1. Introduction
Vinegar is among the earliest known fermented products, with historical records tracing its use back to approximately 3000 BC [
1]. It is a liquid fermentation product, typically containing 4% to 9% acetic acid, that is widely consumed globally, both as a condiment and for its potential health benefits [
2,
3]. Sharf & Malerich [
4] mentioned that to be sold in stores, vinegar must contain at least 4 g·mL
−1 of acetic acid. Among the diverse types of vinegar, fruit-based vinegars are particularly significant due to their content of antioxidant polyphenols, making them of interest to food scientists, nutritionists, consumers, and food producers. The functional benefits of vinegar include antimicrobial, antioxidant, regulation of blood glucose and lipid metabolism, weight loss, anti-inflammatory, and anticancer properties [
3,
5,
6,
7,
8,
9]. The term
functional food was first introduced in Japan during the 1980s to describe processed foods formulated with specific ingredients that offer physiological benefits beyond basic nutrition [
10,
11]. These foods are designed to support targeted body functions and promote overall health. Chen et al. [
3] further categorize functional foods into six types based on their intended health roles and bioactive components: nutritious, medicinal, regulatory, fortified, nutraceuticals, and pharmacological foods. This classification underscores the multifaceted nature of functional foods and their potential contributions to health maintenance, disease prevention, and therapeutic support.
In addition to its recognized functional properties, vinegar holds economic and cultural significance in many countries, including Romania. The Romanian vinegar industry employs traditional and industrial production methods, primarily from grapes and apples. Local vinegar production not only supports food preservation and the culinary sector but also contributes to regional food innovation and the development of functional products. According to Cosmulescu et al. [
12], grape vinegar is among the most widespread types of vinegar produced in Romania, alongside those derived from white and red wine. This reflects the country’s strong viticultural tradition and its integration into vinegar production practices. Other researchers have also highlighted the diversification of vinegar production in Romania, noting the use of berries such as blueberries, blackberries, and raspberries [
13,
14], as well as wild fruits [
12] and even banana peels [
15] as fermentation substrates. Additionally, several Romanian producers actively manufacture artisanal vinegars from apples, quince, roses, black chokeberries, or sour cherries, with or without the addition of honey, available in filtered and raw forms. These fruit vinegars are already well-established in Romanian households and are commonly used not only for traditional vegetable preservation but also in everyday cooking, particularly in dressings, marinades, and various culinary preparations.
Fruit vinegar production, which often utilizes fruit by-products [
7], has become a common practice in the food industry. This method not only prevents food waste by utilizing surplus or lower-quality fruit but also yields a high-quality product [
16]. Traditionally, vinegar was made from cereals [
17]; however, in recent years, fruit vinegar, produced from fruits or fruit juices, has gained increasing popularity [
3]. This shift reflects growing consumer interest in the functional benefits of food products [
18]. The production process involves a two-stage fermentation [
19]. In the first stage, yeasts, such as
Saccharomyces species, convert fermentable sugars into ethanol. In the second stage, acetic acid bacteria, typically
Acetobacter species, oxidize ethanol into acetic acid [
3,
20]. This fermentation process significantly alters the chemical composition of the vinegar, modifying volatile compounds, polyphenols, and organic acids, which are key contributors to its health benefits.
One important issue facing the fruit industry is post-harvest fruit drop, which presents challenges to economic stability and food security. Processing grade two and three fruits into vinegar offers an effective strategy to reduce waste and enhance the value of these fruits [
21]. Jujube (
Ziziphus jujuba Mill.), a widely cultivated tree species in China, is known for its long history of cultivation, high yield, and special fruits, with nutraceutical properties [
22]. However, jujubes are highly perishable, and their storage life is limited [
23,
24]. Consequently, there has been growing interest in processed jujube products that not only extend shelf-life but also provide valuable functional nutrients [
25]. On the other hand, due to cracking or premature dropping of fresh jujube fruits, Xiang et al. [
26] mention the use of defective jujubes for the production of vinegar, as being energy-saving and waste-reducing.
Jujubes are rich in sugars and nutrients, making them ideal for fermentation [
23,
27]. Jujube vinegar, a traditional fermented product, has gained attention due to its potential health benefits, distinctive flavor, and culinary versatility. It is produced from the fruit of the
Ziziphus jujuba tree and is particularly appreciated in East Asian cuisine for its medicinal properties. Rich in polysaccharides, vitamin C, proteins, dietary fibers, fatty acids, and amino acids, jujube vinegar has been traditionally used to promote digestion, improve circulation, and enhance overall wellness [
25]. Flavor, encompassing both taste and aroma, is a critical attribute in determining the vinegar quality. Throughout the production process, a variety of metabolites are generated at different stages, each playing a role in shaping the overall vinegar taste and aroma profile. The fermentation process of jujube vinegar contributes to the development of its sour taste and the formation of bioactive compounds, such as polyphenols, organic acids, and other bioactive substances, which are believed to contribute to its health benefits. Cai et al. [
25] note that jujube vinegar is particularly rich in bioactive compounds, including gallic, chlorogenic, ferulic, and
p-coumaric acid, along with polyphenolics and melanoidins, which contribute to its antibacterial, anti-inflammatory, antioxidant, and anti-diabetic properties. Melanoidins are brown polymers generated through the Maillard reaction during the vinegar production process, constituting one of the primary high-molecular-weight fractions of vinegar [
28]. Furthermore, Samad et al. [
6] reviewed some of the therapeutic properties of vinegar, such as improving lipid profiles and suppressing fat accumulation; reducing hyperglycemia and improving insulin secretion; inhibiting proliferation and inducing apoptosis in human cancer cells; and using it as a natural disinfectant.
