Childhood Obesity and Its Physiological Association with Sugar-Sweetened, Free-Sugar Juice, and Artificially Sweetened Beverages
Abstract
1. Introduction
2. Methods
3. Global Trends on SSBs and ASBs Intake
3.1. Juices as Free Sugars
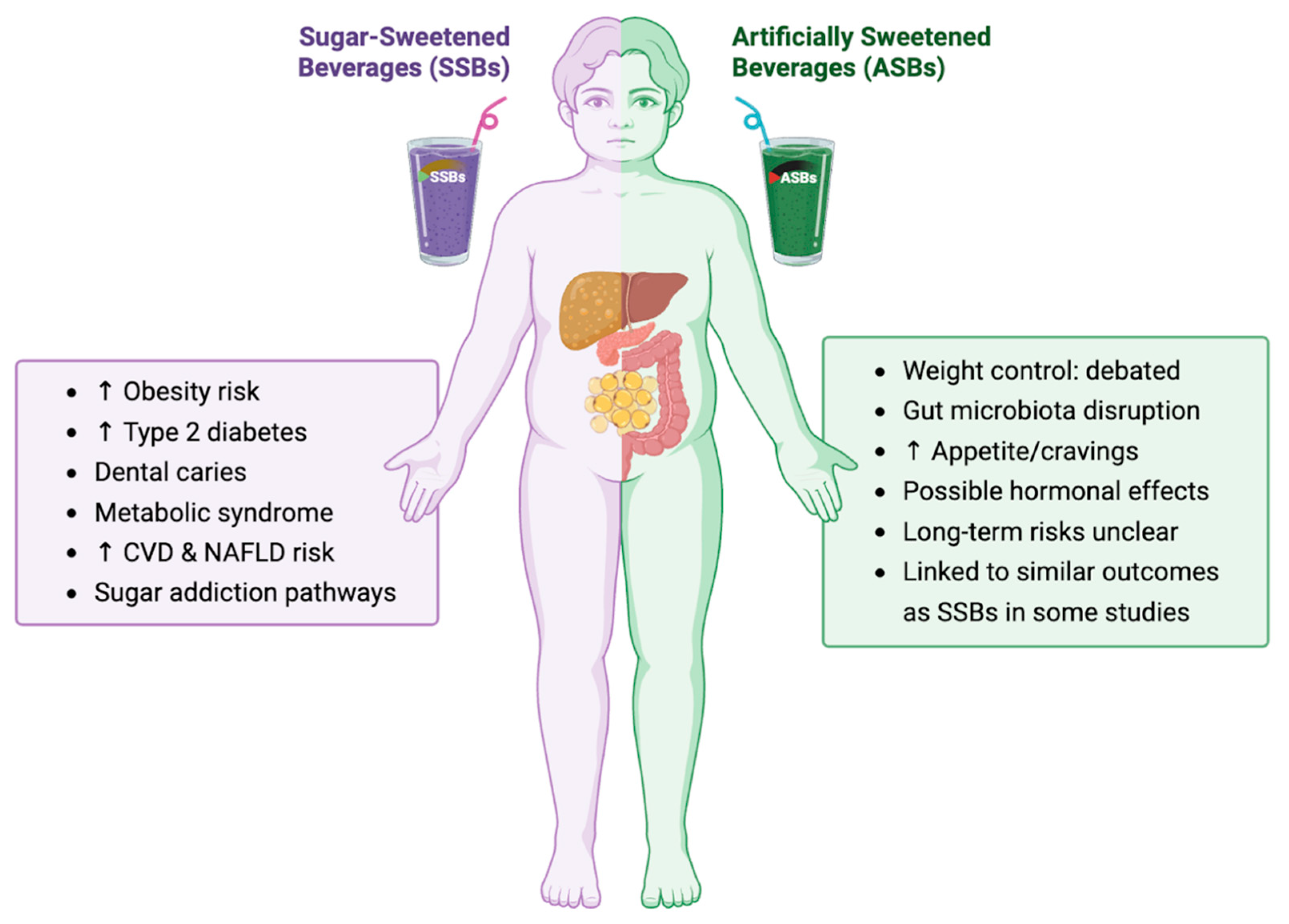
3.2. SSBs and Childhood Obesity
3.3. ASBs: A Risky Alternative?
4. Comparison of SSBs vs. ASBs
| Beverage Type | Example | Sweetener(s) | Energy (kcal) 2 | Total Sugars (g) 2 | Added Sugars (g) 2,3 | Total Carbohydrates (g) 2 |
|---|---|---|---|---|---|---|
| SSB | Cola-type soda | High-fructose corn syrup | 140/39 | 39/11 | 39/11 | 39/11 |
| SSB | Fruit punch–type drink | Sucrose, HFCS 1 | 150/42 | 35/10 | 35/10 | 38/11 |
| SSB | Orange juice (100% juice) | Fructose/glucose | 160/45 | 36/10 | 0/0 | 37/10 |
| ASB | Diet cola | Aspartame | 0/0 | 0/0 | 0/0 | 0/0 |
| ASB | Zero-sugar lemonade | Sucralose, Acesulfame-K | 5/1 | 0/0 | 0/0 | 1/<0.5 |
5. Innate and Modifiable Nature of Sweet Preference
5.1. Hepatokine FGF21: A Fructose Stress Sensor
5.2. Intestinal Sweet-Taste Receptors (T1R2/T1R3) and Incretin–SGLT1 Coupling
5.3. Cortico-Striatal Reward Adaptations
5.4. Fructose, Uric Acid, and Hepatic De Novo Lipogenesis
5.5. GLUT5 Maturation and Epigenetic Programming Across Early Life
5.6. Hypothalamic Gliosis and Appetite Signaling
6. Physiological Alterations Associated with SSBs and ASBs
6.1. Gut Microbiota
6.2. Inflammatory Effects
6.3. Hormonal Disruption and Obesity
6.4. Other Pathologies
7. Public Health Implications
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASBs | Artificially Sweetened Beverages |
| BMI | Body Mass Index |
| CCK | Cholecystokinin |
| CRP | C-Reactive Protein |
| CVD | Cardiovascular Disease |
| GIP | Glucose-Dependent Insulinotropic Polypeptide |
| GLP-1 | Glucagon-Like Peptide-1 |
| HFCS | High-Fructose Corn Syrup |
| MBH | Mediobasal Hypothalamus |
| NAFLD | Nonalcoholic Fatty Liver Disease |
| NHANES | National Health and Nutrition Examination Survey |
| SGLT1 | Sodium-glucose cotransporter 1 |
| SSBs | Sugar-Sweetened Beverages |
| T1R2 | Taste Receptor Type 1 Member 2 |
| T1R3 | Taste Receptor Type 1 Member 3 |
| WHO | World Health Organization |
References
- Malik, V.S.; Pan, A.; Willett, W.C.; Hu, F.B. Sugar-Sweetened Beverages and Weight Gain in Children and Adults: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2013, 98, 1084–1102. [Google Scholar] [CrossRef]
- Hu, F.B. Resolved: There Is Sufficient Scientific Evidence That Decreasing Sugar-sweetened Beverage Consumption Will Reduce the Prevalence of Obesity and Obesity-related Diseases. Obes. Rev. 2013, 14, 606–619. [Google Scholar] [CrossRef]
- Johnson, R.K.; Appel, L.J.; Brands, M.; Howard, B.V.; Lefevre, M.; Lustig, R.H.; Sacks, F.; Steffen, L.M.; Wylie-Rosett, J. Dietary Sugars Intake and Cardiovascular Health: A Scientific Statement from the American Heart Association. Circulation 2009, 120, 1011–1020. [Google Scholar] [CrossRef]
- Lobstein, T.; Jackson-Leach, R.; Moodie, M.L.; Hall, K.D.; Gortmaker, S.L.; Swinburn, B.A.; James, W.P.T.; Wang, Y.; McPherson, K. Child and Adolescent Obesity: Part of a Bigger Picture. Lancet 2015, 385, 2510–2520. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Peterson, K.E.; Gortmaker, S.L. Relation between Consumption of Sugar-Sweetened Drinks and Childhood Obesity: A Prospective, Observational Analysis. Lancet 2001, 357, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Te Morenga, L.; Mallard, S.; Mann, J. Dietary Sugars and Body Weight: Systematic Review and Meta-Analyses of Randomised Controlled Trials and Cohort Studies. BMJ 2012, 346, e7492. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Cena, H.; Magenes, V.C.; Vincenti, A.; Comola, G.; Beretta, A.; Di Napoli, I.; Zuccotti, G. Sugar-Sweetened Beverages and Metabolic Risk in Children and Adolescents with Obesity: A Narrative Review. Nutrients 2023, 15, 702. [Google Scholar] [CrossRef]
