Unveiling the Regional Identity of Madeira Wine: Insights from Saccharomyces cerevisiae Strains Using Interdelta Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Equipment and Software
2.3. PCR-Interdelta Optimization
2.3.1. Commercial Yeast Strains
2.3.2. Yeast Isolation
2.3.3. DNA Isolation and Purification
2.3.4. Optimization of PCR-Interdelta Conditions
2.4. PCR-Interdelta Analysis of Samples from DRM
2.4.1. Sampling
2.4.2. Microfermentation and Yeast Isolation
2.4.3. PCR-Interdelta Analysis of Samples
3. Results
3.1. Spontaneous Microfermentation and Yeast Isolation
3.2. Optimized PCR-Interdelta Conditions
3.3. PCR-Interdelta Analysis of Endogenous Isolates
4. Discussion
PCR-Interdelta Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perestrelo, R.; Silva, C.; Gonçalves, C.; Castillo, M.; Câmara, J.S. An Approach of the Madeira Wine Chemistry. Beverages 2020, 6, 12. [Google Scholar] [CrossRef]
- Pinheiro de Carvalho, M.Â.A.; Ragonezi, C.; Oliveira, M.C.O.; Reis, F.; Macedo, F.L.; de Freitas, J.G.R.; Nóbrega, H.; Ganança, J.F.T. Anticipating the Climate Change Impacts on Madeira’s Agriculture: The Characterization and Monitoring of a Vine Agrosystem. Agronomy 2022, 12, 2201. [Google Scholar] [CrossRef]
- Direção Regional de Estatística. Estatísticas da Agricultura e Pesca da Região Autónoma da Madeira; Direção Regional de Estatística: Funchal, Portugal, 2024; ISBN 978-989-9188-06-8. Available online: https://estatistica.madeira.gov.pt/download-now/economica/agricultura-floresta-e-pesca/prod-veg-prd-animal-pesca-pt/prod-vegetal-publicacoes-pt/send/74-producao-vegetal-publicacoes/17512-agricultura-e-pesca-2023pdf.html (accessed on 3 May 2025).
- Perestrelo, R.; Silva, C.; Câmara, J.S. Madeira Wine Volatile Profile. A Platform to Establish Madeira Wine Aroma Descriptors. Molecules 2019, 24, 3028. [Google Scholar] [CrossRef]