This study focuses on the chemical composition of homemade and commercially produced jujube vinegar in terms of bioactive compounds. Homemade jujube vinegar is typically produced using traditional fermentation methods, which emphasize natural microbial processes, while commercial jujube vinegar may undergo industrial-scale production methods that could influence its chemical composition, flavor, and health-related properties. By analyzing and comparing the phenolic profiles and organic acids of these two types of vinegar using HPLC-MS, this study aims to provide insights into the impact of production methods on the overall quality and possible health benefits of jujube vinegar. The findings of this research will contribute to a deeper understanding of how production methods influence the bioactive properties of fermented foods, with implications for both consumers and food producers.
2. Materials and Methods
2.1. Materials
Ten kg of fresh jujube fruits (cultivar ‘Xuancheng Jian’ and Fellini selection) were harvested at the full red stage from an orchard located in Bratovoești commune, Dolj County, Oltenia Region, Romania (44°07′ N 23°54′ E).
For comparative analysis, commercially available jujube vinegar was procured from a supermarket in Baoding, Hebei Province, China. According to the product label, the vinegar comprises the following ingredients: jujube, bran, water, sugar, and salt, with an acidity level of ≥4.5 g·100·mL−1.
2.2. Vinegar Preparation
The vinegar production process was adapted from the methods reported by Cosmulescu et al. [
12] and Ren et al. [
29], with slight modifications. Fresh jujube fruits (stones removed) were thoroughly cleaned, chopped, and rinsed, then mixed with potable tap water at a 1:2 ratio. To this mixture, beet sugar (5%
w/
v; sourced from Bod Sugar Factory, Bod, Brasov County, Romania) was added. The resulting blend was homogenized and transferred into glass containers, loosely covered with cloth, and left to ferment at ambient temperature (21–22 °C) for 14 days, with manual stirring performed daily. Following fermentation, the mixture underwent repeated filtration through 3–5 µm pore-size filter paper until a clear liquid free of solid residues was obtained. Brix was adjusted to 12% according to the method described by Budak [
30] to stimulate alcoholic fermentation without the addition of starter culture. The alcoholic fermentation process was conducted over 30 days at a temperature of 21–22 °C. To the obtained jujube wine (10% alcoholic concentration), mother vinegar inoculum (2%) was added to encourage fermentation. The fermentation process was carried out at room temperature (21–22 °C) for 60 days (without stirring). After this, the vinegar was filtered and allowed to age naturally for another 90 days at room temperature (21–22 °C). Three replicate samples were collected from each vinegar batch (homemade and commercial) and analyzed individually to assess physico-chemical characteristics, phenolic profile, and antioxidant activity.
2.3. Physico-Chemical Analysis
Sugar concentration was measured using a Hanna digital refractometer (model HI96801, range 0–85% Brix, Woonsocket, RI, USA), with an accuracy of ±0.2%. Total titratable acidity was assessed through titration with 0.1 N NaOH (Carl Roth, Karlsruhe, Germany), employing phenolphthalein (Merck KGaA, Darmstadt, Germany, purity 98%) as an indicator; results were reported in grams of acetic acid [
31]. The pH level was measured at 25 °C using a HANNA Instruments pH meter (model HI2210, Woonsocket, RI, USA).
2.4. The Determination of the Total Polyphenol Content (TPC)
The vinegar samples were diluted with distilled water at a 1:1 ratio, then filtered through a Chromafil Xtra nylon 0.45 µm filter (Chromafil, Macherey-Nagel, Düren, Germany). A 1 mL aliquot of the filtered sample was used for the determination of total polyphenol content (TPC) based on the method described by Singleton and Rossi [
32].
Total polyphenol content (TPC) was quantified using a Perkin Elmer Lambda 25 double-beam spectrophotometer (PerkinElmer, Waltham, MA, USA). In a 10 mL volumetric flask, 1 mL of the diluted vinegar sample was added, followed by 5 mL of distilled water and 1 mL of 0.1 N Folin–Ciocâlteu reagent, and the mixture was allowed to stand at room temperature for 5 min. Afterward, 1.5 mL of a 7.5% Na2CO3 solution was added, and the volume was adjusted to 10 mL with distilled water. The samples were then kept in the dark for 1 h to allow the reaction to proceed. Absorbance was measured at a wavelength of λ = 765 nm.
Under the same conditions, a calibration curve was constructed using a gallic acid standard dissolved in methanol at five different concentrations ranging from 50 to 750 μg·mL
−1. The resulting calibration equation, with a coefficient of determination R
2 = 0.9935, was used for the quantification of total phenolic content (TPC) in the analyzed samples. The results were expressed as milligrams of gallic acid equivalents per liter (mg GAE·L
−1).
2.5. Antioxidant Activity (DPPH Method)
The vinegar samples were filtered through a 0.45 µm Chromafil Xtra nylon filter (Chromafil, Macherey-Nagel, Düren, Germany), and 0.1 mL of the filtered sample was used to assess DPPH antioxidant activity according to the method described by Brand-Williams et al. and Pekkarinen et al. [
33,
34].
DPPH antioxidant activity was determined using a Perkin Elmer Lambda 25 double-beam spectrophotometer (PerkinElmer, Waltham, MA, USA). An 80 µM DPPH solution was prepared in 96% ethanol and stored in a dark glass bottle at room temperature until use.
