Chemical and Sensory Characterization of Carob Spirits According to Different Distillation Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Carob Syrup and Fermentation
2.3. Distillation Procedure
2.3.1. First Distillation
2.3.2. Second Distillation
- Copper Charantais alembic from Maritas Stills, S.L. (Palmeira, Spain): 9 L of the first distillate was distilled in a 10 L copper Charantais alembic as an individual batch (1 replicate). The base of the boiler was heated by an electrical heating source and tap water was used to cool the total condenser. The heater was set to obtain an average distillation rate of approximately 25 mL min−1. Based on the sensory analysis carried out by expert tasters of spirits, the distillation products were separated into three fractions: head, heart, and tail. The head fractions were defined as the first 300 mL; the following 2700 mL was collected as the heart fraction; and the following 1250 mL was collected as the tail fraction.
- Copper Charantais alembic with column from Maritas Stills, S.L. (Palmeira, Spain): 9 L of first distillate was distilled in a 10 L copper Charantais alembic with column as an individual batch (1 replicate). The base of the boiler was heated by an electrical heating source, and tap water was used to cool the total condenser. The heater was set to obtain an average distillation rate of approximately 26 mL min−1. On the basis of sensorial analysis, the distillation products were separated into three fractions: head, heart, and tail. The head fractions were defined as the first 200 mL; the following 3050 mL was collected as the heart fraction; and the following 1000 mL was collected as the tail fraction.
- Both distillation methods are characterized by similar operating parameters, such as energy consumption and processing time.
2.4. Chemical Analysis of Distillates
Sample Preparation for Liquid–Liquid Extraction for Gas Chromatography/Mass Spectrometry (GC/MS)
2.5. Sensory Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Carob Wort and Fermentation
3.2. Ethanol Yield for Alembic and Alembic with Column Distillation Systems
3.3. Carob Distillates Volatiles Content
3.3.1. Esters
3.3.2. Alcohols
3.3.3. Terpenes
3.3.4. Aldehydes and Ketones
3.3.5. Acetals
3.3.6. Furanic Compounds
3.3.7. Not Identified Compounds
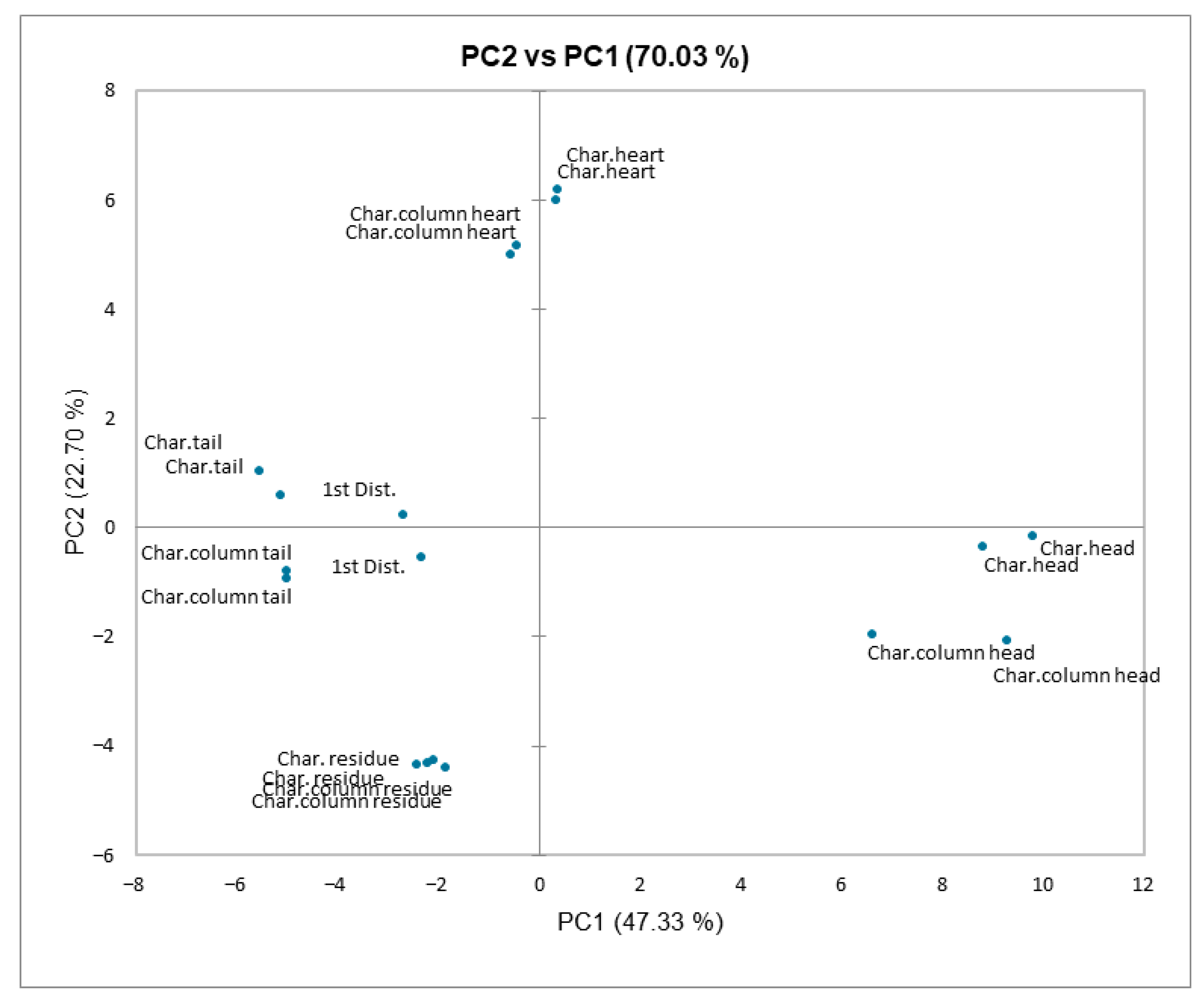
3.4. Principal Component Analysis (PCA)
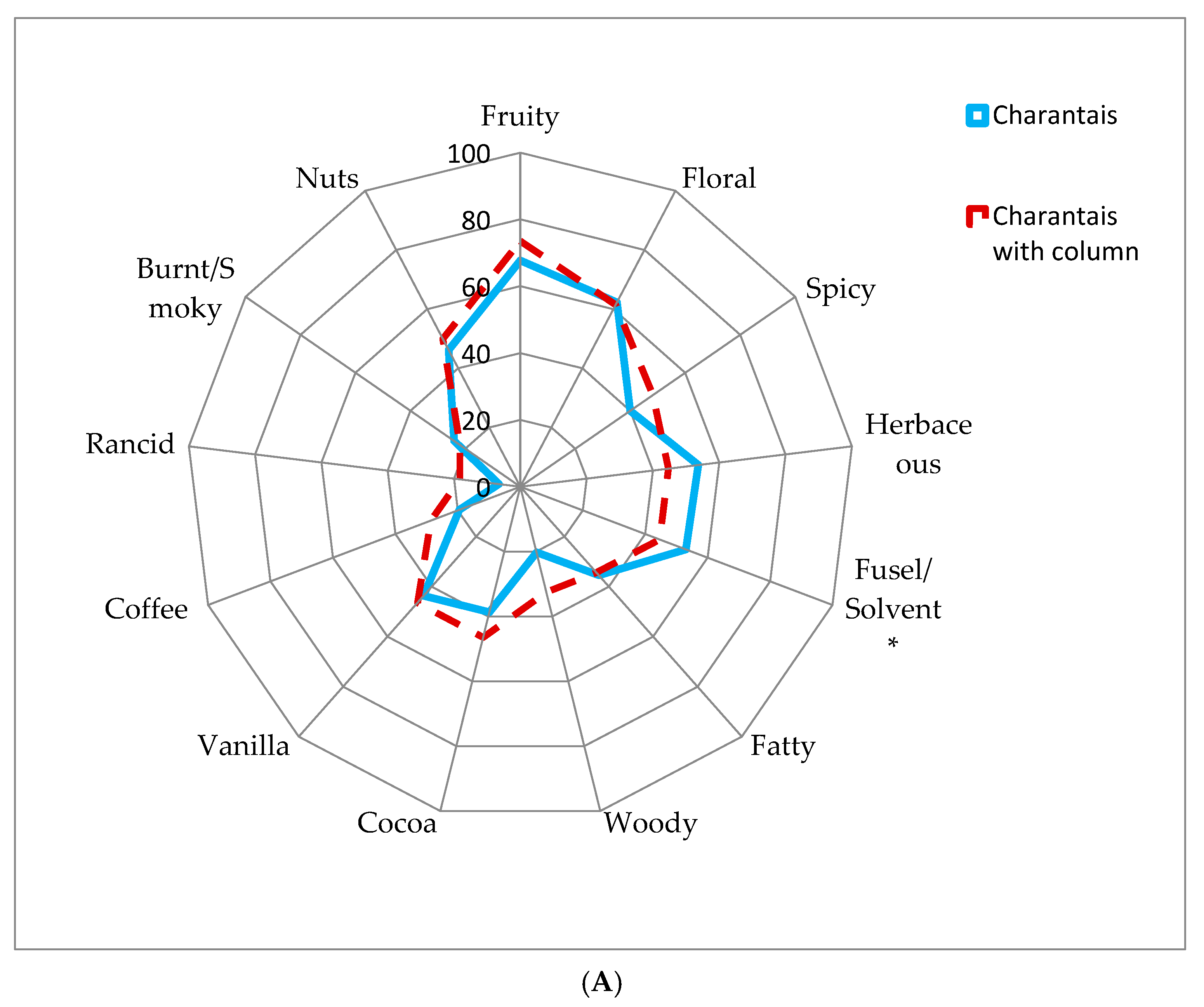
3.5. Sensory Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-AFC | Two-Alternative Forced Choice |
| S. | Saccharomyces |