- Miranda, A.; Pereira, V.; Jardim, H.; Malfeito-Ferreira, M.; Marques, J.C. Impact of Non-Saccharomyces Yeast Fermentation in Madeira Wine Chemical Composition. Processes 2023, 11, 482. [Google Scholar] [CrossRef]
- Perestrelo, R.; Jaouhari, Y.; Abreu, T.; Castillo, M.M.; Travaglia, F.; Pereira, J.A.M.; Câmara, J.S.; Bordiga, M. The Fingerprint of Fortified Wines—From the Sui Generis Production Processes to the Distinctive Aroma. Foods 2023, 12, 2558. [Google Scholar] [CrossRef]
- de Celis, M.; Ruiz, J.; Martín-Santamaría, M.; Alonso, A.; Marquina, D.; Navascués, E.; Gómez-Flechoso, M.; Belda, I.; Santos, A. Diversity of Saccharomyces cerevisiae Yeasts Associated to Spontaneous and Inoculated Fermenting Grapes from Spanish Vineyards. Lett. Appl. Microbiol. 2019, 68, 580–588. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, J.; Song, Y.; Zang, X.; Wang, G.; Pei, Y.; Song, Y.; Qin, Y.; Liu, Y. Yeast Diversity during Spontaneous Fermentations and Oenological Characterisation of Indigenous Saccharomyces cerevisiae for Potential as Wine Starter Cultures. Microorganisms 2022, 10, 1455. [Google Scholar] [CrossRef]
- Pinto, C.; Pinho, D.; Cardoso, R.; Custódio, V.; Fernandes, J.; Sousa, S.; Pinheiro, M.; Egas, C.; Gomes, A.C. Wine Fermentation Microbiome: A Landscape from Different Portuguese Wine Appellations. Front. Microbiol. 2015, 6, 905. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.S.; Hatziloukas, E. Saccharomyces cerevisiae and Its Industrial Applications. AIMS Microbiol 2020, 6, 1–31. [Google Scholar] [CrossRef]
- Xufre, A.; Albergaria, H.; Gírio, F.; Spencer-Martins, I. Use of Interdelta Polymorphisms of Saccharomyces cerevisiae Strains to Monitor Population Evolution during Wine Fermentation. J. Ind. Microbiol. Biotechnol. 2011, 38, 127–132. [Google Scholar] [CrossRef]