- Leung, C.W.; DiMatteo, S.G.; Gosliner, W.A.; Ritchie, L.D. Sugar-Sweetened Beverage and Water Intake in Relation to Diet Quality in U.S. Children. Am. J. Prev. Med. 2018, 54, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Laverty, A.A.; Magee, L.; Monteiro, C.A.; Saxena, S.; Millett, C. Sugar and Artificially Sweetened Beverage Consumption and Adiposity Changes: National Longitudinal Study. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 137. [Google Scholar] [CrossRef]
- Zhu, Y.; Olsen, S.F.; Mendola, P.; Halldorsson, T.I.; Rawal, S.; Hinkle, S.N.; Yeung, E.H.; Chavarro, J.E.; Grunnet, L.G.; Granström, C.; et al. Maternal Consumption of Artificially Sweetened Beverages during Pregnancy, and Offspring Growth through 7 Years of Age: A Prospective Cohort Study. Int. J. Epidemiol. 2017, 46, 1499–1508. [Google Scholar] [CrossRef]
- Scharf, R.J.; DeBoer, M.D. Sugar-Sweetened Beverages and Children’s Health. Annu. Rev. Public Health 2016, 37, 273–293. [Google Scholar] [CrossRef]
- Della Corte, K.; Fife, J.; Gardner, A.; Murphy, B.L.; Kleis, L.; Della Corte, D.; Schwingshackl, L.; LeCheminant, J.D.; Buyken, A.E. World Trends in Sugar-Sweetened Beverage and Dietary Sugar Intakes in Children and Adolescents: A Systematic Review. Nutr. Rev. 2021, 79, 274–288. [Google Scholar] [CrossRef]
- Ricciuto, L.; Fulgoni, V.L.; Gaine, P.C.; Scott, M.O.; DiFrancesco, L. Trends in Added Sugars Intake and Sources Among US Children, Adolescents, and Teens Using NHANES 2001–2018. J. Nutr. 2022, 152, 568–578. [Google Scholar] [CrossRef]
- Park, S.; Zhao, L.; Lee, S.H.; Hamner, H.C.; Moore, L.V.; Galuska, D.A.; Blanck, H.M. Children and Adolescents in the United States with Usual High Added Sugars Intake: Characteristics, Eating Occasions, and Top Sources, 2015–2018. Nutrients 2023, 15, 274. [Google Scholar] [CrossRef]
- Ricciuto, L.; Fulgoni, V.L.; Gaine, P.C.; Scott, M.O.; DiFrancesco, L. Intakes of Added Sugars, with a Focus on Beverages and the Associations with Micronutrient Adequacy in US Children, Adolescents, and Teens (NHANES 2003–2018). Nutrients 2023, 15, 3285. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.; Lowthian, E.; Hawkins, J.; Hallingberg, B.; Alhumud, M.; Roberts, C.; Murphy, S.; Moore, G. Sugar-Sweetened Beverage Consumption from 1998–2017: Findings from the Health Behaviour in School-Aged Children/School Health Research Network in Wales. PLoS ONE 2021, 16, e0248847. [Google Scholar] [CrossRef]
- Chatelan, A.; Lebacq, T.; Rouche, M.; Kelly, C.; Fismen, A.-S.; Kalman, M.; Dzielska, A.; Castetbon, K. Long-Term Trends in the Consumption of Sugary and Diet Soft Drinks among Adolescents: A Cross-National Survey in 21 European Countries. Eur. J. Nutr. 2022, 61, 2799–2813. [Google Scholar] [CrossRef]
- Rocha, L.L.; Pessoa, M.C.; Gratão, L.H.A.; Carmo, A.S.D.; Cunha, C.D.F.; Oliveira, T.R.P.R.D.; Mendes, L.L. Health Behavior Patterns of Sugar-Sweetened Beverage Consumption among Brazilian Adolescents in a Nationally Representative School-Based Study. PLoS ONE 2021, 16, e0245203. [Google Scholar] [CrossRef]