| RID | Refractive Index Detector |
| UV-DAD | Ultraviolet Diode Array Detector |
| HPLC | High-Performance Liquid Chromatography |
| GC/MS | Gas Chromatography/Mass Spectrometry |
| m/z | Mass-to-charge ratio |
| SIM | Selected Ion Monitoring |
| NIST | National Institute of Standards and Technology |
| ANOVA | Analysis of Variance |
| ISO | Internal Organization for Standardization |
| EU | European Union |
| Dist. | Distillation |
| Char. | Charantais |
| PCA | Principal Component Analysis |
| PC1 | Principal Component 1 |
| PC2 | Principal Component 2 |
| QDA | Quantitative Descriptive Analysis |
References
- Sánchez-Segado, S.; Lozano, L.J.; de los Ríos, A.P.; Hernández-Fernández, F.J.; Godínez, C.; Juan, D. Process design and economic analysis of a hypothetical bioethanol production plant using carob pod as feedstock. Bioresour. Technol. 2012, 104, 324–328. [Google Scholar] [CrossRef]
- Korkmaz, N.; Akin, M.; Koc, A.; Eyduran, S.P.; Ilhan, G.; Sagbas, H.I.; Ercisli, S. Morphological and biochemical diversity among wild-grown carob trees (Ceratonia siliqua L.). Folia Hort. 2020, 32, 69–78. [Google Scholar] [CrossRef]
- Rovira, M.; Ninot, A.; Romero, A.; Batlle, I. Varietats de garrofer dels Països Catalans: Caracterització i estructura poblacional. Quad. Agrar. 2021, 51, 7–30. [Google Scholar] [CrossRef]
- Benkovic, M.; Bosiljkov, T.; Semic, A.; Ježek, D.; Srecec, S. Influence of Carob Flour and Carob Bean Gum on Rheological Properties of Cocoa and Carob Pastry Fillings. Foods 2019, 8, 66. [Google Scholar] [CrossRef]
- Bouzouita, N.; Khaldi, A.; Zgoulli, S.; Chebil, L.; Chekki, R.; Chaabouni, M.M.; Thonart, P. The analysis of crude and purified locust bean gum: A comparison of samples from different carob tree populations in Tunisia. Food Chem. 2007, 101, 1508–1515. [Google Scholar] [CrossRef]
- Goulas, V.; Stylos, E.; Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Functional Components of Carob Fruit: Linking the Chemical and Biological Space. Int. J. Mol. Sci. 2016, 17, 1875. [Google Scholar] [CrossRef]
- Roseiro, L.B.; Duarte, L.C.; Oliveira, D.L.; Roque, R.; Bernardo-Gil, M.G.; Martins, A.I.; Sepúlveda, C.; Almeida, J.; Meireles, M.; Gírio, F.M.; et al. Supercritical, ultrasound and conventional extracts from carob (Ceratonia siliqua L.) biomass: Effect on the phenolic profile and antiproliferative activity. Ind. Crops Prod. 2013, 47, 132–138. [Google Scholar] [CrossRef]
- Sánchez, S.; Lozano, L.J.; Godínez, C.; Juan, D.; Pérez, A.; Hernández, F.J. Carob pod as a feedstock for the production of bioethanol in Mediterranean areas. Appl. Energy 2010, 87, 3417–3424. [Google Scholar] [CrossRef]