- Querol, A.; Huerta, T.; Barrio, E.; Ramón, D. Dry Yeast Strain for use in Fermentation of Alicante Wines: Selection and DNA Patterns. J. Food Sci. 1992, 57, 183–185. [Google Scholar] [CrossRef]
- Capece, A.; Siesto, G.; Poeta, C.; Pietrafesa, R.; Romano, P. Indigenous Yeast Population from Georgian Aged Wines Produced by Traditional “Kakhetian” Method. Food Microbiol. 2013, 36, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Schuller, D.; Valero, E.; Dequin, S.; Casal, M. Survey of Molecular Methods for the Typing of Wine Yeast Strains. FEMS Microbiol. Lett. 2004, 231, 19–26. [Google Scholar] [CrossRef]
- Ness, F.; Lavallee, F.; Dubourdieu, D.; Aigle, M.; Dulaub, L. Identification of Yeast Strains Using the Polymerase Chain Reaction. J. Sci. Food Agric. 1993, 62, 89–94. [Google Scholar] [CrossRef]
- Lazar, I., Jr.; Lazar, I., Sr. GelAnalyzer, Veision 19.1. 2010. Available online: www.gelanalyzer.com (accessed on 14 September 2023).
- Castillo, M.; da Silva, E.; Câmara, J.S.; Khadem, M. Molecular Identification and VOMs Characterization of Saccharomyces cerevisiae Strains Isolated from Madeira Region Winery Environments. Processes 2020, 8, 1058. [Google Scholar] [CrossRef]
- Löoke, M.; Kristjuahan, K.; Kristjuhan, A. Extraction of Genomic DNA from Yeasts for PCR-Based Applications. Biotechniques 2011, 50, 325–328. [Google Scholar] [CrossRef]
- Legras, J.L.; Karst, F. Optimisation of Interdelta Analysis for Saccharomyces cerevisiae Strain Characterisation. FEMS Microbiol. Lett. 2003, 221, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology—The Microbiology of Wine and Vinifications, 2nd ed.; John Wiley and Sons, Ltd.: West Sussex, UK, 2005; Volume 1, p. viii. [Google Scholar]