- Ventura, A.K.; Mennella, J.A. Innate and Learned Preferences for Sweet Taste during Childhood. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-T.; Kao, Y.-H.; Li, M.S.; Luo, T.; Lin, H.-Y.; Lee, C.-H.; Seal, D.W.; Hu, C.; Chen, L.-S.; Tseng, T.-S. Sugar-Sweetened Beverages Intake, Abdominal Obesity, and Inflammation among US Adults without and with Prediabetes—An NHANES Study. Int. J. Environ. Res. Public. Health 2022, 20, 681. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015; ISBN 978-92-4-154902-8. [Google Scholar]
- Scientific Advisory Committee on Nutrition (SACN). Carbohydrates and Health; The Stationery Office: London, UK, 2015; ISBN 978-0-11-708284-7. [Google Scholar]
- Keller, A.; Bucher Della Torre, S. Sugar-Sweetened Beverages and Obesity among Children and Adolescents: A Review of Systematic Literature Reviews. Child. Obes. 2015, 11, 338–346. [Google Scholar] [CrossRef]
- Ruanpeng, D.; Thongprayoon, C.; Cheungpasitporn, W.; Harindhanavudhi, T. Sugar and Artificially Sweetened Beverages Linked to Obesity: A Systematic Review and Meta-Analysis. QJM Int. J. Med. 2017, 110, 513–520. [Google Scholar] [CrossRef]
- de Ruyter, J.C.; Olthof, M.R.; Seidell, J.C.; Katan, M.B. A Trial of Sugar-Free or Sugar-Sweetened Beverages and Body Weight in Children. N. Engl. J. Med. 2012, 367, 1397–1406. [Google Scholar] [CrossRef]
- Marshall, T.A.; Curtis, A.M.; Cavanaugh, J.E.; Warren, J.J.; Levy, S.M. Child and Adolescent Sugar-Sweetened Beverage Intakes Are Longitudinally Associated with Higher Body Mass Index z Scores in a Birth Cohort Followed 17 Years. J. Acad. Nutr. Diet. 2019, 119, 425–434. [Google Scholar] [CrossRef]
- Bray, G.A.; Popkin, B.M. Dietary Sugar and Body Weight: Have We Reached a Crisis in the Epidemic of Obesity and Diabetes?: Health Be Damned! Pour on the Sugar. Diabetes Care 2014, 37, 950–956. [Google Scholar] [CrossRef]
- Bleich, S.N.; Vercammen, K.A. The Negative Impact of Sugar-Sweetened Beverages on Children’s Health: An Update of the Literature. BMC Obes. 2018, 5, 6. [Google Scholar] [CrossRef]
- Vos, M.B.; Kaar, J.L.; Welsh, J.A.; Van Horn, L.V.; Feig, D.I.; Anderson, C.A.M.; Patel, M.J.; Cruz Munos, J.; Krebs, N.F.; Xanthakos, S.A.; et al. Added Sugars and Cardiovascular Disease Risk in Children: A Scientific Statement from the American Heart Association. Circulation 2017, 135, e1017–e1034. [Google Scholar] [CrossRef]
- Pereira, M.A.; Fulgoni, V.L. Consumption of 100% Fruit Juice and Risk of Obesity and Metabolic Syndrome: Findings from the National Health and Nutrition Examination Survey 1999–2004. J. Am. Coll. Nutr. 2010, 29, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Fleming-Milici, F.; Phaneuf, L.; Harris, J.L. Marketing of Sugar-Sweetened Children’s Drinks and Parents’ Misperceptions about Benefits for Young Children. Matern. Child. Nutr. 2022, 18, e13338. [Google Scholar] [CrossRef] [PubMed]
- Marshall, T.A.; Levy, S.M.; Broffitt, B.; Warren, J.J.; Eichenberger-Gilmore, J.M.; Burns, T.L.; Stumbo, P.J. Dental Caries and Beverage Consumption in Young Children. Pediatrics 2003, 112, e184–e191. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.E.; Perez, V. Low-Calorie Sweeteners and Body Weight and Composition: A Meta-Analysis of Randomized Controlled Trials and Prospective Cohort Studies. Am. J. Clin. Nutr. 2014, 100, 765–777. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Lutsey, P.L.; Wang, Y.; Lima, J.A.; Michos, E.D.; Jacobs, D.R. Diet Soda Intake and Risk of Incident Metabolic Syndrome and Type 2 Diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2009, 32, 688–694. [Google Scholar] [CrossRef]