- Richane, A.; Mansour, R.B.; Megdiche, W.; Ksouri, R.; Khaoula, A.; Nizar, M.; Hanen, B.I. Variability of phenolic compounds and antioxidant activities of ten Ceratonia siliqua L. provenances. Biochem. Syst. Ecol. 2022, 104, 104486. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Agapiou, A.; Giannopoulos, S.; Kokkinofta, R. Nutritional characterization of carobs and traditional carob products. Food Sci. Nutr. 2018, 6, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Yatmaz, E. Continuous ethanol fermentation from carob pod extract medium at different hydraulic residence time (HRT). Gida-J. Food 2019, 44, 93–103. [Google Scholar] [CrossRef]
- López, F.; Rodríguez-Bencomo, J.J.; Orriols, I.; Pérez-Correa, J.R. Chapter 10—Fruit brandies. In Science and Technology of Fruit Wine Production; Kosseva, M.R., Joshi, V.K., Panesar, P.S., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 531–556. [Google Scholar]
- Mrvčić, J.; Srečec, S.; Hanousek Čiča, K.; Devčić, B.; Petravić-Tominac, V.; Trontel, A.; Bosiljkov, T.; Blažić, M.; Čanadi Jurešić, G.; Stanzer, D. Characterization of the Fermentation Process and Aroma Profile of Carob Brandy. In Proceedings of the 10th Central European Congress on Food, Online, 10–11 June 2021; CE-Food 2020. Brka, M., Sarić, Z., Oručević Žuljević, S., Omanović-Mikličanin, E., Taljić, I., Biber, L., Mujčinović, A., Eds.; Springer: Cham, Switzerland, 2020; pp. 68–87. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Dantas, M.; Romano, A. Influence of Carob Pod (Ceratonia siliqua L.) Variety and Processing on the Antioxidant Capacity and Total Phenolic Content of Carob Liquors. In INCREaSE: Proceedings of the 1st International Congress on Engineering and Sustainability in the XXI Century-INCREaSE, Faro, Portugal, 11–13 October 2017; Mortal, A., Aníbal, J., Monteiro, J., Sequeira, C., Semião, J., Moreira da Silva, M., Oliveira, M., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 216–226. [Google Scholar] [CrossRef]
- Hanousek Čiča, K.; Mrvčić, J.; Srečec, S.; Filipan, K.; Blažić, M.; Stanzer, D. Physicochemical and aromatic characterization of carob macerates produced by different maceration conditions. Food Sci. Nutr. 2020, 8, 942–954. [Google Scholar] [CrossRef]
- ISO 11035:1994; Sensory Analysis—Identification and Selection of Descriptors for Establishing a Sensory Profile by a Multidimensional Approach. ISO (International Organization for Standardization): Geneva, Switzerland, 2015.