- Zhang, J.; Plowman, J.E.; Tian, B.; Clerens, S.; On, S.L.W. Genotyping and Phenotyping of Indigenous Saccharomyces cerevisiae from a New Zealand Organic Winery and Commercial Sources Using Inter-Delta and MALDI-TOF MS Typing. Microorganisms 2024, 12, 1299. [Google Scholar] [CrossRef]
- Querol, A.; Barrio, E.; Huerta, T.; Ramón, D. Molecular Monitoring of Wine Fermentations Conducted by Active Dry Yeast Strains. Appl. Environ. Microbiol. 1992, 58, 2948–2953. [Google Scholar] [CrossRef]
- Querol, A.; Barrio, E.; Ramón, D. Population Dynamics of Natural Saccharomyces Strains during Wine Fermentation. Int. J. Food Microbiol. 1994, 21, 315–323. [Google Scholar] [CrossRef]
- Versavaud, A.; Courcoux, P.; Roulland, C.; Dulau, L.; Hallet, J.N. Genetic Diversity and Geographical Distribution of Wild Saccharomyces cerevisiae Strains from the Wine-Producing Area of Charentes, France. Appl. Environ. Microbiol. 1995, 61, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Espinar, M.T.; Llopis, S.; Querol, A.; Barrio, E. Molecular Identification and Characterization of Wine Yeasts. In Molecular Wine Microbiology; Carrascosa, A.V., Muñoz, R., González, R., Eds.; Elsevier Science Publishing Co Inc.: New York, NY, USA, 2011; pp. 111–141. ISBN 9780123750211. [Google Scholar]
- Schuller, D.; Alves, H.; Dequin, S.; Casal, M. Ecological Survey of Saccharomyces cerevisiae Strains from Vineyards in the Vinho Verde Region of Portugal. FEMS Microbiol. Ecol. 2005, 51, 167–177. [Google Scholar] [CrossRef]
- Cappello, M.S.; Bleve, G.; Grieco, F.; Dellaglio, F.; Zacheo, G. Characterization of Saccharomyces cerevisiae Strains Isolated from Must of Grape Grown in Experimental Vineyard. J. Appl. Microbiol. 2004, 97, 1274–1280. [Google Scholar] [CrossRef]