- Zhang, G.-H.; Chen, M.-L.; Liu, S.-S.; Zhan, Y.-H.; Quan, Y.; Qin, Y.-M.; Deng, S.-P. Effects of Mother’s Dietary Exposure to Acesulfame-K in Pregnancy or Lactation on the Adult Offspring’s Sweet Preference. Chem. Senses 2011, 36, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.P.; Williams, K.; Resendez, R.G.; Hunt, K.J.; Hazuda, H.P.; Stern, M.P. Fueling the Obesity Epidemic? Artificially Sweetened Beverage Use and Long-Term Weight Gain. Obesity 2008, 16, 1894–1900. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Tiemann, C.D.; Patterson, B.W.; Wice, B.M.; Klein, S. Sucralose Affects Glycemic and Hormonal Responses to an Oral Glucose Load. Diabetes Care 2013, 36, 2530–2535. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial Sweeteners Induce Glucose Intolerance by Altering the Gut Microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Brown, R.J.; De Banate, M.A.; Rother, K.I. Artificial Sweeteners: A Systematic Review of Metabolic Effects in Youth. Int. J. Pediatr. Obes. 2010, 5, 305–312. [Google Scholar] [CrossRef]
- Zheng, M.; Rangan, A.; Olsen, N.J.; Andersen, L.B.; Wedderkopp, N.; Kristensen, P.; Grøntved, A.; Ried-Larsen, M.; Lempert, S.M.; Allman-Farinelli, M.; et al. Substituting Sugar-Sweetened Beverages with Water or Milk Is Inversely Associated with Body Fatness Development from Childhood to Adolescence. Nutrition 2015, 31, 38–44. [Google Scholar] [CrossRef]
- Schneider, S.; Mata, J.; Kadel, P. Relations between Sweetened Beverage Consumption and Individual, Interpersonal, and Environmental Factors: A 6-Year Longitudinal Study in German Children and Adolescents. Int. J. Public Health 2020, 65, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Mullee, A.; Romaguera, D.; Pearson-Stuttard, J.; Viallon, V.; Stepien, M.; Freisling, H.; Fagherazzi, G.; Mancini, F.R.; Boutron-Ruault, M.-C.; Kühn, T.; et al. Association Between Soft Drink Consumption and Mortality in 10 European Countries. JAMA Intern. Med. 2019, 179, 1479. [Google Scholar] [CrossRef]
- Rathaus, M.; Azem, L.; Livne, R.; Ron, S.; Ron, I.; Hadar, R.; Efroni, G.; Amir, A.; Braun, T.; Haberman, Y.; et al. Long-Term Metabolic Effects of Non-Nutritive Sweeteners. Mol. Metab. 2024, 88, 101985. [Google Scholar] [CrossRef]
- Hunter, S.R.; Reister, E.J.; Cheon, E.; Mattes, R.D. Low Calorie Sweeteners Differ in Their Physiological Effects in Humans. Nutrients 2019, 11, 2717. [Google Scholar] [CrossRef] [PubMed]
- Raynor, H.A.; Deierlein, A.L.; Gardner, C.D.; Giovannucci, E.; Taylor, C.A.; Hoelscher, D.M.; Anderson, C.A.M.; Booth, S.L.; Fung, T.T.; Stanford, F.C.; et al. Low- and No-Calorie Sweetened Beverages and Growth, Body Composition, and Risk of Obesity: A Systematic Review; USDA Nutrition Evidence Systematic Review (NESR) Systematic Reviews, Rapid Reviews, and Evidence Scans; USDA Nutrition Evidence Systematic Review: Alexandria, VA, USA, 2024. [Google Scholar]
- Murphy, M.M.; Barrett, E.C.; Bresnahan, K.A.; Barraj, L.M. 100% Fruit Juice and Measures of Glucose Control and Insulin Sensitivity: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. J. Nutr. Sci. 2017, 6, e59. [Google Scholar] [CrossRef]
- Chakravartti, S.P.; Jann, K.; Veit, R.; Liu, H.; Yunker, A.G.; Angelo, B.; Monterosso, J.R.; Xiang, A.H.; Kullmann, S.; Page, K.A. Non-Caloric Sweetener Effects on Brain Appetite Regulation in Individuals across Varying Body Weights. Nat. Metab. 2025, 7, 574–585. [Google Scholar] [CrossRef]
- Reference Amounts Customarily Consumed per Eating Occasion (21 CFR §101.12). US Food and Drug Administration. 2020. Available online: https://www.law.cornell.edu/cfr/text/21/101.12 (accessed on 14 July 2025).
