Ripening Kinetics and Grape Chemistry of Virginia Petit Manseng
Abstract
1. Introduction
1.1. Grape Ripening Kinetics
1.2. Petit Manseng Grapes
1.3. Project Objective
2. Materials and Methods
2.1. Grape Sample Collection and Processing
2.1.1. Grape Collection
2.1.2. Grape Processing
2.2. Analysis of Grape Samples
2.2.1. Grape Acids and pH
Titratable Acidity (TA)
Grape pH Measurements
Malic Acid
2.2.2. Grape Sugars
Total Soluble Solids (TSS)
Glucose and Fructose
2.2.3. Calculations of Alcohol Content and Grape Maturity
Estimation of Alcohol Content
Grape Maturity and Wine Quality Correlation
2.3. Statistics
2.3.1. Boosted Tree Models
2.3.2. Segmented Regression
2.3.3. Linear Discriminant Analysis (LDA)
3. Results and Discussion
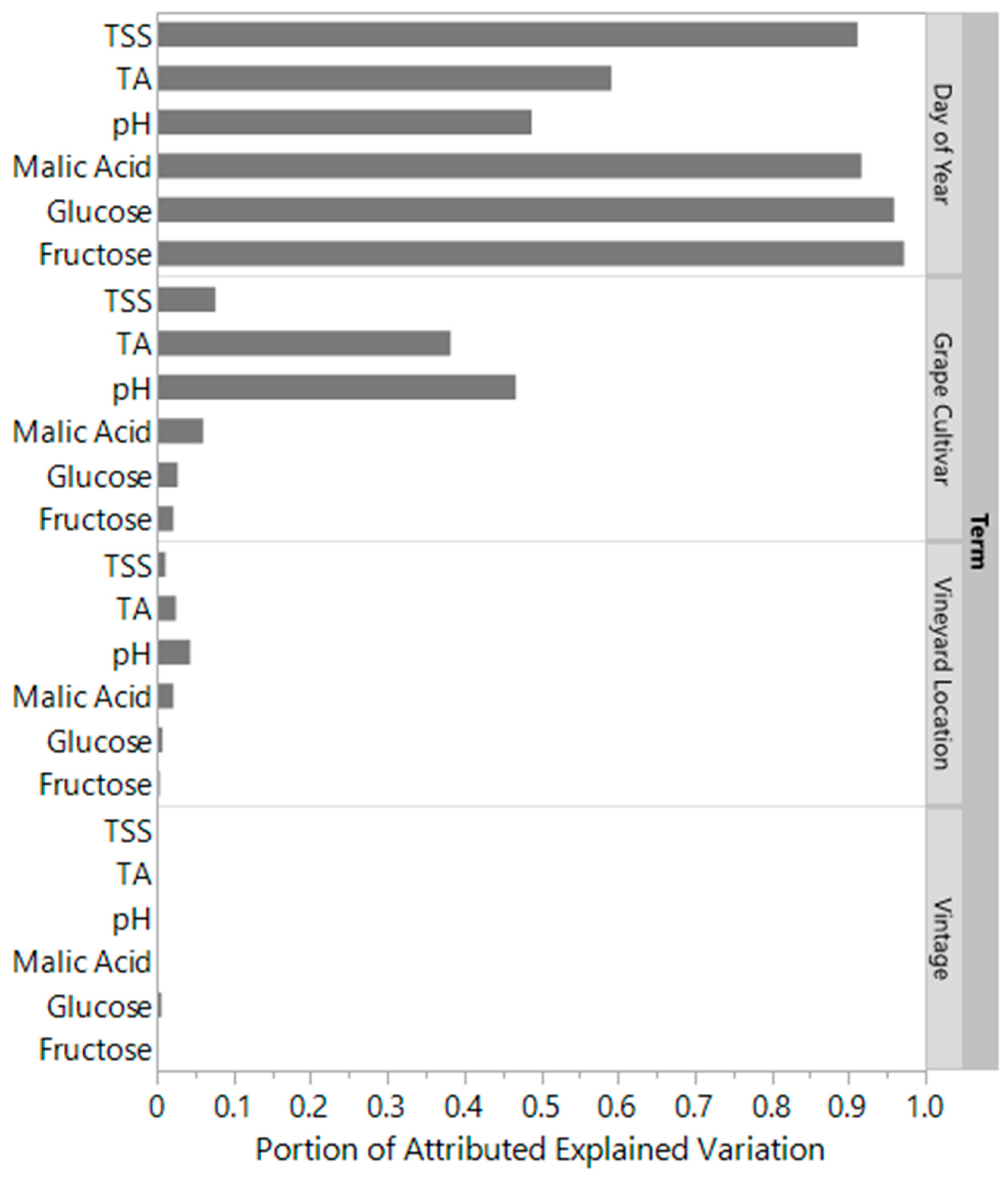
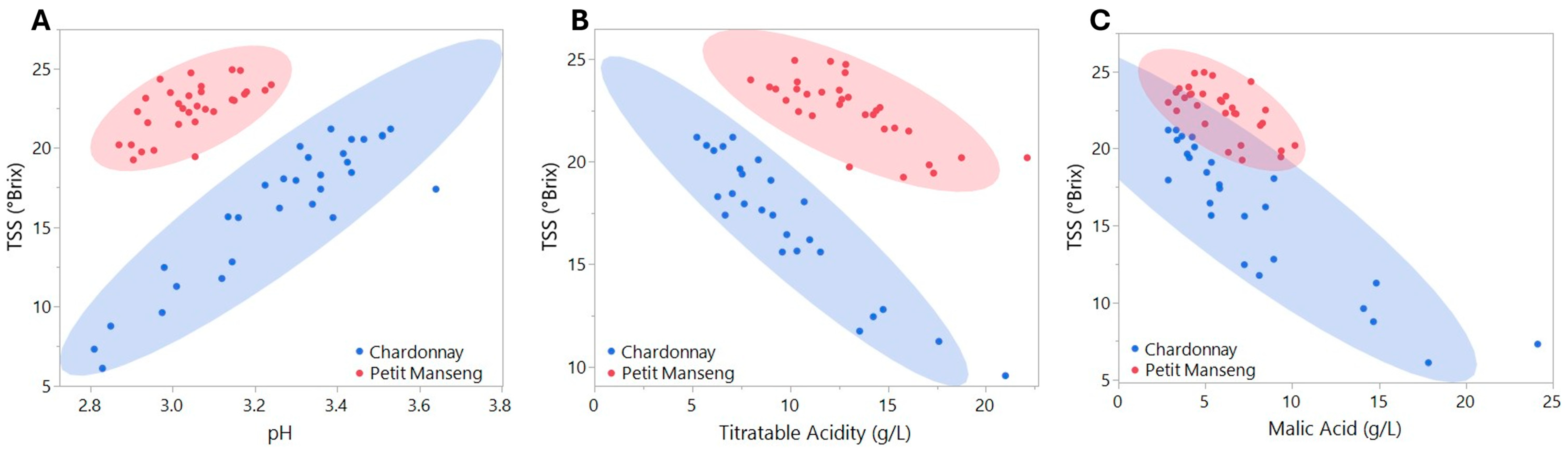
3.1. Grape Chemistry
Boosted Tree Models
3.2. Ripening Kinetics
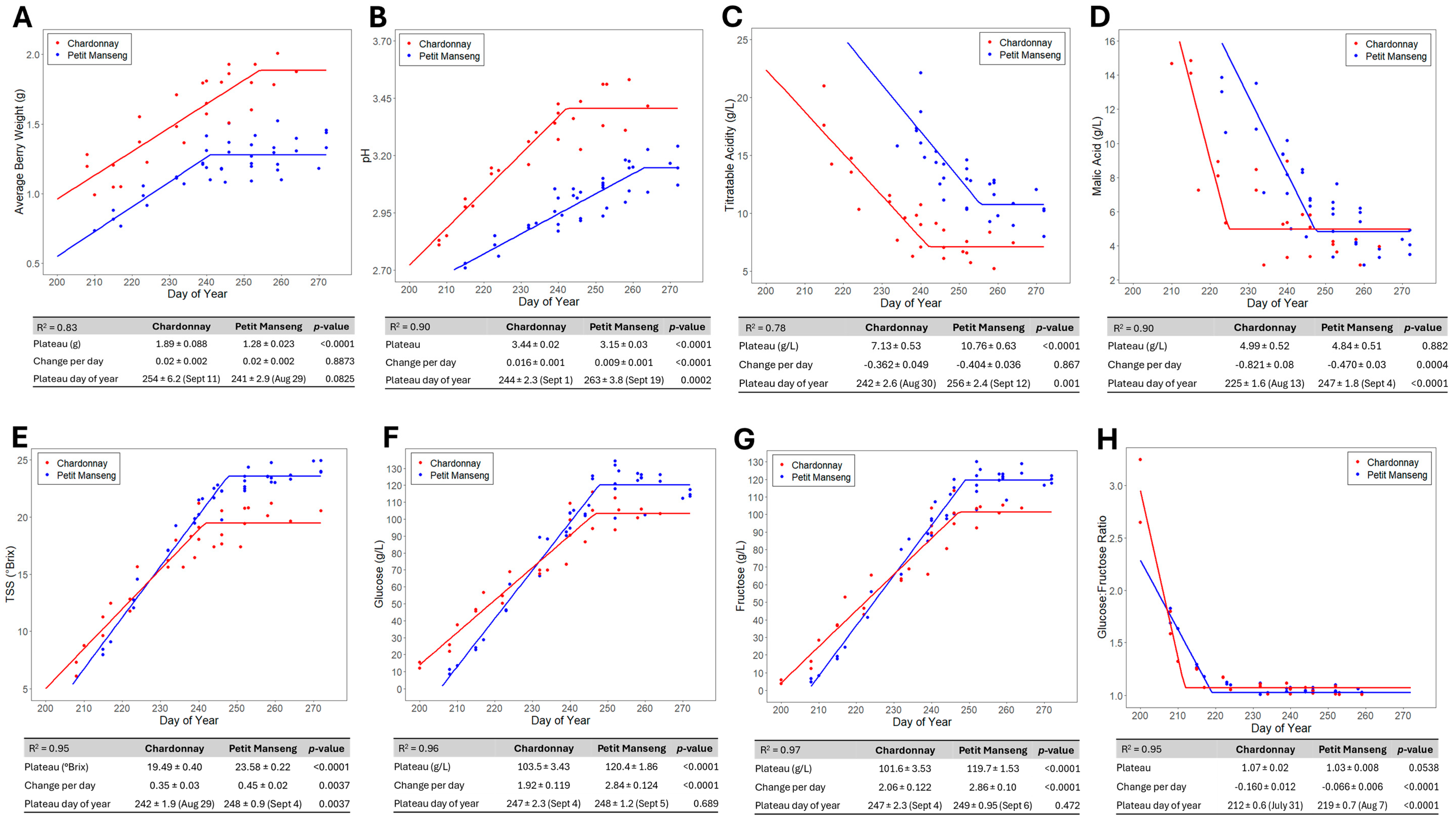
3.2.1. Grape Berry Weights
3.2.2. Grape Acid Kinetics
3.2.3. Grape Sugar Kinetics
3.3. Fundamental Differences in Chardonnay and Petit Manseng Grapes
3.4. Implications for Winemakers
3.4.1. Grape Chemistry and Fermentation to Make Dry-Style Wine
3.4.2. Predicting Wine Quality Based on Grape Chemistry
