chromatographia 2010, 72, 55–62. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Sasaki, T.; Li, W.; Zaike, S.; Asada, Y.; Li, Q.; Ma, F.; Zhang, Q.; Koike, K. Antioxidant lignoids from leaves of Ribes nigrum. Phytochemistry 2013, 95, 333–340. [Google Scholar] [CrossRef]
- Lin, L.; Sun, J.; Chen, P.; Monagas, M.J.; Harnly, J.M. UHPLC-PDA-ESI/HRMS Profiling method to identify and quantify oligomeric proanthocyanidins in plant products. J. Agric. Food Chem. 2014, 62, 9387–9400. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, D.; Xiong, B.; Kong, L.; Zhu, X. Isolation of new flavan-3-ol and lignan glucoside from Loropetalum chinense and their antimicrobial activities. Fitoterapia 2013, 90, 228–232. [Google Scholar] [CrossRef]
- Bernardo, J.; Ferreres, F.; Gil-Izquierdo, A.; Videira, R.M.; Valentao, P.; Veiga, F.; Andrade, P.B. In vitro multimodal-effect of Trichilia catigua A. Juss. (Meliaceae) bark aqueous extract in CNS targets. J. Ethnopharmacol. 2018, 211, 247–255. [Google Scholar] [CrossRef]
- Burgos-Edwards, A.; Theoduloz, C.; Miño, S.; Ghosh, D.; Shulaev, V.; Ramírez, C.; Sánchez-Jardón, L.; Rozzi, R.; Schmeda-Hirschmann, G. Phenolic composition and bioactivity of Ribes magellanicum fruits from Southern Patagonia. Heliyon 2024, 10, e25542. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Edwards, A.; Jiménez-Aspee, F.; Thomas-Valdés, S.; Schmeda-Hirschmann, G.; Theoduloz, C. Qualitative and quantitative changes in polyphenol composition and bioactivity of Ribes magellanicum and R. punctatum after in vitro gastrointestinal digestion. Food Chem. 2017, 237, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Jáuregui, O.; Di Lecce, G.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M. Screening of the polyphenol content of tomato-based products through accurate-mass spectrometry (HPLC–ESI-QTOF). Food Chem. 2011, 129, 877–883. [Google Scholar] [CrossRef]
- Shelke, V.; Kale, A.; Kulkarni, Y.A.; Gaikwad, A.B. Phloretin: A comprehensive review of its potential against diabetes and associated complications. J. Pharm. Pharmacol. 2024, 76, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Terazawa, M. Dihydroroseoside, a new cyclohexanone glucoside, from the leaves of shirakamba (Betula platyphylla Sukatchev var. japonica Hara). J. Wood Sci. 2001, 47, 145–148. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Galeron, M.A.; Aubert, C. Electron ionization mass spectrometric fragmentation and multiple reaction monitoring quantification of trimethylsilyl derivatives of cucurbic acid and its 6,7-stereoisomers. Rapid Commun. Mass Spectrom. 2016, 30, 2253–2264. [Google Scholar] [CrossRef]
- Dong, H.W.; Wang, K.; Chang, X.X.; Jin, F.-F.; Wang, Q.; Jiang, X.-F.; Liu, J.-R.; Wu, Y.-H.; Yang, C. Beta-ionone-inhibited proliferation of breast cancer cells by inhibited COX-2 activity. Arch. Toxicol. 2019, 93, 2993–3003. [Google Scholar] [CrossRef]
- Shimomura, H.; Sashida, Y.; Adachi, T. Phenolic glucosides from Prunus grayana. Phytochemistry 1986, 26, 249–251. [Google Scholar] [CrossRef]
- Kasajima, N.; Ito, H.; Hatano, T.; Yoshida, T. Phloroglucinol diglycosides accompanying hydrolyzable tannins from Kunzea ambigua. Phytochemistry 2008, 69, 3080–3086. [Google Scholar] [CrossRef] [PubMed]
- Staszowska-Karkut, M.; Materska, M. Phenolic composition, mineral content, and beneficial bioactivities of leaf extracts from black currant (Ribes nigrum L.), raspberry (Rubus idaeus), and aronia (Aronia melanocarpa). Nutrients 2020, 12, 463. [Google Scholar] [CrossRef]
- Declume, C. Anti-inflammatory evaluation of a hydroalcoholic extract of black currant leaves (Ribes nigrum). J. Ethnopharmacol. 1989, 27, 91–98. [Google Scholar] [CrossRef]