- U.S. Department of Agriculture, Agricultural Research Service. Beverages, Carbonated, Cola, Fast-Food Cola. FoodData Cent. 2019. Available online: https://fdc.nal.usda.gov/food-search?type=SR%20Legacy&query=cola (accessed on 14 July 2025).
- Coca-Cola®Coke—Nutrition Facts—SmartLabelSmartLabelTM. Coca-Cola®Coke. 2024. Available online: https://smartlabel.coca-colaproductfacts.com/nutrition/index.html?CocaCola-6760fluidounce&upc=049000050103 (accessed on 10 July 2025).
- Espinosa, A.; Mendoza, K.; Laviada-Molina, H.; Rangel-Méndez, J.A.; Molina-Segui, F.; Sun, Q.; Tobias, D.K.; Willett, W.C.; Mattei, J. Effects of Nonnutritive Sweeteners on the BMI of Children and Adolescents: A Systematic Review and Meta-Analysis of Randomized Controlled Trials and Prospective Cohort Studies. Adv. Nutr. 2024, 15, 100292. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J.D.; Carruth, B.R. A Longitudinal Study of Children’s Juice Intake and Growth. J. Am. Diet. Assoc. 2001, 101, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Shefferly, A.; Scharf, R.J.; DeBoer, M.D. Longitudinal Evaluation of 100% Fruit Juice Consumption on BMI Status in 2-5-Year-Old Children. Pediatr. Obes. 2016, 11, 221–227. [Google Scholar] [CrossRef]
- Nguyen, M.; Jarvis, S.E.; Chiavaroli, L.; Mejia, S.B.; Zurbau, A.; Khan, T.A.; Tobias, D.K.; Willett, W.C.; Hu, F.B.; Hanley, A.J.; et al. Consumption of 100% Fruit Juice and Body Weight in Children and Adults: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2024, 178, 237–246. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Mennella, J.A. Early Flavor Learning and Its Impact on Later Feeding Behavior. J. Pediatr. Gastroenterol. Nutr. 2009, 48 (Suppl. 1), S25–S30. [Google Scholar] [CrossRef]
- Mueller, C.; Zeinstra, G.G.; Forde, C.G.; Jager, G. Sweet and Sour Sips: No Effect of Repeated Exposure to Sweet or Sour-Tasting Sugary Drinks on Children’s Sweetness Preference and Liking. Appetite 2024, 196, 107277. [Google Scholar] [CrossRef]
- Mennella, J.A.; Bobowski, N.K. The Sweetness and Bitterness of Childhood: Insights from Basic Research on Taste Preferences. Physiol. Behav. 2015, 152, 502–507. [Google Scholar] [CrossRef]
- Stribiţcaia, E.; Evans, C.E.L.; Gibbons, C.; Blundell, J.; Sarkar, A. Food Texture Influences on Satiety: Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 12929. [Google Scholar] [CrossRef] [PubMed]
- Søberg, S.; Sandholt, C.H.; Jespersen, N.Z.; Toft, U.; Madsen, A.L.; Von Holstein-Rathlou, S.; Grevengoed, T.J.; Christensen, K.B.; Bredie, W.L.P.; Potthoff, M.J.; et al. FGF21 Is a Sugar-Induced Hormone Associated with Sweet Intake and Preference in Humans. Cell Metab. 2017, 25, 1045–1053.e6. [Google Scholar] [CrossRef] [PubMed]
- Giannini, C.; Feldstein, A.E.; Santoro, N.; Kim, G.; Kursawe, R.; Pierpont, B.; Caprio, S. Circulating Levels of FGF-21 in Obese Youth: Associations with Liver Fat Content and Markers of Liver Damage. J. Clin. Endocrinol. Metab. 2013, 98, 2993–3000. [Google Scholar] [CrossRef]
- Feldstein Ewing, S.W.; Claus, E.D.; Hudson, K.A.; Filbey, F.M.; Yakes Jimenez, E.; Lisdahl, K.M.; Kong, A.S. Overweight Adolescents’ Brain Response to Sweetened Beverages Mirrors Addiction Pathways. Brain Imaging Behav. 2017, 11, 925–935. [Google Scholar] [CrossRef]
- Bobbert, T.; Schwarz, F.; Fischer-Rosinsky, A.; Pfeiffer, A.F.H.; Möhlig, M.; Mai, K.; Spranger, J. Fibroblast Growth Factor 21 Predicts the Metabolic Syndrome and Type 2 Diabetes in Caucasians. Diabetes Care 2013, 36, 145–149. [Google Scholar] [CrossRef]
- Jang, H.-J.; Kokrashvili, Z.; Theodorakis, M.J.; Carlson, O.D.; Kim, B.-J.; Zhou, J.; Kim, H.H.; Xu, X.; Chan, S.L.; Juhaszova, M.; et al. Gut-Expressed Gustducin and Taste Receptors Regulate Secretion of Glucagon-like Peptide-1. Proc. Natl. Acad. Sci. USA 2007, 104, 15069–15074. [Google Scholar] [CrossRef]
- Margolskee, R.F.; Dyer, J.; Kokrashvili, Z.; Salmon, K.S.H.; Ilegems, E.; Daly, K.; Maillet, E.L.; Ninomiya, Y.; Mosinger, B.; Shirazi-Beechey, S.P. T1R3 and Gustducin in Gut Sense Sugars to Regulate Expression of Na+-Glucose Cotransporter 1. Proc. Natl. Acad. Sci. USA 2007, 104, 15075–15080. [Google Scholar] [CrossRef]