- Tristezza, M.; Fantastico, L.; Vetrano, C.; Bleve, G.; Corallo, D.; Mita, G.; Grieco, F. Molecular and Technological Characterization of Saccharomyces cerevisiae Strains Isolated from Natural Fermentation of Susumaniello Grape Must in Apulia, Southern Italy. Int. J. Microbiol. 2014, 2014, 897428. [Google Scholar] [CrossRef] [PubMed]
- Bougreau, M.; Ascencio, K.; Bugarel, M.; Nightingale, K.; Loneragan, G. Yeast Species Isolated from Texas High Plains Vineyards and Dynamics during Spontaneous Fermentations of Tempranillo Grapes. PLoS ONE 2019, 14, 0216246. [Google Scholar] [CrossRef]
- Van Der Westhuizen, T.J.; Augustyn, O.P.H.; Pretorius, I.S. Geographical Distribution of Indigenous Saccharomyces cerevisiae Strains Isolated from Vineyards in the Coastal Regions of the Western Cape in South Africa. S. Afr. J. Enol. Vitic. 2000, 21, 3–9. [Google Scholar] [CrossRef][Green Version]
- Del Mónaco, S.M.; Curilen, Y.; Maturano, R.; Simes, A.; Caballero, A. The Use of Indigenous Yeast to Develop High-Quality Patagonian Chapter Book. In Grape and Wine Biotechnology; Morato, A., Loira, I., Eds.; InTechOpen: London, UK, 2016. [Google Scholar]
- Feng, L.; Wang, J.; Ye, D.; Song, Y.; Qin, Y.; Liu, Y. Yeast Population Dynamics during Spontaneous Fermentation of Icewine and Selection of Indigenous Saccharomyces cerevisiae Strains for the Winemaking in Qilian, China. J. Sci. Food Agric. 2020, 100, 5385–5394. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Hao, M.; Pretorius, I.S.; Chen, S. Identification of Yeast Population Dynamics of Spontaneous Fermentation in Beijing Wine Region, China. Ann. Microbiol. 2009, 59, 69–76. [Google Scholar] [CrossRef]
- Van Zandycke, S.; Bertrand, D.; Daniel, H.; Douglas, P.; Helber, J.; Jenkins, D.; Kanda, H.; Pawlowski, K.; Rainieri, S.; Rosti, J.; et al. PCR Applications to Brewing: Differentiation of Brewing Yeast Strains by PCR Fingerprinting. Am. Soc. Brew. Chemists J. 2008, 66, 266–270. [Google Scholar] [CrossRef]