For the assay, 3.9 mL of DPPH solution was added to a 15 mL Eppendorf tube (Eppendorf, Hamburg, Germany), followed by the addition of 0.1 mL of the vinegar sample. The contents of the tube were mixed and then incubated in the dark at room temperature for 30 min. A control solution was prepared similarly, replacing the vinegar sample with 0.1 mL of 96% ethanol. After the 30 min incubation, the absorbance of the sample was measured at a wavelength of λ = 518 nm, using the control solution as a reference.
The antioxidant potential of the vinegar samples was expressed as % DPPH inhibition, calculated using the following formula:
where
A0 is the absorbance of the control solution at 518 nm;
As is the absorbance of the sample at 518 nm.
2.6. Determination of Carbohydrates and Organic Acids—HPLC-RID Method
The vinegar samples were filtered through a 0.45 µm Chromafil Xtra nylon filter (Chromafil, Macherey-Nagel, Düren, Germany), and 20 µL of each filtered vinegar sample was injected into the HPLC system.
High-performance liquid chromatography (HPLC) analysis was carried out using an Agilent 1200 system (Agilent Technologies, Santa Clara, CA, USA), which included a quaternary pump, solvent degasser, manual injector, and a refractive index detector (RID). Compound separation was achieved using a Polaris Hi-Plex H column (300 × 7.7 mm; Agilent Technologies, CA, USA) with a 5 mM sulfuric acid (H2SO4) solution as the mobile phase, delivered at a flow rate of 0.6 mL·min−1. The column was maintained at 70 °C, and the RID was operated at 35 °C. The total run time for compound elution was 25 min.
Data acquisition and analysis were performed using OpenLab–ChemStation software (Agilent Technologies, Santa Clara, CA, USA, version C.01.09). The identification of compounds in the vinegar samples was achieved by comparing their retention times with those of standard chemical compounds.
Quantification of carbohydrates and organic acids was performed using external calibration. Calibration curves were constructed by injecting five different concentrations of each standard compound. The equations obtained from the calibration curves were used to determine the concentrations of the respective compounds in the analyzed samples. The calibration equations and corresponding coefficients of determination (R
2) were as follows:
2.7. Determination of Individual Phenolic Compounds Using HPLC-DAD-ESI+
Vinegar samples were filtered through a 0.45 µm Chromafil Xtra nylon filter (Chromafil, Macherey-Nagel, Düren, Germany). An aliquot of 20 µL from each filtered sample was injected into the high-performance liquid chromatography (HPLC) system, with triplicate injections performed for each sample.
Chromatographic analysis was conducted using an Agilent 1200 HPLC system (Agilent Technologies, Santa Clara, CA, USA), equipped with a quaternary pump, solvent degasser, autosampler, and a UV–vis photodiode array detector (DAD), coupled with an Agilent 6110 single-quadrupole mass spectrometer (MS). The separation of compounds was performed on a Kinetex XB C18 column (4.6 × 150 mm, 5 μm particles; Phenomenex, Torans, CA, USA) using a gradient elution system. The mobile phases consisted of (A) water containing 0.1% acetic acid and (B) acetonitrile containing 0.1% acetic acid. The gradient program, applied over a 30 min run time, (expressed as % B) was as follows: 0 min, 5% B; 0–2 min, 5% B; 2–18 min, 5–40% B; 18–20 min, 40–90% B; 20–24 min, 90% B; 24–25 min, 90–5% B; 25–30 min, 5% B. The column temperature was maintained at 25 °C, with a flow rate of 0.5 mL·min−1.
Spectral data were acquired over a wavelength range of 200–600 nm for all detected peaks, with chromatograms recorded at 280 nm and 340 nm (
Appendix B,
Figure A3 and
Figure A4).
Mass spectrometry analysis was conducted in positive electrospray ionization (ESI) mode, using a full scan acquisition method. The following instrumental conditions were employed: capillary voltage set to 3000 V, source temperature of 350 °C, nitrogen flow rate of 7 L·min−1, and mass-to-charge (m/z) range of 120–1200.
Data acquisition and subsequent analysis were conducted using Agilent ChemStation software (Agilent Technologies, Santa Clara, CA, USA), version Rev B.02.01-SR2.
Calibration curves for phenolic compounds were constructed by injecting five different concentrations of standard solutions dissolved in methanol. The standards used were gallic acid (R2 = 0.9978, LOD = 0.35 μg·mL−1, LOQ = 1.05 μg·mL−1), chlorogenic acid (R2 = 0.9937, LOD = 0.41 μg·mL−1, LOQ = 1.64 μg·mL−1), and rutin (R2 = 0.9981, LOD = 0.21 μg·mL−1, LOQ = 0.84 μg·mL−1). The equations obtained from the calibration curves were used for the quantitative determination of each phenolic compound. Hydroxybenzoic acids were quantified as gallic acid equivalents, hydroxycinnamic acids as chlorogenic acid equivalents, and flavonols as rutin equivalents. Identification of phenolic compounds was performed by comparing retention times, UV–vis absorption spectra, and mass spectra with those of the corresponding standard compounds and literature data.