- Kemp, S.E.; Hollowood, T.; Hort, J. Sensory Evaluation: A Practical Handbook, 1st ed.; Willey Blackwell: Chichester, UK, 2009; pp. 147–157. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2, The Chemistry of Wine Stabilization and Treatments, 2nd ed.; John Willey & Sons Ltd.: West Sussex, UK, 2006; Volume 2, pp. 53–66. [Google Scholar]
- Li, X.; Lim, S.; Yu, B.; Curran, P.; Liu, S. Impact of pulp on the chemical profile of mango wine. S. Afr. J. of Enol. Vitic. 2013, 34, 119–128. [Google Scholar] [CrossRef][Green Version]
- Duarte, W.F.; Dias, D.R.; Oliveira, J.M.; Teixeira, J.A.; e Silva, J.B.A.; Schwan, R.F. Characterization of different fruit wines made from cacao, cupuassu, gabiroba, jaboticaba and umbu. LWT-Food Sci. Technol. 2010, 43, 1564–1572. [Google Scholar] [CrossRef]
- Regmi, U.; Palma, M.; Barroso, C.G. Direct determination of organic acids in wine and wine-derived products by Fourier transform infrared (FT-IR) spectroscopy and chemometric techniques. Anal. Chim. Acta 2012, 732, 137–144. [Google Scholar] [CrossRef]
- Miljić, U.D.; Puškaš, V.S. Influence of fermentation conditions on production of plum (Prunus domestica L.) wine: A response surface methodology approach. Hem. Ind. 2014, 68, 199–206. [Google Scholar] [CrossRef]
- Garai-Ibabe, G.; Ibarburu, I.; Berregi, I.; Claisse, O.; Lonvaud-Funel, A.; Irastorza, A.; Duenas, M.T. Glycerol metabolism and bitterness producing lactic acid bacteria in cidermaking. Int. J. Food Microbiol. 2008, 121, 253–261. [Google Scholar] [CrossRef]
- Llompart, B.; Dalmau, E.; Umaña, M.; Femenia, A. Physicochemical Characterization and Antioxidant Properties of Cellulose-Rich Extracts Obtained from Carob (Ceratonia siliqua L.) Pulp for Preparation of Cellulose-Rich Gels. Gels 2025, 11, 145. [Google Scholar] [CrossRef]
- Maksimovic, V.; Dragisic Maksimovic, J. Chapter 4 Composition, Nutritional, and Therapeutic Values of Fruit and Berry Wines. In Science and Technology of Fruit Wine Production; Kosseva, M.R., Joshi, V.K., Panesar, P.S., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 178–179. [Google Scholar]
- Botelho, G.; Anjos, O.; Estevinho, L.; Caldeira, I. Methanol in Grape Derived, Fruit and Honey Spirits: A Critical Review on Source, Quality Control, and Legal Limits. Processes 2020, 8, 1609. [Google Scholar] [CrossRef]
- García-Llobodanin, L.; Roca, J.; López, J.R.; Pérez-Correa, J.R.; López, F. The lack of reproducibility of different distillation techniques and its impact on pear spirit composition. Int. J. Food Sci. Technol. 2011, 46, 1956–1963. [Google Scholar] [CrossRef]
- Arrieta-Garay, Y.; López-Vázquez, C.; Blanco, P.; Perez-Correa, J.R.; Orriols, I.; Lopez, F. Kiwi spirits with stronger floral and fruity characters were obtained with a packed column distillation system. J. Inst. Brew. 2014, 120, 111–118. [Google Scholar] [CrossRef]
- Wang, X.; Cui, W.; Guo, W.; Sun, B.; Huang, M.; Li, J.; Li, H.; Meng, N. Separation techniques for manufacturing fruit spirits: From traditional distillation to advanced pervaporation process. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13278. [Google Scholar] [CrossRef]
- Spaho, N. Chapter 6 Distillation Techniques in the Fruit Spirits Production. In Distillation—Innovative Applications and Modeling; Fernandes-Mendes, M., Ed.; IntechOpen: London, UK, 2017; pp. 129–152. [Google Scholar] [CrossRef]