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| %ABV | Percent alcohol by volume |
| LDA | Linear discriminant analysis |
| TA | Titratable acidity |
| TSS | Total soluble solids |
References
- Moreno-Arribas, M.V.; Polo, M.C. Wine Chemistry and Biochemistry, 1st ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Kunter, B.; Unal, O.B.; Keskin, S.; Hatterman-Valenti, H.; Kaya, O. Comparison of the sugar and organic acid components of seventeen table grape varieties produced in Ankara (Türkiye): A study over two consecutive seasons. Front. Plant Sci. 2024, 15, 1321210. [Google Scholar] [CrossRef] [PubMed]
- Perrot, N.; Tonda, A.; Brunetti, I.; Guillemin, H.; Perret, B.; Goulet, E.; Guerin, L.; Picque, D. A decision-support system to predict grape berry quality and wine potential for a Chenin vineyard. Comput. Electron. Agric. 2022, 200, 107167. [Google Scholar] [CrossRef]
- Bigard, A.; Romieu, C.; Sire, Y.; Veyret, M.; Ojeda, H.; Torregrosa, L. The kinetics of grape ripening revisited through berry density sorting. OENO One 2019, 53, 709–724. [Google Scholar] [CrossRef]
- Kennedy, J. Understanding Grape Berry Development. Practical Winery and Vineyard. 2002. Available online: https://jcast.fresnostate.edu/ve/documents/outreach/Understanding-page%2014.pdf (accessed on 28 April 2025).