2.8. Chemicals and Reagents
Folin–Ciocâlteu 1N reagent was purchased from Supelco (Darmstadt, Germany). Methanol and HPLC-grade acetonitrile were sourced from Merck (Darmstadt, Germany). Gallic acid (purity > 98%, HPLC grade) was obtained from Sigma (Sigma-Aldrich Chemie GmbH, Steinheim, Germany), as were chlorogenic acid (purity > 98%, HPLC grade) and rutin (purity > 99%, HPLC grade). Sodium carbonate and sulfuric acid were purchased from Chempur (Piekary Śląskie, Poland). Glucose, fructose, and citric acid standards (99% purity) were purchased from Merck (Darmstadt, Germany), lactic acid from MP Biomedicals (Illkirch-Graffenstaden, France), and acetic acid from Fluka (Taufkirchen, Germany). Ultrapure water was purified using the Direct-Q UV system (Millipore, Burlington, MA, USA), with acidified water also purified by the Direct-Q UV system (Millipore, USA).
2.9. Statistical Analysis
Statistical analyses were conducted using IBM SPSS version 26.0 (SPSS Inc., Chicago, IL, USA). A one-way analysis of variance (ANOVA) was performed to compare the chemical composition between homemade and commercial vinegars. A significance level of p < 0.05 was used for all statistical tests. Pearson correlation coefficients were calculated to assess potential relationships between the parameters studied. All measurements were performed in triplicate, and the results are presented as the mean ± standard deviation.
3. Results
3.1. Analysis of the Phytochemical Compositions of the Jujube Vinegars
The results regarding the phytochemical composition of homemade and commercial jujube vinegars are presented in
Table 1. The Brix value of the commercial jujube vinegar (10.70%) was significantly higher than that of the homemade vinegar (8.65%), indicating a greater concentration of soluble solids in the commercial variety. This suggests that the commercial vinegar may be sweeter and denser. Both vinegars exhibited similar pH values, with the homemade vinegar having a slightly higher pH (2.97) compared to the commercial vinegar (2.89), though the difference was minimal and unlikely to significantly affect their overall acidity.
The homemade jujube vinegar showed a higher total titratable acidity (4.76 g acetic acid·100 mL−1) compared to the commercial vinegar (3.95 g acetic acid·100 mL−1), suggesting that the homemade vinegar had a stronger acidic profile. In terms of total phenolic content (TPC), the commercial vinegar contained significantly higher levels (306.64 mg GAE·L−1) than the homemade vinegar (219.09 mg GAE·L−1), indicating that the commercial vinegar may have more bioactive compounds contributing to its antioxidant properties.
The DPPH assay revealed that the commercial vinegar had much higher antioxidant activity (36.98%), which is consistent with its higher TPC, compared to the homemade vinegar (10.62%). Statistical analysis showed significant differences in Brix (p = 0.003), total phenolic content (p = 0.005), and DPPH (p = 0.001) activity between the two jujube vinegars; no significant differences were found in pH or total titratable acidity.
Overall, these results suggest that the commercial jujube vinegar contains higher concentrations of soluble solids, phenolic compounds, and antioxidant activity compared to the homemade variety, while the homemade vinegar has a slightly stronger acidic profile. These findings highlight the potential impact of production methods and the raw material used (only fresh jujube fruits in the homemade vinegar versus jujube and bran in the commercial one) on the phytochemical composition and health-related properties of jujube vinegar.
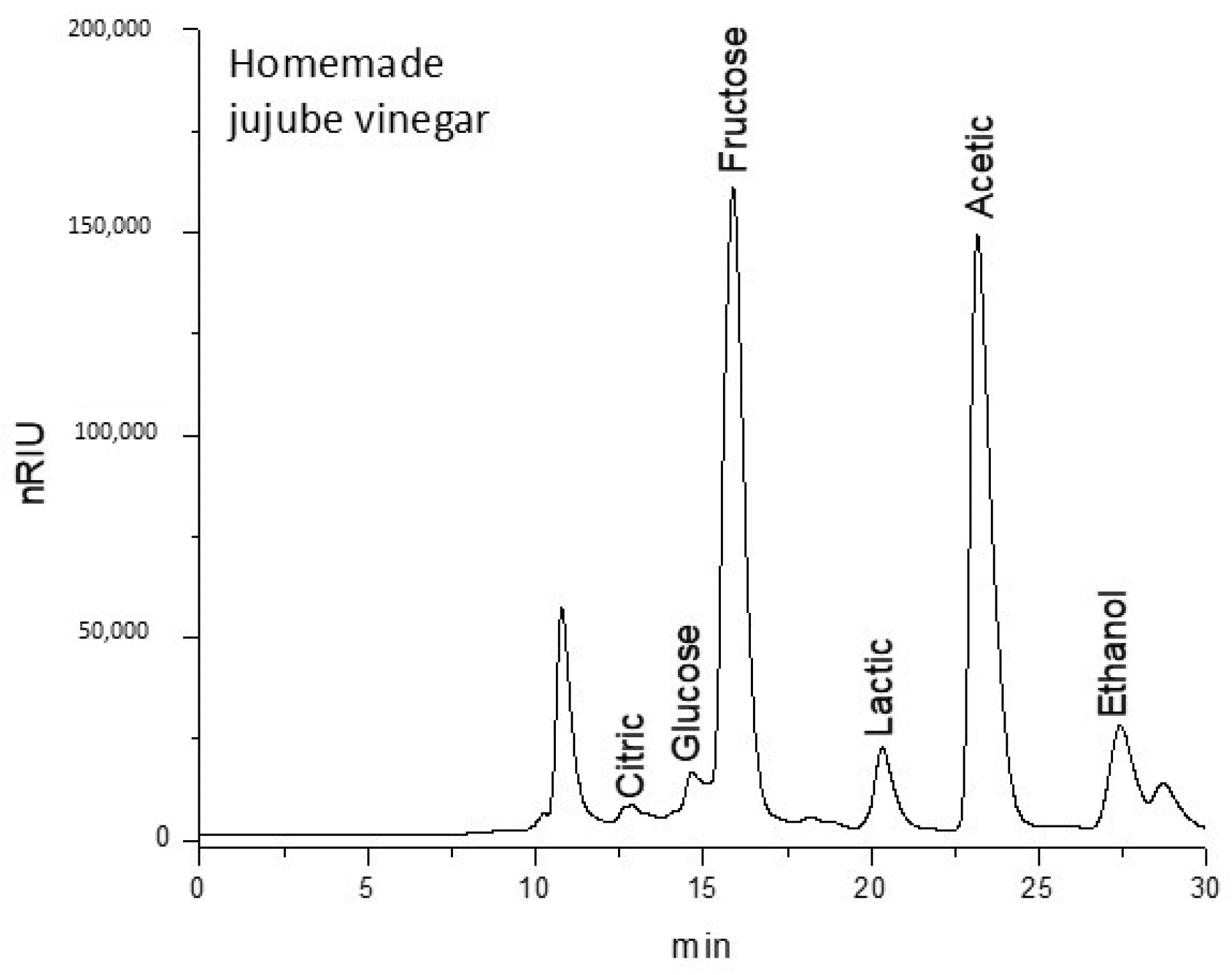
3.2. Analysis of the Carbohydrates and Organic Acids of Jujube Vinegars
The results regarding the carbohydrates and organic acids of homemade and commercial jujube vinegar are presented in
Table 2 and
Appendix A (
Figure A1 and
Figure A2, respectively).
The homemade jujube vinegar contained significantly lower glucose (1.57 mg·mL−1) compared to the commercial vinegar (13.72 mg·mL−1) (p = 0.001). In contrast, fructose levels were significantly higher in the homemade vinegar (24.15 mg·mL−1) compared to the commercial vinegar (20.07 mg·mL−1) (p = 0.001).