- Le Jeune, C.; Erny, C.; Demuyter, C.; Lollier, M. Evolution of the Population of Saccharomyces cerevisiae from Grape to Wine in a Spontaneous Fermentation. Food Microbiol. 2006, 23, 709–716. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, G.; Siesto, G.; Romano, P. Diversity of Saccharomyces cerevisiae Yeasts Associated to Spontaneously Fermenting Grapes from an Italian “Heroic Vine-Growing Area”. Food Microbiol. 2012, 31, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lappa, I.K.; Kachrimanidou, V.; Pateraki, C.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Indigenous Yeasts: Emerging Trends and Challenges in Winemaking. Curr. Opin. Food Sci. 2020, 32, 133–143. [Google Scholar] [CrossRef]
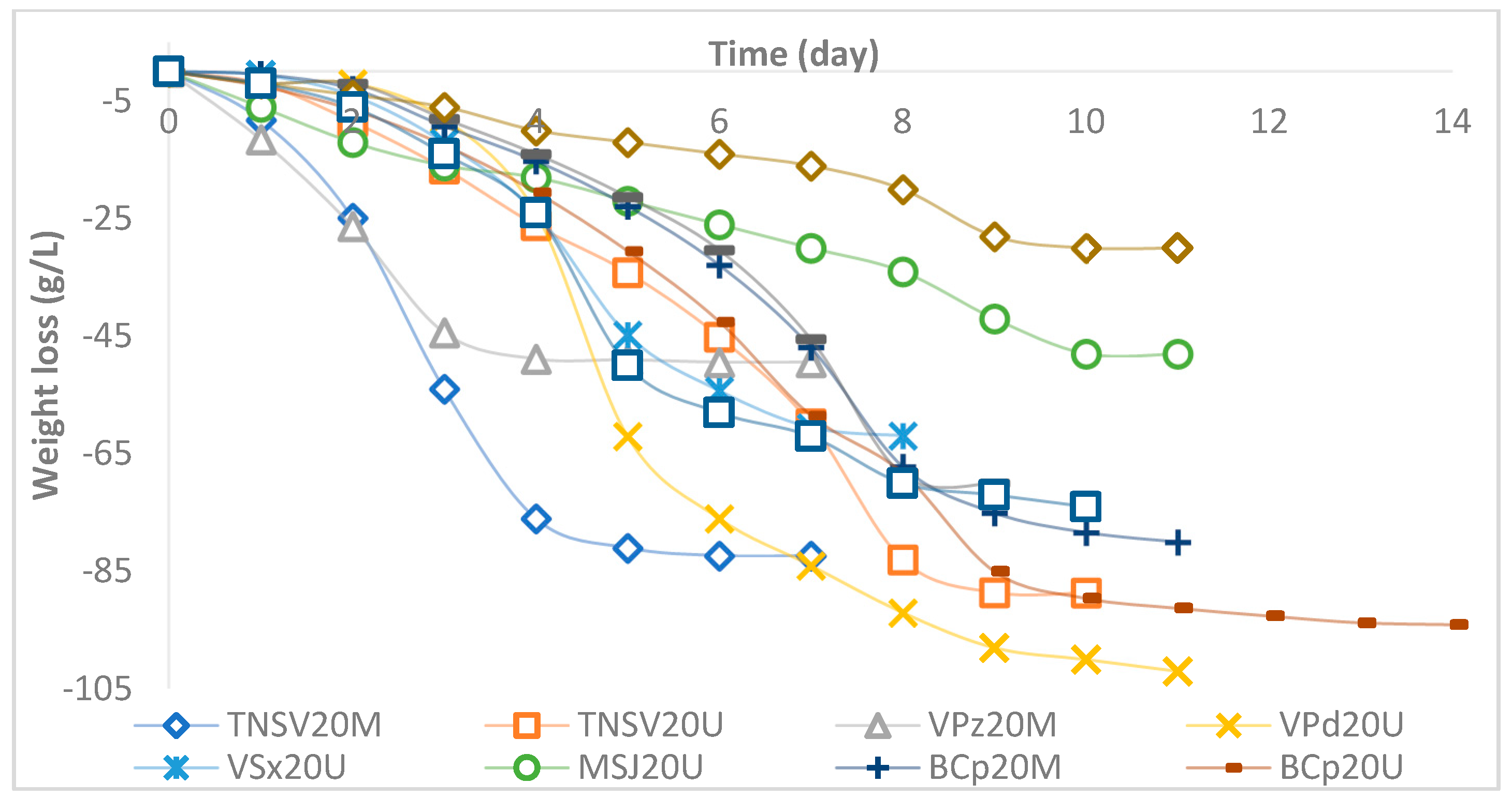
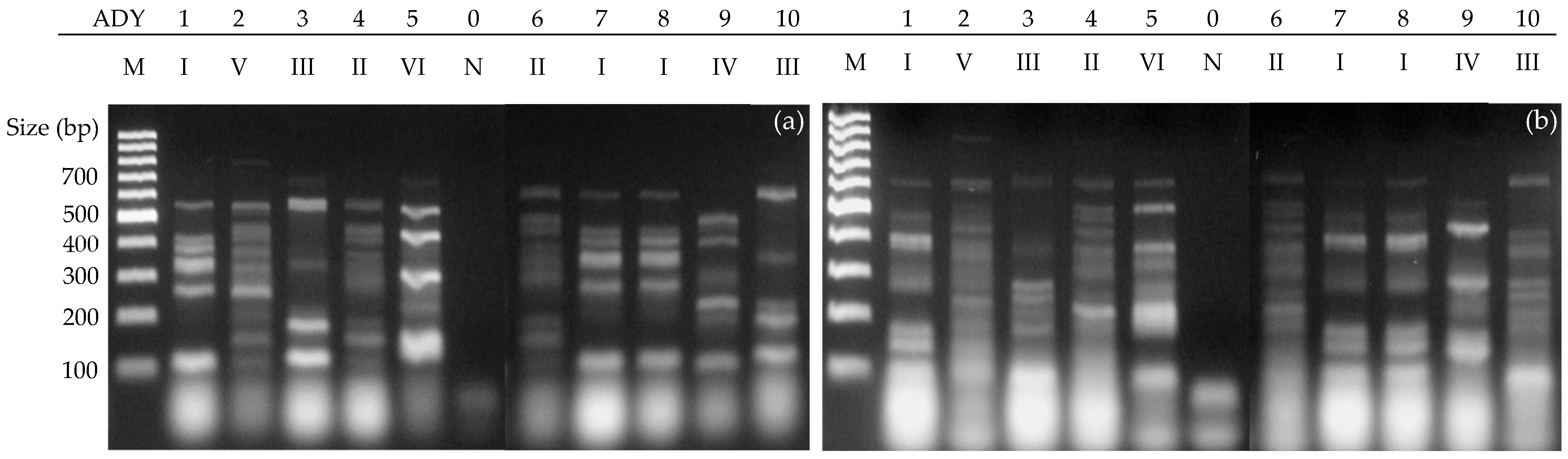
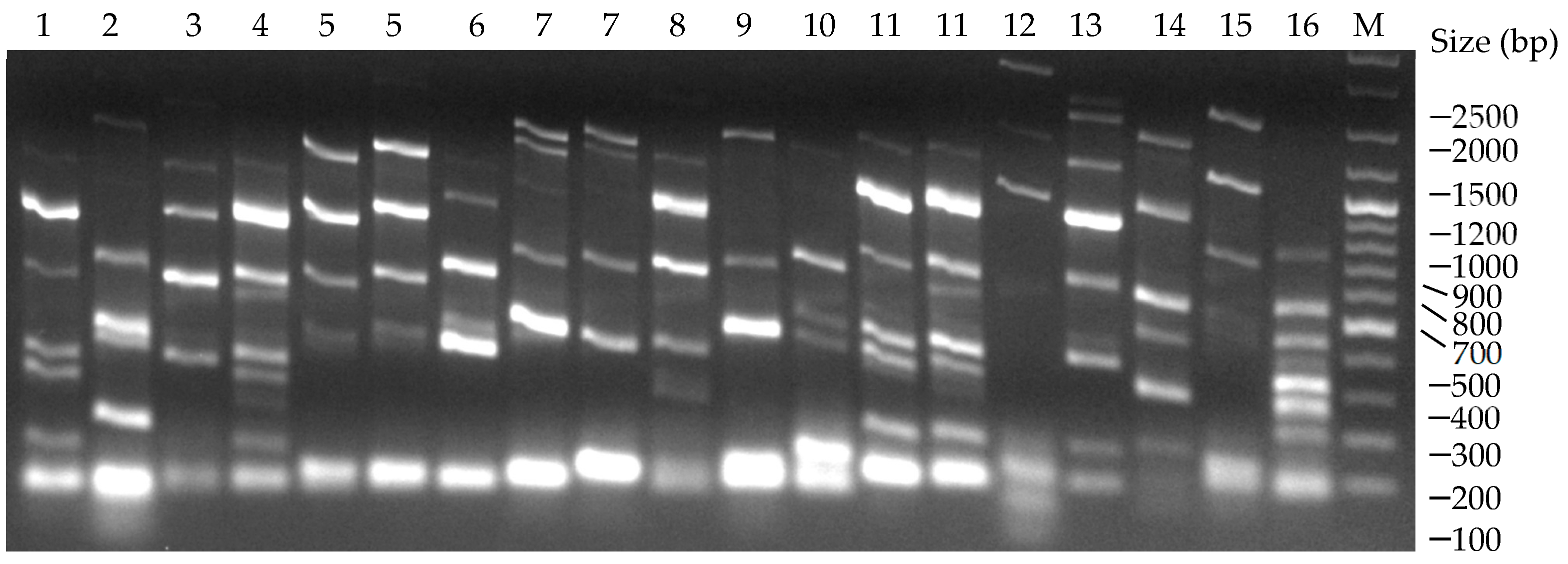
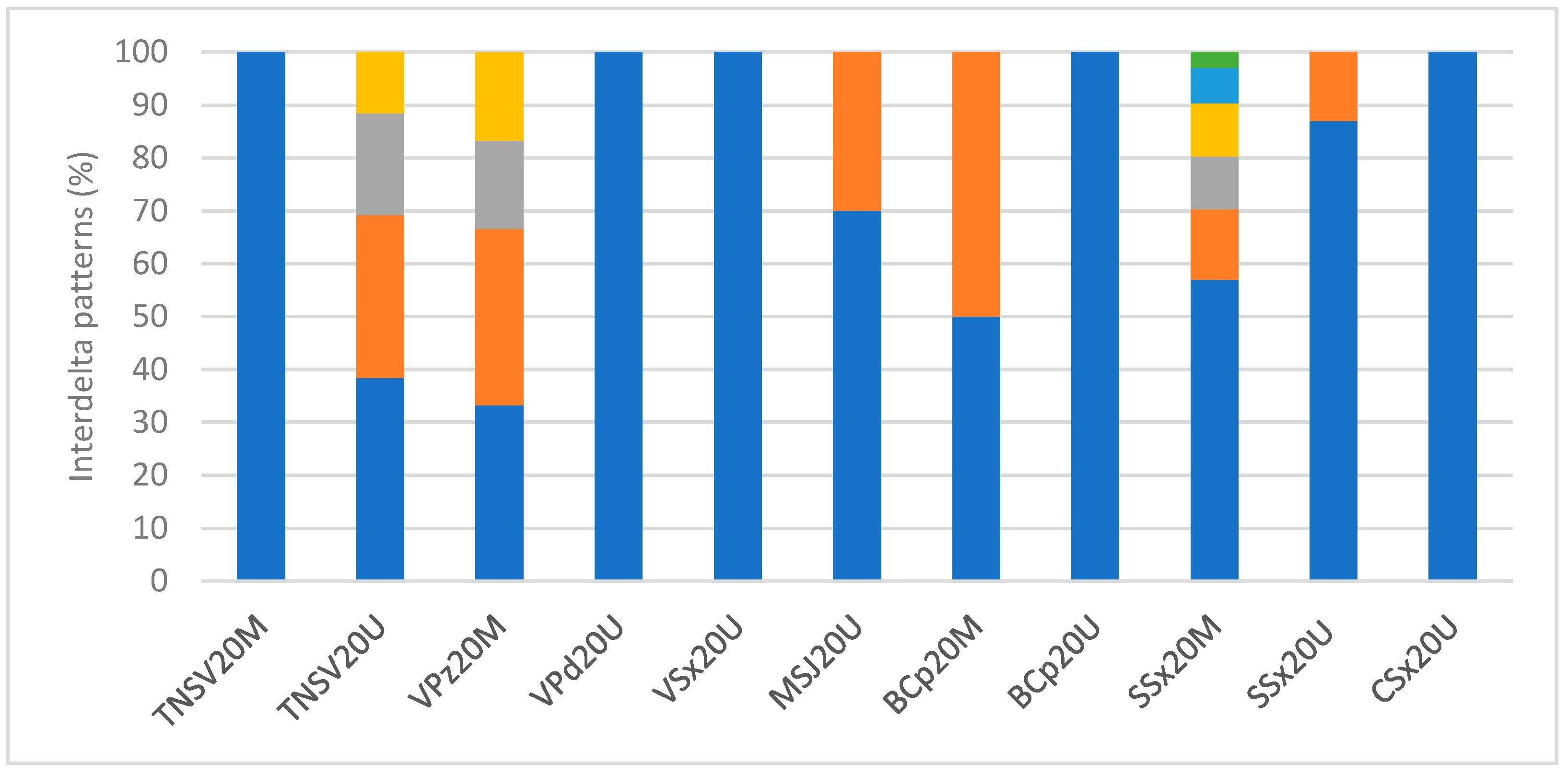
| # | Variety | Type 1 | Zone | Coordinates 2 | Id. 3 |
|---|---|---|---|---|---|
| 1 | Tinta Negra | Must | São Vicente | 32.47° N 17.01° W | TNSV20M |
| 2 | Tinta Negra | Berries | São Vicente | TNSV20U | |
| 3 | Verdelho | Must | Prazeres | 32.45° N 17.12° W | VPz20M |
| 4 | Verdelho | Berries | Pedregal | 32.39° N 16.59° W | VPd20U |
| 5 | Verdelho | Berries | Seixal | 32.49° N 17.06° W | VSx20U |
| 6 | Malvasia SJ * | Berries | São Jorge | 32.49° N 16.54° W | MSJ20U |
| 7 | Boal | Must | Campanário | 32.40° N 17.02° W | BCp20M |
| 8 | Boal | Berries | Campanário | BCp20U | |
| 9 | Sercial | Must | Seixal | 32.49° N 17.06° W | SSx20M |
| 10 | Sercial | Berries | Seixal | SSx20U | |
| 11 | Complexa | Berries | Seixal | CSx20U |