- Sercan, D.; Yahya, K.A. Characterization of Aroma Profile of Bogma, Traditional Homemade Turkish Spirit. Greener J. Agric. Sci. 2017, 7, 263–270. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Galego, L.; Pérez-Santín, E.; Romano, A. Production method and varietal source influence the volatile profiles of spirits prepared from fig fruits (Ficus carica L.). Eur. Food Res. Technol. 2018, 244, 2213–2229. [Google Scholar] [CrossRef]
- Stanojević, J.; Karabegović, I.; Danilović, B.; Zvezdanović, J.; Stanojević, L.; Cvetković, D. Comparison of Gas Chromatography–Mass Spectrometry and Headspace-Solid phase Microextraction Methods for the Qualitative and Semi-Quantitative Determination of Aroma Compounds in Fruit-Based Spirits (Rakija) from Serbia. J. Anal. Chem. 2024, 79, 81–94. [Google Scholar] [CrossRef]
- Coelho, E.; Pinto, M.; Bastos, R.; Cruz, M.; Nunes, C.; Rocha, S.M.; Coimbra, M.A. Concentrate Apple Juice Industry: Aroma and Pomace Valuation as Food Ingredients. Appl. Sci. 2021, 11, 2443. [Google Scholar] [CrossRef]
- Da Porto, C. Grappa: Production, sensory properties and market development. In Alcoholic Beverages Sensory Evaluation and Consumer Research; Piggott, J., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2012; Volume 3, pp. 299–314. [Google Scholar] [CrossRef]
- Cortés, S.; Rodríguez, R.; Salgado, J.M.; Domínguez, J.M. Comparative study between Italian and Spanish grape marc spirits in terms of major volatile compounds. Food Control. 2011, 22, 673–680. [Google Scholar] [CrossRef]
- López-Vázquez, C.; García-Llobodanin, L.; Pérez-Correa, J.R.; López, F.; Blanco, P.; Orriols, I. Aromatic Characterization of Pot Distilled Kiwi Spirits. J. Agric. Food. Chem. 2012, 60, 2242–2247. [Google Scholar] [CrossRef]
- Diéguez, S.C.; De la Peña, M.L.; Gómez, E. Volatile Composition and Sensory Characters of Commercial Galician Orujo Spirits. J. Agric. Food Chem. 2005, 53, 6759–6765. [Google Scholar] [CrossRef]
- Masino, F.; Montevecchi, G.; Riponi, C.; Antonelli, A. Composition of some commercial grappas (grape marc spirit): The anomalous presence of 1,1-diethoxy-3-methylbutane: A case study. Eur. Food Res. Technol. 2009, 228, 565–569. [Google Scholar] [CrossRef]
- Spaho, N.; Gaši, F.; Leitner, E.; Blesić, M.; Akagić, A.; Žuljević, S.O.; Kurtović, M.; Ratković, D.Ð.; Murtić, M.S.; Akšić, M.F.; et al. Characterization of Volatile Compounds and Flavor in Spirits of Old Apple and Pear Cultivars from the Balkan Region. Foods 2021, 10, 1258. [Google Scholar] [CrossRef]
- Okaru, A.O.; Lachenmeier, D.W. The Food and Beverage Occurrence of Furfuryl Alcohol and Myrcene—Two Emerging Potential Human Carcinogens? Toxics 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Stanzer, D.; Hanousek Čiča, K.; Blesić, M.; Smajić Murtić, M.; Mrvčić, J.; Spaho, N. Alcoholic Fermentation as a Source of Congeners in Fruit Spirits. Foods 2023, 12, 1951. [Google Scholar] [CrossRef]

| Carob Composition Prior Distillation | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fructose (g/L) | Sucrose (g/L) | Glucose (g/L) | Malic Acid (g/L) | Succinic Acid (g/L) | Glycerol (g/L) | Methanol (g/L) | Ethanol (g/L) | |
| Carob wort | 71.83 ± 1.73 | 4.04 ± 0.08 | 69.89 ± 1.68 | <LOD a (0.625) | <LOD a (0.625) | 0.75 ± 0.03 | n.d. b | n.d. b |
| Fermented carob wort | 2.74 ± 0.09 | 3.28 ± 0.10 | 0.43± 0.01 | 2.55 ± 0.10 | 4.99 ± 0.16 | 6.22 ± 0.19 | 1.01 ± 0.02 | 60.46 ± 1.59 |
| Distillation Type | Amount Distilled (L) | Alcoholic Strength (% vol) | Distilled Volume (L) | Alcoholic Heart Strength (% vol) | Theoretical Ethanol Yield (L a.a.) | Absolute Heart Ethanol Yield (L a.a.) | Heart Ethanol Yield (%) |
|---|---|---|---|---|---|---|---|
| Alembic first distillation * | 79.1 * | 7.3 * | 20.68 * | 26.3 * | 5.76 * | 5.5 * | 94.9 * |