Regarding organic acids, the commercial jujube vinegar contained significantly higher citric acid (2.47 mg·mL−1) than the homemade vinegar (0.64 mg·mL−1), which could contribute to a distinct flavor profile (p < 0.001). Lactic acid levels were similar between the two samples, with the homemade vinegar containing 2.27 mg·mL−1 and the commercial vinegar containing 2.36 mg·mL−1, showing no significant difference. Acetic acid, a key component of vinegar, was found to be higher in the homemade vinegar (36.48 mg·mL−1) compared to the commercial vinegar (28.98 mg·mL−1), indicating a stronger acidic profile in the homemade product (p = 0.001). Finally, the ethanol content was significantly higher (p < 0.001) in the homemade vinegar (2.65 mg·mL−1) compared to the commercial vinegar (1.10 mg·mL−1), likely reflecting differences in fermentation processes or raw materials used.
The statistical analysis indicated significant differences (p < 0.05) in glucose, fructose, citric acid, acetic acid, and ethanol content between the two vinegar products. In contrast, no significant differences were found in lactic acid content. These findings reflect the influence of production methods and fermentation conditions on the carbohydrate and organic acid profiles of jujube vinegar.
3.3. Correlation Analysis
To evaluate possible relationships between the various components of homemade and commercial jujube vinegars, Pearson correlation analysis was conducted, and the results are presented in
Figure 1. The analysis revealed several strong and significant correlations between the variables.
There is a very strong positive correlation between Brix and Total Phenolic Content (TPC) (r = 0.998), as well as between Brix and DPPH (r = 0.999), suggesting that higher concentrations of soluble solids (Brix) are associated with increased phenolic content and antioxidant activity. Additionally, Brix shows a very strong negative correlation with fructose (r = −0.998), indicating that higher Brix values are linked to lower fructose levels in the vinegar. Acetic acid shows a strong negative correlation with Brix (r = −0.993), indicating that higher concentrations of acetic acid are typically associated with lower Brix values. Acetic acid also demonstrates a positive correlation with ethanol (r = 0.998), highlighting a strong relationship between acetic acid production and ethanol levels during fermentation. The total phenolic content (TPC) is strongly positively correlated with DPPH radical scavenging activity (r = 0.997) and glucose (r = 0.998), suggesting that higher phenolic content is associated with greater antioxidant activity and higher glucose content. On the other hand, TPC negatively correlates with fructose (r = −0.999), indicating an inverse relationship between these two compounds.
There is a very strong negative correlation between fructose and glucose (r = −1.000), meaning that as glucose levels increase, fructose levels decrease, and vice versa. Citric acid shows a positive correlation with Brix (r = 0.996), implying that higher Brix values are linked to higher citric acid content, which could contribute to the overall acidity and flavor profile of the vinegar. The relationship between lactic acid and other variables is somewhat weaker, with significant correlations observed with pH (r = −0.911) and citric acid (r = 0.880), suggesting that lactic acid concentrations are moderately influenced by the acidity and other organic acids in the vinegar.
Overall, these results indicate that many of the key components of jujube vinegar, such as sugars (glucose and fructose), organic acids (acetic acid and citric acid), and bioactive compounds (TPC), are strongly correlated with one another, reflecting complex interactions in the fermentation process. These correlations could provide insights into the factors that influence the chemical composition and antioxidant activity of jujube vinegar, which may vary between homemade and commercial varieties.