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking, 1st ed.; Springer: Boston, MA, USA, 1999. [Google Scholar]
- Plane, R.; Mattick, L.; Weirs, L. An Acidity Index For The Taste Of Wines. Am. J. Enol. Vitic. 1980, 31, 265–268. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry, 2nd ed; Wiley: Hoboken, NJ, USA, 2024. [Google Scholar]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.; Tavares, R.; Sousa, M.; Agasse, A.; Delrot, S.; Geros, H. Biochemical Changes throughout Grape Berry Development and Fruit and Wine Quality. Food 2007, 1, 1–22. Available online: https://hdl.handle.net/1822/6820 (accessed on 28 April 2025).
- Bisson, L. In Search of Optimal Grape Maturity. Pract. Winery Vineyard J. 2001, 23, 32–43. [Google Scholar]
- Jordao, A.; Vilela, A.; Cosme, F. From Sugar of Grape to Alcohol of Wine: Sensorial Impact of Alcohol in Wine. Beverages 2015, 1, 292–310. [Google Scholar] [CrossRef]
- Du Plessis, C.S. Optimum Maturity and Quality Parameters in Grapes: A Review. S. Afr. J. Enol. Vitic. 1984, 5, 34–42. [Google Scholar] [CrossRef]
- Wolf, T.K. Wine Grape Production Guide for Eastern North America, 1st ed.; Plant and Life Sciences Publishing: Ithaca, NY, USA, 2008. [Google Scholar]