- Ma, J.; Bellon, M.; Wishart, J.M.; Young, R.; Blackshaw, L.A.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effect of the Artificial Sweetener, Sucralose, on Gastric Emptying and Incretin Hormone Release in Healthy Subjects. Am. J. Physiol.-Gastrointest. Liver Physiol. 2009, 296, G735–G739. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.L.; Vialou, V.; Rios, L.; Carle-Florence, T.L.; Chakravarty, S.; Kumar, A.; Graham, D.L.; Green, T.A.; Kirk, A.; Iñiguez, S.D.; et al. The Influence of ΔFosB in the Nucleus Accumbens on Natural Reward-Related Behavior. J. Neurosci. 2008, 28, 10272–10277. [Google Scholar] [CrossRef]
- Salinas-Velarde, I.D.; Bernal-Morales, B.; Pacheco-Cabrera, P.; Sánchez-Aparicio, P.; Pascual-Mathey, L.I.; Venebra-Muñoz, A. Lower ΔFosB Expression in the Dopaminergic System after Stevia Consumption in Rats Housed under Environmental Enrichment Conditions. Brain Res. Bull. 2021, 177, 172–180. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Cicerchi, C.; Li, N.; Roncal-Jimenez, C.A.; Ishimoto, T.; Le, M.; Garcia, G.E.; Thomas, J.B.; Rivard, C.J.; et al. Uric Acid Stimulates Fructokinase and Accelerates Fructose Metabolism in the Development of Fatty Liver. PLoS ONE 2012, 7, e47948. [Google Scholar] [CrossRef]
- Cohen, C.C.; Li, K.W.; Alazraki, A.L.; Beysen, C.; Carrier, C.A.; Cleeton, R.L.; Dandan, M.; Figueroa, J.; Knight-Scott, J.; Knott, C.J.; et al. Dietary Sugar Restriction Reduces Hepatic de Novo Lipogenesis in Adolescent Boys with Fatty Liver Disease. J. Clin. Investig. 2021, 131, e150996. [Google Scholar] [CrossRef]
- Douard, V.; Ferraris, R.P. Regulation of the Fructose Transporter GLUT5 in Health and Disease. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E227–E237. [Google Scholar] [CrossRef]
- Socha-Banasiak, A.; Sakowicz, A.; Gaj, Z.; Kolejwa, M.; Gach, A.; Czkwianianc, E. Intestinal Fructose Transporters GLUT5 and GLUT2 in Children and Adolescents with Obesity and Metabolic Disorders. Adv. Med. Sci. 2024, 69, 349–355. [Google Scholar] [CrossRef]
- Azad, M.B.; Sharma, A.K.; De Souza, R.J.; Dolinsky, V.W.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Lefebvre, D.L.; Sears, M.R.; et al. Association Between Artificially Sweetened Beverage Consumption During Pregnancy and Infant Body Mass Index. JAMA Pediatr. 2016, 170, 662. [Google Scholar] [CrossRef]
- Sewaybricker, L.E.; Schur, E.A.; Melhorn, S.J.; Campos, B.M.; Askren, M.K.; Nogueira, G.A.S.; Zambon, M.P.; Antonio, M.A.R.G.M.; Cendes, F.; Velloso, L.A.; et al. Initial Evidence for Hypothalamic Gliosis in Children with Obesity by Quantitative T2 MRI and Implications for Blood Oxygen-level Dependent Response to Glucose Ingestion. Pediatr. Obes. 2019, 14, e12486. [Google Scholar] [CrossRef] [PubMed]
- Valdearcos, M.; McGrath, E.; Brown Mayfield, S.; Folick, A.; Cheang, R.; Li, L.; Bachor, T.; Lippert, R.; Xu, A.; Koliwad, S. Microglia Mediate the Early-Life Programming of Adult Glucose Control. Cell Rep. 2024, 44, 115409. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Romero, H.N.; Biddinger, J.E.; Bedenbaugh, M.N.; Simerly, R. Microglia Are Required for Developmental Specification of AgRP Innervation in the Hypothalamus of Offspring Exposed to Maternal High-Fat Diet during Lactation. eLife 2025, 13, RP101391. [Google Scholar] [CrossRef] [PubMed]
- Shearrer, G.E.; Daniels, M.J.; Toledo-Corral, C.M.; Weigensberg, M.J.; Spruijt-Metz, D.; Davis, J.N. Associations among Sugar Sweetened Beverage Intake, Visceral Fat, and Cortisol Awakening Response in Minority Youth. Physiol. Behav. 2016, 167, 188–193. [Google Scholar] [CrossRef]
- Martin, A.A.; Hamill, L.R.; Davies, S.; Rogers, P.J.; Brunstrom, J.M. Energy-Dense Snacks Can Have the Same Expected Satiation as Sugar-Containing Beverages. Appetite 2015, 95, 81–88. [Google Scholar] [CrossRef]