3.4. Analysis of the Individual Phenols of Jujube Vinegars
The analysis of individual phenols in homemade and commercial jujube vinegars reveals significant differences in the concentration of various phenolic compounds, as presented in
Table 3 and
Appendix B (
Figure A3 and
Figure A4, respectively).
In the homemade vinegar, several phenolic compounds were found in notably higher concentrations compared to the commercial vinegar. For instance, syringaldehyde, a hydroxybenzaldehyde, was significantly higher in the homemade vinegar than in the commercial vinegar (p < 0.001). Similarly, protocatechuic aldehyde (p = 0.001) and gallic acid (p = 0.002), both hydroxybenzoic acids, were more concentrated in the homemade vinegar than in the commercial vinegar.
In contrast, the commercial vinegar exhibited much higher levels of some phenolic compounds, such as 2-hydroxybenzoic acid (p < 0.001) and 2,4-dihydroxybenzoic acid (p = 0.001), which were found at significantly lower concentrations in the homemade vinegar. While these compounds are naturally present in fruits, factors such as extended fermentation time and the controlled conditions of industrial production may contribute to the increased levels observed in the commercially produced vinegar. Other compounds, such as 3,4-dihydroxybenzoic acid (protocatechuic acid) (p = 0.004) and 3-hydroxybenzoic acid (p = 0.001) also showed higher concentrations in the homemade jujube vinegar, compared to the commercial vinegar.
Several compounds were not detected in the commercial vinegar, including 5-caffeoylquinic acid (chlorogenic acid), caffeic acid-glucoside, p-coumaric acid, and quercetin-glucoside, which were present in the homemade vinegar. Additionally, 4-hydroxy-3-methoxybenzoic acid (vanillic acid) was present in higher concentrations in the homemade vinegar compared to the commercial vinegar (p = 0.003), while 4-hydroxybenzoic acid was also more abundant in the homemade vinegar than in the commercial vinegar (p < 0.001).
Overall, the data indicate that the homemade vinegar has a broader range of phenolic compounds, with some key compounds present at higher concentrations compared to the commercial vinegar. The commercial vinegar, on the other hand, contains specific phenols at significantly higher levels, suggesting potential differences in the raw materials and fermentation processes used in their production.
4. Discussion
The present study highlights significant differences in the chemical composition between homemade and commercial jujube vinegar. These variations are primarily attributed to differences in processing methods, traditional or industrial, along with the choice of jujube cultivars (raw materials) and the environmental conditions during vinegar production. Various types of vinegar are produced worldwide, including black vinegar, rice vinegar, balsamic vinegar, and white wine vinegar. The production of vinegar involves different raw materials, yeast strains, and fermentation methods, each contributing to its distinct flavors and characteristics [
35]. Vinegar is primarily classified into grain vinegar and fruit vinegar based on the raw materials used. Both types are produced through the anaerobic fermentation of saccharides into ethanol by yeast, followed by the aerobic oxidation of ethanol to acetic acid by specific bacterial species [
36]. The antioxidant activity of fruit vinegar is linked to the presence of bioactive compounds, including phenolic acids and flavonoids [
37]. Decoction, storing, and aging may also affect vinegar’s antioxidant activity [
28]. Cai et al. [
25] note that microbial fermentation products, owing to their intricate metabolic pathways and the presence of various biological enzymes, have the potential to enhance product flavor and taste while enriching their nutritional value.
The physico-chemical properties of the homemade and commercial jujube vinegar in this study were consistent with those reported in the literature. For instance, Basiri [
21] mentioned a pH of 3.02 and an acidity of 0.139% in the analyzed jujube vinegar, while Karadag et al. [
38] identified a pH of 2.64 and a 4.53° Brix. Shahi et al. [
39] mentioned a pH of 3.27, a Brix of 14.47%, and a total titratable acidity of 3.02% for the analyzed jujube vinegar. Duan and Li [
36] report that jujube vinegar produced by the Shanxi Agricultural University’s Vinegar Research Centre has a pH of 2.82, a total sugar content of 1.82%, and a total titratable acidity of 3.81. Budak [
30] also reported that the analyzed jujube vinegar had a pH value of 3.24 and a Brix of 4.75°. The results of this study (8.65–10.70% Brix; 2.97–2.89 pH; and 4.76–3.95 TTA g acetic acid·100 mL
−1) fall within the range of previously reported values, further validating the consistency of the vinegar’s physico-chemical properties. These findings underscore the importance of production methods and raw material quality in influencing vinegar’s chemical profile, as mentioned by Duan and Li [
36].