| Heart alembic second distillation | 9 | 26.4 | 2.70 | 65.8 | 2.4 | 1.8 | 74.8 |
| Heart alembic with column second distillation | 9 | 26.3 | 3.05 | 66.6 | 2.4 | 2.0 | 85.8 |
| Compound | 1st Dist. ** 24 | 2nd Dist. Char. *** Head 24 | 2nd Dist. Char. Heart 24 | 2nd Dist. Char. Tail 24 | 2nd Dist. Char. Residue 24 | 2nd Dist. Char. Column Head 24 | 2nd Dist. Char. Column Heart 24 | 2nd Dist. Char. Column Tail 24 | 2nd Dist. Char. Column Residue 24 |
|---|---|---|---|---|---|---|---|---|---|
| 1-propanol a | 0.22 ± 0.03 | 0.49 ± 0.02 | 0.49 ± 0.05 | 0.10 ± 0.01 | n.d. | 0.58 ± 0.00 | 0.54 ± 0.06 | 0.08 ± 0.01 | n.d. |
| Isobutanol a | 3.65 ± 0.58 | 10.38 ± 0.39 | 7.86 ± 0.36 | 0.31 ± 0.01 | n.d. | 12.66 ± 0.97 | 8.09 ± 0.76 | 0.26 ± 0.08 | n.d. |
| 1-Butanol a | n.d. | 0.09 ± 0.00 | 0.10 ± 0.01 | n.d. | n.d. | 0.10 ± 0.01 | 0.11 ± 0.02 | n.d. | n.d. |
| 3-Methyl-1-pentanol a,b,c | n.d. | 0.07 ± 0.00 | 0.07 ± 0.00 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 1-Hexanol a | n.d. | 0.06 ± 0.00 | 0.07 ± 0.00 | n.d. | n.d. | 0.06 ± 0.00 | 0.07 ± 0.00 | n.d. | n.d. |
| Phenylethyl alcohol a,b,c | 28.71 ± 4.95 | 2.06 ± 0.43 | 9.08 ± 0.78 | 50.93 ± 2.02 | 19.00 ± 3.47 | 2.54 ± 0.04 | 9.59 ± 0.23 | 59.31 ± 1.09 | 27.91 ± 2.23 |
| 2-Heptanol a,b,c | 0.09 ± 0.01 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.14 ± 0.00 | n.d. | n.d. |
| ∑ Alcohols | 32.67 ± 5.58 | 13.15 ± 0.85 | 17.66 ± 1.22 | 51.34 ± 2.05 | 19.00 ± 3.47 | 15.93 ± 1.03 | 18.54 ± 1.07 | 59.65 ± 1.18 | 27.91 ± 2.23 |
| Ethyl acetate a | 18.39 ± 1.18 | 89.27 ± 16.89 | 16.08 ± 1.26 | 1.18 ± 0.01 | 0.15 ± 0.15 | 99.95 ± 2.20 | 17.85 ± 0.40 | 2.18 ± 0.35 | 0.24 ± 0.01 |
| Ethylbutanoate a | 0.11 ± 0.01 | 1.10 ± 0.06 | 0.07 ± 0.00 | n.d. | n.d. | 1.40 ± 0.21 | 0.08 ± 0.00 | n.d. | n.d. |
| Isobutylacetate a,b,c | n.d. | 1.04 ± 0.04 | n.d. | n.d. | n.d. | 1.29 ± 0.01 | n.d. | n.d. | n.d. |
| Ethyl hexanoate a | 0.22 ± 0.00 | 3.19 ± 0.21 | 0.19 ± 0.01 | 0.04 ± 0.00 | n.d. | 3.65 ± 0.42 | 0.20 ± 0.00 | n.d. | n.d. |
| Ethyl octanoate a | 0.31 ± 0.02 | 4.89 ± 0.10 | 0.31 ± 0.06 | 0.11 ± 0.01 | n.d. | 4.78 ± 1.41 | 0.33 ± 0.03 | 0.13 ± 0.01 | n.d. |
| Ethyl decanoate a | 0.13 ± 0.02 | 3.29 ± 0.12 | 0.21 ± 0.07 | 0.05 ± 0.01 | n.d. | 2.21 ± 0.87 | 0.14 ± 0.01 | 0.03 ± 0.00 | n.d. |
| Ethyl 9-decenoate a | n.d. | 0.13 ± 0.00 | n.d. | n.d. | n.d. | 0.10 ± 0.04 | n.d. | n.d. | n.d. |
| 2-Phenylethyl acetate a,b,c | 0.28 ± 0.01 | 0.21 ± 0.01 | 0.62 ± 0.02 | 0.39 ± 0.02 | n.d. | 0.11 ± 0.02 | 0.54 ± 0.03 | 0.17 ± 0.01 | n.d. |
| Ethyl benzenepropanoate a,b,c | 0.03 ± 0.00 | n.d. | 0.07 ± 0.00 | n.d. | n.d. | n.d. | 0.05 ± 0.00 | n.d. | n.d. |
| Ethyl dodecanoate a | n.d. | 0.27 ± 0.01 | 0.02 ± 0.01 | n.d. | n.d. | 0.17 ± 0.11 | n.d. | n.d. | n.d. |
| Ethyl tetradecanoate a,b,c | n.d. | 0.06 ± 0.00 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ethyl 9-hexadecenoate a,b,c | n.d. | 0.24 ± 0.00 | 0.05 ± 0.02 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ethyl hexadecanoate a | n.d. | 0.63 ± 0.02 | n.d. | n.d. | n.d. | 0.36 ± 0.25 | 0.03 ± 0.01 | n.d. | n.d. |
| Ethyl isobutyrate a | n.d. | n.d. | n.d. | 0.06 ± 0.00 | n.d. | n.d. | n.d. | 0.06 ± 0.01 | n.d. |
| Diethyl succinate a,b,c | n.d. | n.d. | n.d. | 0.03 ± 0.00 a | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ethyl 2-hydroxy-4-methyl pentanoate a,b,c | n.d. | 0.02 ± 0.00 | 0.03 ± 0.00 | n.d. | n.d. | n.d. | 0.03 ± 0.00 | n.d. | n.d. |
| Ethyl lactate a,b,c | 0.41 ± 0.07 | 0.11 ± 0.00 | n.d. | 0.99 ± 0.07 | 0.09 ± 0.09 | n.d. | 0.42 ± 0.02 | 1.03 ± 0.10 | 0.22 ± 0.05 |