- Virginia Vineyards Association. Virginia Wine 2023 Commercial Wine Grape Report. 2024. Available online: https://virginiavineyardsassociation.org/commercial-report/ (accessed on 10 June 2025).
- Robinson, J. The Oxford Companion to Wine, 4th ed.; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Robinson, J.; Harding, J.; Vouillamoz, J. Wine Grapes: A Complete Guide to 1368 Vine Varieties, including their Origins and Flavours, 1st ed.; Penguin Books: London, UK, 2012. [Google Scholar]
- U.S. Wine Market: Size, Share & Trends Analysis Report, By Product (Table Wine, Dessert Wine Sparkling Wine), By Distribution (On-Trade, Off-Trade), Competitive Landscape And Segment Forecasts, 2018–2025; Grand Review Research: San Francisco, CA, USA, 2018.
- McMillan, R. State of the US Wine Industry 2020. 2021. Available online: https://www.svb.com/globalassets/library/uploadedfiles/reports/svb-2020-state-of-the-wine-industry-report-final.pdf (accessed on 28 April 2025).
- Ting, J.H. Trial Evaluates Optimum Time To Harvest Dry Petit Manseng. 2020. Available online: https://virginiavineyardsassociation.org/wp-content/uploads/2020/12/Winter2020_GrapePress.pdf (accessed on 28 April 2025).