| # | Id. | SCS 1 (g/L) | SCF 2 (g/L) | Dilution | Col. No. 3 | CFU/mL 4 |
|---|---|---|---|---|---|---|
| 1 | TNSV20M | 151.47 | 42.01 | 10−5 | 109 | 1.09 × 107 |
| 2 | TNSV20U | 166.62 | 45.44 | 10−6 | 298 | 2.98 × 108 |
| 3 | VPz20M | 168.30 | 40.39 | 10−5 | 137 | 1.37 × 107 |
| 4 | VPd20U | 193.55 | 47.12 | 10−7 | 95 | 9.50 × 108 |
| 5 | VSx20U | 154.84 | 33.66 | 10−5 | 114 | 1.14 × 107 |
| 6 | MSJ20U | 151.47 | 37.03 | 10−5 | 37 | 3.70 × 106 |
| 7 | BCp20M | 168.30 | 47.12 | 10−5 | 104 | 1.04 × 107 |
| 8 | BCp20U | 185.13 | 50.50 | 10−4 | 164 | 1.64 × 106 |
| 9 | SSx20M | 151.47 | 33.66 | 10−5 | 212 | 2.12 × 107 |
| 10 | SSx20U | 151.47 | 50.50 | 10−4 | 30 | 3.00 × 105 |
| 11 | CSx20U | 153.15 | 37.03 | 10−5 | 67 | 6.70 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, M.M.; Parra, N.; Câmara, J.S.; Khadem, M. Unveiling the Regional Identity of Madeira Wine: Insights from Saccharomyces cerevisiae Strains Using Interdelta Analysis. Beverages 2025, 11, 84. https://doi.org/10.3390/beverages11030084
Castillo MM, Parra N, Câmara JS, Khadem M. Unveiling the Regional Identity of Madeira Wine: Insights from Saccharomyces cerevisiae Strains Using Interdelta Analysis. Beverages. 2025; 11(3):84. https://doi.org/10.3390/beverages11030084
Chicago/Turabian StyleCastillo, Mariangie M., Nikol Parra, José S. Câmara, and Mahnaz Khadem. 2025. "Unveiling the Regional Identity of Madeira Wine: Insights from Saccharomyces cerevisiae Strains Using Interdelta Analysis" Beverages 11, no. 3: 84. https://doi.org/10.3390/beverages11030084
APA StyleCastillo, M. M., Parra, N., Câmara, J. S., & Khadem, M. (2025). Unveiling the Regional Identity of Madeira Wine: Insights from Saccharomyces cerevisiae Strains Using Interdelta Analysis. Beverages, 11(3), 84. https://doi.org/10.3390/beverages11030084