| ∑ Esters | 19.87 ± 1.30 | 104.45 ± 17.47 | 17.66 ± 1.45 | 2.85 ± 0.12 | 0.24 ± 0.24 | 114.01 ± 5.55 | 19.66 ± 0.51 | 3.60 ± 0.48 | 0.46 ± 0.07 |
| cis-Linalooloxide a,b,c | 0.87 ± 0.07 | 0.74 ± 0.01 | 1.64 ± 0.02 | 0.90 ± 0.03 | n.d. | 0.44 ± 0.05 | 1.71 ± 0.06 | 0.50 ± 0.00 | n.d. |
| trans-Linalooloxide a,b,c | 0.51 ± 0.05 | 0.30 ± 0.01 | 0.84 ± 0.01 | 0.86 ± 0.04 | 0.06 ± 0.02 | 0.16 ± 0.02 | 0.89 ± 0.00 | 0.60 ± 0.02 | 0.01 ± 0.01 |
| p-Menth-1-en-9-al a,b,c | 0.07 ± 0.01 | n.d. | 0.14 ± 0.00 | 0.08 ± 0.00 | n.d. | n.d. | 0.13 ± 0.01 | n.d. | n.d. |
| D-nerolidol a,b,c | n.d. | n.d. | 0.04 ± 0.01 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2,3-Dihydrofarnesol a,c | n.d. | n.d. | 0.17 ± 0.03 | n.d. | n.d. | n.d. | 0.10 ± 0.01 | n.d. | n.d. |
| Linalool a,b | n.d. | 0.01 ± 0.00 | 0.01 ± 0.00 | n.d. | n.d. | 0.01 ± 0.00 | 0.01 ± 0.00 | n.d. | n.d. |
| Alfa-Terpineol a,c | n.d. | 0.04 ± 0.00 a | 0.02 ± 0.00 a | 0.01 ± 0.00 a | 0.00 ± 0.00 * | 0.03 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.01 | n.d. |
| ∑ Terpenes | 1.44 ± 0.13 | 1.09 ± 0.02 | 2.86 ± 0.07 | 1.84 ± 0.07 | 0.06 ± 0.02 | 0.64 ± 0.08 | 2.84 ± 0.08 | 1.12 ± 0.03 | 0.01 ± 0.01 |
| 3-methylbutanal + 2-methylbutanal a,b,c | 0.48 ± 0.02 | 5.20 ± 0.03 | 0.35 ± 0.04 | n.d. | n.d. | 7.21 ± 0.43 | 0.39 ± 0.01 | n.d. | n.d. |
| Benzaldehyde a,b,c | 0.09 ± 0.01 | 0.26 ± 0.01 | 0.45 ± 0.01 | 0.25 ± 0.01 | 0.03 ± 0.03 | 0.22 ± 0.03 | 0.45 ± 0.00 | 0.05 ± 0.01 | 0.08 ± 0.00 |
| (E)-2-Hexenal a,b,c | n.d. | n.d. | n.d. | 0.02 ± 0.00 | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2-Cyclopentene-1,4-dione a,b,c | 0.08 ± 0.01 | n.d. | 0.07 ± 0.00 | 0.17 ± 0.01 | n.d. | n.d. | 0.09 ± 0.00 | 0.10 ± 0.01 | n.d. |
| ∑ Aldehydes + Ketones | 0.64 ± 0.04 | 5.46 ± 0.04 | 0.87 ± 0.05 | 0.45 ± 0.02 | 0.03 ± 0.03 | 7.43 ± 0.45 | 0.93 ± 0.02 | 0.15 ± 0.02 | 0.08 ± 0.00 |
| Acetal a | 9.53 ± 1.17 | 78.28 ± 7.38 | 16.70 ± 1.93 | 0.85 ± 0.07 | 1.55 ± 0.24 | 91.40 ± 1.68 | 17.65 ± 0.11 | 1.48 ± 0.33 | 1.28 ± 0.04 |
| Isobutanal diethyl acetal a | n.d. | 2.96 ± 0.18 | 0.14 ± 0.01 | n.d. | n.d. | 3.51 ± 0.46 | 0.14 ± 0.01 | n.d. | n.d. |
| 3-Methyl butanal diethyl acetal a | 0.15 ± 0.01 | 3.31 ± 0.17 | 0.18 ± 0.01 | n.d. | n.d. | 3.80 ± 0.56 | 0.19 ± 0.01 | n.d. | n.d. |
| 2-Methylbutanal diethyl acetal a | n.d. | 1.01 ± 0.03 | n.d. | n.d. | n.d. | 1.25 ± 0.19 | 0.05 ± 0.00 | n.d. | n.d. |
| 1,1,3-triethoxypropane a,c | 0.04 ± 0.00 | 0.10 ± 0.00 | 0.05 ± 0.00 | 0.03 ± 0.00 | n.d. | 0.12 ± 0.01 | 0.05 ± 0.00 | n.d. | n.d. |
| Phenylacetaldehyde diethyl acetal a,b,c | 0.08 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.00 | n.d. | n.d. | n.d. | 0.04 ± 0.00 | n.d. | n.d. |
| ∑ Acetals | 9.79 ± 1.19 | 85.70 ± 7.76 | 17.11 ± 1.95 | 0.88 ± 0.08 | 1.55 ± 0.24 | 100.08 ± 2.89 | 18.12 ± 0.14 | 1.48 ± 0.33 | 1.28 ± 0.04 |
| Furfurylformate a | 0.37 ± 0.03 | 1.36 ± 0.12 | 0.69 ± 0.04 | 0.32 ± 0.01 | n.d. | 1.61 ± 0.08 | 0.64 ± 0.02 | 0.25 ± 0.02 | n.d. |
| Dihydro-2-methyl-3(2H)-furanone a,c | 0.44 ± 0.06 | 0.26 ± 0.01 | 0.56 ± 0.09 | 0.91 ± 0.09 | n.d. | n.d. | 0.68 ± 0.03 | 0.98 ± 0.04 | 0.07 ± 0.01 |
| 1-(2-furanyl)-1-propanone a,b,c | 0.17 ± 0.02 | 0.11 ± 0.01 | 0.27 ± 0.00 | 0.31 ± 0.00 | n.d. | 0.07 ± 0.01 | 0.30 ± 0.01 | 0.23 ± 0.00 | n.d. |
| unknown/furanic a | 0.22 ± 0.01 | 1.83 ± 0.09 | 0.92 ± 0.06 | n.d. | n.d. | 2.09 ± 0.20 | 0.91 ± 0.05 | 0.07 ± 0.01 | n.d. |
| 5-Methyl furfural a,c | 1.78 ± 0.26 | 0.65 ± 0.03 | 1.89 ± 0.09 | 4.65 ± 0.12 | 0.23 ± 0.23 | 0.36 ± 0.02 | 2.25 ± 0.00 | 4.64 ± 0.02 | 0.31 ± 0.00 |