- Serrano, J.; Smith, K.R.; Crouch, A.L.; Sharma, V.; Yi, F.; Vargova, V.; LaMoia, T.E.; Dupont, L.M.; Serna, V.; Tang, F.; et al. High-Dose Saccharin Supplementation Does Not Induce Gut Microbiota Changes or Glucose Intolerance in Healthy Humans and Mice. Microbiome 2021, 9, 11. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Rother, K.I. Trends in the Consumption of Low-Calorie Sweeteners. Physiol. Behav. 2016, 164, 446–450. [Google Scholar] [CrossRef]
- Lustig, R.H.; Schmidt, L.A.; Brindis, C.D. The Toxic Truth about Sugar. Nature 2012, 482, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L. Sugar Consumption, Metabolic Disease and Obesity: The State of the Controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef]
- Colley, D.L.; Castonguay, T.W. Effects of Sugar Solutions on Hypothalamic Appetite Regulation. Physiol. Behav. 2015, 139, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Dufour, S.; Mehal, W.Z.; Shulman, G.I. Glucagon Promotes Increased Hepatic Mitochondrial Oxidation and Pyruvate Carboxylase Flux in Humans with Fatty Liver Disease. Cell Metab. 2024, 36, 2359–2366.e3. [Google Scholar] [CrossRef]
- Greenhill, C. Not so Sweet—Artificial Sweeteners Can Cause Glucose Intolerance by Affecting the Gut Microbiota. Nat. Rev. Endocrinol. 2014, 10, 637. [Google Scholar] [CrossRef]
- Mandrioli, D.; Kearns, C.E.; Bero, L.A. Relationship between Research Outcomes and Risk of Bias, Study Sponsorship, and Author Financial Conflicts of Interest in Reviews of the Effects of Artificially Sweetened Beverages on Weight Outcomes: A Systematic Review of Reviews. PLoS ONE 2016, 11, e0162198. [Google Scholar] [CrossRef] [PubMed]
- InterAct Consortium; Romaguera, D.; Norat, T.; Wark, P.A.; Vergnaud, A.C.; Schulze, M.B.; van Woudenbergh, G.J.; Drogan, D.; Amiano, P.; Molina-Montes, E.; et al. Consumption of Sweet Beverages and Type 2 Diabetes Incidence in European Adults: Results from EPIC-InterAct. Diabetologia 2013, 56, 1520–1530. [Google Scholar] [CrossRef]
- Pacheco, L.S.; Tobias, D.K.; Haslam, D.E.; Drouin-Chartier, J.-P.; Li, Y.; Bhupathiraju, S.N.; Willett, W.C.; Ludwig, D.S.; Ebbeling, C.B.; Hu, F.B.; et al. Sugar-Sweetened or Artificially Sweetened Beverage Consumption, Physical Activity and Risk of Type 2 Diabetes in US Adults. Diabetologia 2025, 68, 792–800. [Google Scholar] [CrossRef]
- Malik, V.S.; Li, Y.; Pan, A.; De Koning, L.; Schernhammer, E.; Willett, W.C.; Hu, F.B. Long-Term Consumption of Sugar-Sweetened and Artificially Sweetened Beverages and Risk of Mortality in US Adults. Circulation 2019, 139, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
- López-Pascual, E.; Rienda, I.; Perez-Rojas, J.; Rapisarda, A.; Garcia-Llorens, G.; Jover, R.; Castell, J.V. Drug-Induced Fatty Liver Disease (DIFLD): A Comprehensive Analysis of Clinical, Biochemical, and Histopathological Data for Mechanisms Identification and Consistency with Current Adverse Outcome Pathways. Int. J. Mol. Sci. 2024, 25, 5203. [Google Scholar] [CrossRef]
- Emamat, H.; Ghalandari, H.; Tangestani, H.; Abdollahi, A.; Hekmatdoost, A. Artificial Sweeteners Are Related to Non-Alcoholic Fatty Liver Disease: Microbiota Dysbiosis as a Novel Potential Mechanism. EXCLI J. 2020, 19, 620–626. [Google Scholar] [CrossRef]
- Vos, M.B.; Abrams, S.H.; Barlow, S.E.; Caprio, S.; Daniels, S.R.; Kohli, R.; Mouzaki, M.; Sathya, P.; Schwimmer, J.B.; Sundaram, S.S.; et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J. Pediatr. Gastroenterol. Nutr. 2017, 64, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.-S.; Lin, W.-T.; Ting, P.-S.; Huang, C.-K.; Chen, P.-H.; Gonzalez, G.V.; Lin, H.-Y. Sugar-Sweetened Beverages and Artificially Sweetened Beverages Consumption and the Risk of Nonalcoholic Fatty Liver (NAFLD) and Nonalcoholic Steatohepatitis (NASH). Nutrients 2023, 15, 3997. [Google Scholar] [CrossRef]
- World Health Organization. Oral Health Data Portal. The Global Health Observatory. 2023. Available online: https://www.who.int/data/gho/data/themes/oral-health-data-portal (accessed on 10 July 2025).