- Garbacki, N.; Tits, M.; Angenot, N.; Damas, J. Inhibitory effects of proanthocyanidins from Ribes nigrum leaves on carrageenan acute inflammatory reactions induced in rats. BMC Pharmacol. 2004, 4, 1471–2210. [Google Scholar] [CrossRef]
- Semwal, R.; Joshi, S.K.; Semwal, R.B.; Semwal, D.K. Health benefits and limitations of rutin—A natural flavonoid with high nutraceutical value. Phytochem. Lett. 2021, 46, 119–128. [Google Scholar] [CrossRef]
- Farha, A.K.; Gan, R.Y.; Li, H.B.; Wu, D.T.; Atanasov, A.G.; Gul, K.; Zhang, J.-R.; Yang, Q.-Q.; Corke, H. The anticancer potential of the dietary polyphenol rutin: Current status, challenges, and perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 832–859. [Google Scholar] [CrossRef]
- Dhanya, R. Quercetin for managing type 2 diabetes and its complications, an insight into multitarget therapy. Biomed. Pharmacother. 2022, 146, 112560. [Google Scholar] [CrossRef] [PubMed]
- Şöhretoğlu, D.; Sari, S. Flavonoids as alpha-glucosidase inhibitors: Mechanistic approaches merged with enzyme kinetics and molecular modelling. Phytochem. Rev. 2020, 19, 1081–1092. [Google Scholar] [CrossRef]
- Magar, R.T.; Sohng, J.K. A review on structure, modifications and structure-activity relation of quercetin and its derivatives. J. Microbiol. Biotechnol. 2020, 30, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kostić, A.Ž.; Arserim-Uçar, D.K.; Materska, M.; Sawicka, B.; Skiba, D.; Milinčić, D.D.; Pešić, M.B.; Pszczółkowski, P.; Moradi, D.; Ziarati, P.; et al. Unlocking quercetin’s neuroprotective potential: A focus on bee-collected pollen. Chem. Biodivers. 2024, 21, e202400114. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 9580–9604. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef]
- Son, T.G.; Camandola, S.; Mattson, M.P. Hormetic dietary phytochemicals. Neuromol. Med. 2008, 10, 236–246. [Google Scholar] [CrossRef]
- Barreiro-Sisto, U.; Fernández-Fariña, S.; González-Noya, A.M.; Pedrido, R.; Maneiro, M. Enemies or allies? Hormetic and ap parent non-dose-dependent effects of natural bioactive antioxidants in the treatment of inflammation. Int. J. Mol. Sci. 2024, 25, 1892. [Google Scholar] [CrossRef]
- Dilberger, B.; Weppler, S.; Eckert, G.P. Phenolic acid metabolites of polyphenols act as inductors for hormesis in C. elegans. Mech. Ageing Dev. 2021, 198, 111518. [Google Scholar] [CrossRef]
- Mattson, M.P. Dietary factors, hormesis and health. Ageing Res. Rev. 2008, 7, 43–48. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Iavicoli, I.; Calabrese, V. Hormesis: Its impact on medicine and health. Hum. Exp. Toxicol. 2013, 32, 120–152. [Google Scholar] [CrossRef] [PubMed]
- Pande, S.; Raisuddin, S. The underexplored dimensions of nutritional hormesis. Curr. Nutr. Rep. 2022, 11, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, D.M.; Porzel, A.; Frolov, A.; El Seedi, H.R.; Ludger, A.; Farag, M.A. Comparative analysis of Hibiscus sabdariffa (roselle) hot and cold extracts in respect to their potential for α-glucosidase inhibition. Food Chem. 2018, 250, 236–244. [Google Scholar] [CrossRef]
- Orita, T.; Chogahara, S.; Okuda, M.; Sakao, K.; Miyata, T.; Hou, D.-X. Extraction efficiency and alpha-glucosidase inhibitory activities of green tea catechins by different infusion methods. Foods 2023, 12, 2611. [Google Scholar] [CrossRef]
- Jiang, S.; Zhao, X.; Liu, C.; Dong, Q.; Mei, L.; Chen, C.; Shao, Y.; Tao, Y.; Yue, H. Identification of phenolic compounds in fruits of Ribes stenocarpum Maxim. by UHPLC-QTOF/MS and their hypoglycemic effects in vitro and in vivo. Food Chem. 2021, 344, 128568. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).