| Furfural a | 3.74 ± 0.59 | 2.16 ± 0.05 | 4.78 ± 0.41 | 7.07 ± 0.85 | 0.26 ± 0.26 | 1.82 ± 0.04 | 6.21 ± 0.06 | 7.66 ± 0.11 | 0.38 ± 0.06 |
| Acetylfuran a | 1.97 ± 0.30 | 0.83 ± 0.02 | 2.22 ± 0.12 | 4.50 ± 0.42 | 0.21 ± 0.21 | 0.55 ± 0.06 | 2.69 ± 0.03 | 4.77 ± 0.11 | 0.28 ± 0.02 |
| Furfuryl alcohol a,b,c | 0.32 ± 0.06 | n.d. | n.d. | 0.38 ± 0.03 | 0.08 ± 0.08 | n.d. | 0.13 ± 0.01 | 0.43 ± 0.02 | 0.27 ± 0.07 |
| ∑ Furanic compounds | 9.02 ± 1.34 | 7.20 ± 0.33 | 11.34 ± 0.81 | 18.14 ± 1.53 | 0.79 ± 0.79 | 6.50 ± 0.40 | 13.80 ± 0.21 | 19.04 ± 0.34 | 1.31 ± 0.16 |
| not identified a | n.d. | n.d. | n.d. | 0.58 ± 0.07 | n.d. | n.d. | n.d. | 0.58 ± 0.00 | n.d. |
| not identified a,b,c | 0.09 ± 0.00 | n.d. | 0.11 ± 0.00 | n.d. | n.d. | n.d. | n.d. | 0.15 ± 0.01 | n.d. |
| not identified/mixture a,b,c | n.d. | 1.47 ± 0.08 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| ∑ Not identified | 0.09 ± 0.00 | 1.47 ± 0.08 | 0.11 ± 0.00 | 0.58 ± 0.07 | n.d. | n.d. | n.d. | 0.73 ± 0.01 | n.d. |
| Charantais Alembic | Charantais Alembic with Column | |
|---|---|---|
| Sensory properties | Slightly higher intensity in herbaceous and fusel/chemical/solvent notes. | Slightly higher intensity in spicy, woody, cocoa, coffee, and rancid notes. |
| Both distillates are characterized by higher intensity in floral, fruity, and alcoholic notes. The effect of the distillation system did not show significant differences between both distillation products regarding their organoleptic properties. | ||
| Heart Ethanol yield | 74.8% | 85.8% |
| Alcoholic heart strength | 65.8% | 66.6% |
| Chemical properties heart fractions | Alcohols concentration: | Alcohols concentration: |
| 17.66 ± 1.22 mg/L | 18.54 ± 1.07 mg/L | |
| 3-Methyl-1-pentanol detected | 3-Methyl-1-pentanol detected | |
| 2-Heptanol not detected | 2-Heptanol detected | |
| Esters concentration: | Esters concentration: | |
| 17.66 ± 1.45 mg/L | 19.66 ± 0.51 mg/L | |
| Terpenes concentration: | Terpenes concentration: | |
| 2.86 ± 0.07 mg/L | 2.84 ± 0.08 mg/L | |
| D-nerolidol detected | D-nerolidol not detected | |
| Aldehydes and ketones concentration: | Aldehydes and ketones concentration: | |
| 0.87 ± 0.05 mg/L | 0.93 ± 0.02 mg/L | |
| Acetals concentration: | Acetals concentration: | |
| 17.11 ± 1.95 mg/L | 18.12 ± 0.14 mg/L | |
| Furanic compounds concentration: | Furanic compounds concentration: | |
| 11.34 ± 0.81 mg/L | 13.80 ± 0.21 mg/L | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Colom, C.; Andazola, J.; Bargalló-Guinjoan, C.; Rodríguez-Bencomo, J.J.; López, F. Chemical and Sensory Characterization of Carob Spirits According to Different Distillation Systems. Beverages 2025, 11, 119. https://doi.org/10.3390/beverages11040119
López-Colom C, Andazola J, Bargalló-Guinjoan C, Rodríguez-Bencomo JJ, López F. Chemical and Sensory Characterization of Carob Spirits According to Different Distillation Systems. Beverages. 2025; 11(4):119. https://doi.org/10.3390/beverages11040119
Chicago/Turabian StyleLópez-Colom, Clara, Julio Andazola, Carles Bargalló-Guinjoan, Juan José Rodríguez-Bencomo, and Francisco López. 2025. "Chemical and Sensory Characterization of Carob Spirits According to Different Distillation Systems" Beverages 11, no. 4: 119. https://doi.org/10.3390/beverages11040119
APA StyleLópez-Colom, C., Andazola, J., Bargalló-Guinjoan, C., Rodríguez-Bencomo, J. J., & López, F. (2025). Chemical and Sensory Characterization of Carob Spirits According to Different Distillation Systems. Beverages, 11(4), 119. https://doi.org/10.3390/beverages11040119