Organic acids play a key role in determining the volatility and flavor of fruit vinegar [
40]. According to Ge et al. [
41], they are the key functional and flavor components of vinegar, with fruit vinegars generally containing a higher total acid content compared to grain vinegars (such as those made from sorghum, rice, or wheat). Organic acids in fruit vinegar are classified into volatile acids, which are primarily produced during fermentation, including acetic, formic, propionic, butyric, and quinic acid, and non-volatile acids, such as lactic, malic, pyroglutamic, citric, and succinic acid [
41]. Acetic acid is the primary flavor compound in vinegar and has a long-established history as a key food additive used to acidify foods for preservation [
9]. According to Wu et al. [
42], most organic acids in vinegar are produced from proteins, starches, and fats in the raw materials through microbial activity during fermentation. In this study, we found that homemade jujube vinegar contained higher levels of acetic acid, whereas lactic and citric acids were higher in the commercial jujube vinegar. Several authors have focused on identifying organic acids present in jujube vinegar. In their analysis of green jujube vinegar, Zhang et al. [
40] reported the following concentrations: 1.211 g·100 mL
−1 of lactic acid, 1.001 g·100 mL
−1 of acetic acid, 0.032 g·100 mL
−1 of tartaric acid, 0.020 g·100 mL
−1 of citric acid, and 0.042 g·100 mL
−1 of malic acid. Their study ranked the acids in descending order as follows: acetic acid > lactic acid > succinic acid > oxalic acid > malic acid > citric acid > tartaric acid. Budak [
30] reported the following acid concentrations in the analyzed jujube vinegar: acetic acid at 46,375.3 mg·L
−1, lactic acid at 2030.1 mg·L
−1, tartaric acid at 993.5 mg·L
−1, succinic acid at 843.4 mg·L
−1, malic acid at 655.2 mg·L
−1, formic acid at 538.5 mg·L
−1, citric acid at 530.7 mg·L
−1 and, in smaller amounts, oxalic acid (75 mg·L
−1), ascorbic acid (57.3 mg·L
−1), and fumaric acid (3.2 mg·L
−1). Zhao et al. [
23] identified seven organic acids in jujube vinegar at various fermentation stages, with the following concentrations: acetic acid (1910.20–4547.60 mg·L
−1), malic acid (700.70–1191.20 mg·L
−1), citric acid (541.7–815.30 mg·L
−1), tartaric acid (291.89–409.40 mg·L
−1), lactic acid (289.90–417.46 mg·L
−1), succinic acid (95.44–196.99 mg·L
−1), and oxalic acid (27.68–37.94 mg·L
−1). A study by Xiang et al. [
26] on the main organic acids in vinegar produced from both defective and high-quality fresh jujube fruits found no significant differences between the two types of vinegar. Ten organic acids were identified [
26], with the following concentrations (mg·100 mL
−1) for vinegar from defective fruits and high-quality fruits, respectively: acetic acid (4399.52–4467.06), lactic acid (121.81–117.93), malic acid (101.26–112.12), tartaric acid (50.86–61.92), formic acid (42.88–23.35), oxalic acid (0.19–0.32), succinic acid (24.85–20.12), citric acid (24.85–15.57), and fumaric acid (0.25–0.29). These variations are likely due to differences in fermentation processes, fermentation times, and raw materials used, supporting the hypothesis that organic acid content is closely linked to the production method.
Phenolic compounds, key bioactive components in vinegars that mainly originate from raw materials, contain one or more phenolic hydroxyl groups on aromatic rings and contribute significantly to antioxidant activity [
43,
44,
45]. Current literature underscores the detection of phenolic compounds in jujube vinegar, as identified through liquid chromatography techniques. Zhang et al. [
46] reported that the phenolic compounds identified in their study, such as caffeic, gallic, sinapic, syringic, ferulic acid, catechin, and rutin, exhibited high antioxidant activity. The individual phenolics present in the traditional jujube vinegar identified by Karadag et al. [
38] (whole fruits without the addition of preservatives) were gallic acid (10.9 mg·mL
−1), protocatechuic acid (6.83 mg·mL
−1), caffeic acid (10.9 mg·mL
−1), and rutin (7.72 mg·mL
−1), while
p-hydroxybenzoic, syringic,
p-coumaric acid, and kaempferol were not detected.
The major phenolic compounds identified by Budak [
30] were epicatechin (8.61 mg·L
−1), chlorogenic acid (7.92 mg·L
−1),
p-hydroxybenzoic acid (5.83 mg·L
−1), protocatechuic acid (2.42 mg·L
−1), and, in smaller amounts, benzoic acid (1.90 mg·L
−1), quercetin (1.52 mg·L
−1), caffeic acid (0.80 mg·L
−1), kaempferol (0.70 mg·L
−1), syringic acid (0.30 mg·L
−1), vanillin (0.10 mg·L
−1), and
p-coumaric acid (0.21 mg·L
−1). Noteworthy in the above-mentioned study is that chlorogenic acid content was much higher compared with the analyzed jujube juice and jujube wine (2.01 and 2.30 mg·L
−1, respectively). Zhao et al. [
23] identified 13 phenolic compounds in jujube vinegar at different fermentation stages, as follows: gallic acid (0.86–1.37 mg·L
−1), caffeic acid (2.23–2.86 mg·L
−1),
p-coumaric acid (4.15–6.58 mg·L
−1), ferulic acid (4.72–16.16 mg·L
−1), protocatechuic acid (1.21–6.89 mg·L
−1), chlorogenic acid (2.09–4.23 mg·L
−1), catechin (31.93–58.18 mg·L
−1), epicatechin (39.67–43.77 mg·L
−1), resveratrol (0.96–1.12 mg·L
−1), rutin (0.46–0.74 mg·L
−1), quercetin (1.11–6.79 mg·L
−1), kaempferol (3.76–5.14 mg·L
−1), and isorhamnetin (4.42–5.77 mg·L
−1). In the study by Liu et al. [
18], the most commonly detected phenolic compounds in the 23 types of fruit vinegar analyzed were gallic, protocatechuic, chlorogenic, caffeic, and
p-coumaric acid. Yıkmış et al. [
47] identified caffeic and ferulic acids as the predominant phenolic components in jujube vinegar samples. Based on the analyzed literature and the results obtained in this study, it can be concluded that homemade jujube vinegar is a source of bioactive compounds, primarily vanillic acid (18.0 mg·L
−1), caffeic acid (12.9 mg·L
−1), gallic acid (12.9 mg·L
−1), protocatechuic acid (9.3 mg·L
−1), chlorogenic acid (3.5 mg·L
−1),
p-coumaric acid (2.7 mg·L
−1), and quercetin (2.6 mg·L
−1). On the other hand, in the commercial jujube vinegar, only gallic acid (5.9 mg·L
−1), vanillic acid (5.3 mg·L
−1), and protocatechuic acid (2.0 mg·L
−1) were found in smaller amounts.