- Bisson, L. Stuck and sluggish fermentations. Am. J. Enol. Vitic. 1999, 50, 107–119. [Google Scholar] [CrossRef]
- Virginia Vineyards Association. Virginia Wine 2021 Commercial Wine Grape Report. 2022. Available online: https://virginiavineyardsassociation.org/commercial-reports/ (accessed on 10 June 2025).
- Virginia Vineyards Association. Virginia Wine 2022 Commercial Wine Grape Report. 2023. Available online: https://virginiavineyardsassociation.org/commercial-reports/ (accessed on 10 June 2025).
- AOAC International. Official Methods of Analysis of AOAC International, 22nd ed.; Official Method 962.12; AOAC International: Gaithersburg, MD, USA, 2023. [Google Scholar]
- ETS labs. Choosing Glucose + Fructose Instead of Brix. Available online: https://www.etslabs.com/publications/publication/8 (accessed on 9 April 2025).
- Elith, J.; Leathwick, J.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 14 April 2025).
- Elzhov, T.V.; Mullen, K.M.; Spiess, A.; Bolker, B. minpack.lm: R Interface to the Levenberg-Marquardt Nonlinear Least-Squares Algorithm Found in MINPACK, Plus Support for Bounds_. R package Version 1.2-4. 2023. Available online: https://CRAN.R-project.org/package=minpack.lm (accessed on 14 April 2025).
- Xanthopoulos, P.; Pardalos, P.M.; Trafalis, T.B. Linear Discriminant Analysis. In Robust Data Mining, 1st ed.; SpringerBriefs in Optimization; Springer: New York, NY, USA, 2013; pp. 27–33. [Google Scholar]
- Deloire, P. The concept of berry sugar loading. WineLand Mag. 2011, 257, 93–95. [Google Scholar]
- Liu, X.Y.; Jia, B.; Sun, X.Y.; Ai, J.Y.; Wang, L.H.; Wang, C.; Zhao, F.; Zhan, J.C.; Huang, W.D. Effect of Initial pH on Growth Characteristics and Fermentation Properties of Saccharomyces cerevisiae. J. Food Sci. 2015, 80, M800–M808. [Google Scholar] [CrossRef] [PubMed]
- Ribereau-Gayon, P.; Dubourdieu, D.; Doneche, B.; Lonvaud, A. Handbook of Enology Volume 1: The Microbiology of Wine and Vinifications, 1st ed.; Wiley: West Sussex, UK, 2006. [Google Scholar]
- Shayanfar, S.; Bodbodak, S. Effect of different physicochemical de-tartration methods on red grape juice quality. J. Food Sci. Technol. 2014, 51, 4084–4089. [Google Scholar] [CrossRef] [PubMed]
- Zoecklein, B. Enology Notes #108. 2005. Available online: https://www.enology.fst.vt.edu/EN/108.html (accessed on 10 April 2025).