- American Dental Association. Dental Erosion 2021. Available online: https://www.ada.org/resources/ada-library/oral-health-topics/dental-erosion (accessed on 9 September 2025).
- Reddy, A.; Norris, D.F.; Momeni, S.S.; Waldo, B.; Ruby, J.D. The pH of Beverages in the United States. J. Am. Dent. Assoc. 2016, 147, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Teng, A.M.; Jones, A.C.; Mizdrak, A.; Signal, L.; Genç, M.; Wilson, N. Impact of Sugar-sweetened Beverage Taxes on Purchases and Dietary Intake: Systematic Review and Meta-analysis. Obes. Rev. 2019, 20, 1187–1204. [Google Scholar] [CrossRef] [PubMed]
- Roberto, C.A.; Lawman, H.G.; LeVasseur, M.T.; Mitra, N.; Peterhans, A.; Herring, B.; Bleich, S.N. Association of a Beverage Tax on Sugar-Sweetened and Artificially Sweetened Beverages with Changes in Beverage Prices and Sales at Chain Retailers in a Large Urban Setting. JAMA 2019, 321, 1799. [Google Scholar] [CrossRef]
- Latner, J.D.; Stunkard, A.J. Getting Worse: The Stigmatization of Obese Children. Obes. Res. 2003, 11, 452–456. [Google Scholar] [CrossRef]
- Puhl, R.M.; Heuer, C.A. The Stigma of Obesity: A Review and Update. Obesity 2009, 17, 941–964. [Google Scholar] [CrossRef] [PubMed]
- Gortmaker, S.L.; Long, M.W.; Resch, S.C.; Ward, Z.J.; Cradock, A.L.; Barrett, J.L.; Wright, D.R.; Sonneville, K.R.; Giles, C.M.; Carter, R.C.; et al. Cost Effectiveness of Childhood Obesity Interventions. Am. J. Prev. Med. 2015, 49, 102–111. [Google Scholar] [CrossRef]
- Powell, L.M.; Wada, R.; Persky, J.J.; Chaloupka, F.J. Employment Impact of Sugar-Sweetened Beverage Taxes. Am. J. Public Health 2014, 104, 672–677. [Google Scholar] [CrossRef]
- Micha, R.; Karageorgou, D.; Bakogianni, I.; Trichia, E.; Whitsel, L.P.; Story, M.; Peñalvo, J.L.; Mozaffarian, D. Effectiveness of School Food Environment Policies on Children’s Dietary Behaviors: A Systematic Review and Meta-Analysis. PLoS ONE 2018, 13, e0194555. [Google Scholar] [CrossRef]
- Pallan, M.; Murphy, M.; Morrison, B.; Sitch, A.; Adamson, A.; Bartington, S.; Dobell, A.; Duff, R.; Frew, E.; Griffin, T.; et al. National School Food Standards in England: A Cross-Sectional Study to Explore Compliance in Secondary Schools and Impact on Pupil Nutritional Intake. Int. J. Behav. Nutr. Phys. Act. 2024, 21, 123. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.E.; Leardo, M.; Aneja, S.; Elbel, B. Effect of a School-Based Water Intervention on Child Body Mass Index and Obesity. JAMA Pediatr. 2016, 170, 220. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Ouardi, M.; Garcia-Llorens, G.; Valls-Belles, V. Childhood Obesity and Its Physiological Association with Sugar-Sweetened, Free-Sugar Juice, and Artificially Sweetened Beverages. Beverages 2025, 11, 137. https://doi.org/10.3390/beverages11050137
El Ouardi M, Garcia-Llorens G, Valls-Belles V. Childhood Obesity and Its Physiological Association with Sugar-Sweetened, Free-Sugar Juice, and Artificially Sweetened Beverages. Beverages. 2025; 11(5):137. https://doi.org/10.3390/beverages11050137
Chicago/Turabian StyleEl Ouardi, Meryem, Guillem Garcia-Llorens, and Victoria Valls-Belles. 2025. "Childhood Obesity and Its Physiological Association with Sugar-Sweetened, Free-Sugar Juice, and Artificially Sweetened Beverages" Beverages 11, no. 5: 137. https://doi.org/10.3390/beverages11050137
APA StyleEl Ouardi, M., Garcia-Llorens, G., & Valls-Belles, V. (2025). Childhood Obesity and Its Physiological Association with Sugar-Sweetened, Free-Sugar Juice, and Artificially Sweetened Beverages. Beverages, 11(5), 137. https://doi.org/10.3390/beverages11050137