Fruit vinegar is popular worldwide for its appealing flavor and health benefits. Phenolic compounds and organic acids are the primary components that influence both the sensory properties and health benefits of fruit vinegar [
18]. According to Cai et al. [
25], jujube vinegar tends to accumulate the most flavor substances in the middle and later stages of fermentation. Cosmulescu et al. [
12] also suggest that homemade vinegar is of much better quality in terms of chemical composition than commercial vinegar and can be further used as functional food [
48] rich in phytochemicals beneficial for human health. According to Hosseini et al. [
5], vinegar is considered a potential bioactive compound in human nutrition and health, primarily due to its antimicrobial and antioxidant properties. Similarly, Duan and Li [
36] report that jujube vinegar may mitigate hyperlipidemia by modulating multiple bioactive components, metabolic pathways, and oxidative stress, in part through the prevention of gut microbiota imbalances.
According to Hosseini et al. [
5], vinegar production methods, such as traditional aging and the optimization of modern fermentation processes, have a substantial impact on its bioactive profile and associated health benefits. Innovations in fermentation technology, including microbial selection, controlled fermentation conditions, and advanced maturation techniques, are vital for maintaining quality and safety, as well as enhancing the health-promoting attributes of vinegar. Ren et al. [
49] suggest that organic acids and phenols found in vinegar can significantly contribute to both their sensory quality and functional activity; additionally, their content is variable and influenced by several factors, including raw materials, processing techniques, and microbiological growth [
36,
37,
50]. Boasiako et al. [
24] highlight that the dynamic interactions between key components, including free amino acids, organic acids, phenolics, volatiles, and microstructure, are central to the transformative nature of fermentation. This intricate reciprocity plays a critical role in shaping the development of flavor, aroma, and texture in fermented foods. Li et al. [
37] analyzed the effect of in vitro gastrointestinal digestion on green jujube vinegar and found that the results complement the scale analysis of the vinegar. Their results emphasize the stability of the functional properties of green jujube vinegar during digestion, suggesting that it could be regarded as a potential functional food and may play a significant role in the treatment of hyperlipidemia. The bioactive compounds identified in this study, present in higher concentrations in homemade jujube vinegar, could play a significant role in alleviating or potentially treating certain illnesses. For example, syringaldehyde, a constituent found in whisky and brandy [
51], may function as a hypoglycemic agent, while protocatechuic aldehyde, as reported by Choi et al. and Masella et al. [
52,
53], exhibits antioxidant, anti-inflammatory, antihyperglycemic, and neuroprotective properties. Additionally, it demonstrates chemopreventive potential by inhibiting in vitro chemical carcinogenesis and exerting pro-apoptotic and anti-proliferative effects across various tissues [
53]. Vanillic acid, a dietary byproduct of benzoic acid frequently employed as a flavoring agent and food stabilizer, exhibits antioxidant and anti-inflammatory properties in human immune cells and plasma, as highlighted by Magiera et al. [
54]. Chlorogenic acid, another compound identified in homemade jujube vinegar, exhibits antioxidant activity, particularly in inhibiting lipid oxidation. Additionally, it offers protective effects against the degradation of other bioactive compounds present in food and demonstrates prebiotic activity [
55]. Caffeic acid exhibits potent antioxidant, anti-inflammatory, and neuroprotective effects [
56], while gallic acid is recognized for its ability to reduce oxidative stress and modulate cellular signaling pathways involved in the prevention of chronic diseases [
57].
p-Coumaric acid demonstrates significant antioxidant properties and may contribute to mitigating the risk of metabolic disorders [
58]. Quercetin, a well-known flavonoid, provides antioxidant and anti-inflammatory benefits and has been associated with improved cardiovascular health and enhanced immune function [
59].
While vinegar has traditionally been utilized as a flavoring agent and preservative, recent studies highlight its potent bioactive properties, suggesting potential health benefits.
Future research should investigate the specific health benefits of jujube vinegar, particularly its antioxidative, antihyperglycemic, anti-obesity, and anti-inflammatory effects. Studies could focus on the bioactive compounds identified in this study, especially those found in higher concentrations in homemade vinegar.
Furthermore, there is a need to optimize fermentation processes and environmental conditions to enhance the levels of these beneficial compounds. At the same time, the vinegar aging process in different wooden barrels could be studied. Research on the bioavailability and stability of these compounds during digestion, as well as their effects on the gut microbiome, could provide deeper insights into the functional potential of jujube vinegar. Additionally, further studies could assess the impact of aging and storage conditions on the stability of these compounds over time, as suggested by Xia et al. [
48]. These research directions could pave the way for the development of jujube vinegar as a natural, functional food with therapeutic applications.