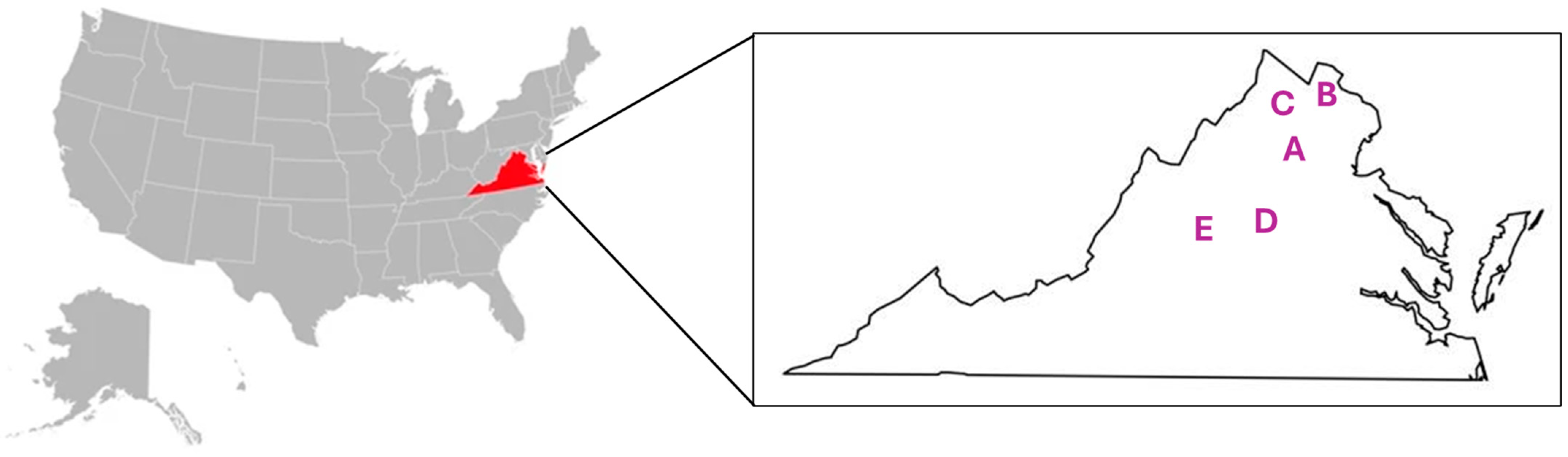

| Vineyard a | Region | AVA | Latitude | Longitude | Acreage | Type |
|---|---|---|---|---|---|---|
| A | Shenendoah Valley | n/a b | 38.88070 | −78.06541 | 1.5 | Commercial |
| B | Northern Virginia | Middleburg AVA | 39.17987 | −77.72390 | 2.0 | Commercial |
| C | Shenendoah Valley | Shenendoah Valley AVA | 39.14478 | −78.29782 | <0.5 | Experimental |
| D | Central Virginia | Monticello AVA | 38.18495 | −78.14491 | 2.5 | Commercial |
| E | Central Virginia | Monticello AVA | 38.06244 | −78.72850 | 2.0 | Commercial |
| Chardonnay | Petit Manseng | |||
|---|---|---|---|---|
| Physiol. Maturity | Plateau | Physiol. Maturity | Plateau | |
| Day of year a | 241 (29 August) | 244 (1 September) | 247 (4 September) | 262 (19 September) |
| °Brix | 19.5 | 19.5 | 23.6 | 23.6 |
| pH | 3.33 | 3.44 | 3.00 | 3.16 |
| TA (g/L) | 7.49 | 7.13 | 13.99 | 10.76 |
| °Brix ∗ pH2 b | 224 | 231 | 212 | 236 |
| °Brix/TA c | 26.0 | 27.3 | 16.9 | 22.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ting, J.H.; Surratt, A.A.; Moccio, L.E.; Sandbrook, A.M.; Chang, E.A.; Cladis, D.P. Ripening Kinetics and Grape Chemistry of Virginia Petit Manseng. Beverages 2025, 11, 108. https://doi.org/10.3390/beverages11040108
Ting JH, Surratt AA, Moccio LE, Sandbrook AM, Chang EA, Cladis DP. Ripening Kinetics and Grape Chemistry of Virginia Petit Manseng. Beverages. 2025; 11(4):108. https://doi.org/10.3390/beverages11040108
Chicago/Turabian StyleTing, Joy H., Alicia A. Surratt, Lauren E. Moccio, Ann M. Sandbrook, Elizabeth A. Chang, and Dennis P. Cladis. 2025. "Ripening Kinetics and Grape Chemistry of Virginia Petit Manseng" Beverages 11, no. 4: 108. https://doi.org/10.3390/beverages11040108
APA StyleTing, J. H., Surratt, A. A., Moccio, L. E., Sandbrook, A. M., Chang, E. A., & Cladis, D. P. (2025). Ripening Kinetics and Grape Chemistry of Virginia Petit Manseng. Beverages, 11(4), 108. https://doi.org/10.3390/beverages11040108







