Abstract
Green tea is one of the most consumed beverages globally. It is very popular due to its specific taste, energizing effect and some health benefits related mainly to the catechins content. Green tea catechins possess antioxidant, anticancer, anti-inflammatory and antimicrobial activity as well as reduce body weight. One of the major and well-studied effects of green tea catechins is their antioxidant effect. However, long-term administration of high doses of antioxidants may result in a pro-oxidant effect and promote cell damage. Therefore, beneficial effects of green tea are directly related to the administered dose of catechins. But it should be noted that consumption of large quantities of green tea beverages or administration of high doses of green tea catechins does not guarantee health benefits and may even lead to adverse effects. This review provides a comprehensive overview of the current knowledge of the antioxidant and pro-oxidant activity of green tea catechins as well as research gaps that require further investigation. In conclusion, despite green tea antioxidant potential, further research is needed to fully understand the benefits and risks associated with the consumption of green tea beverages as well as green tea catechins in different forms and doses.
Keywords:
green tea; catechins; epigallocatechin-3-gallate; antioxidant; pro-oxidant; hepatotoxicity 1. Introduction
Green tea beverages are among the most popular and consumed drinks globally. Green tea is manufactured from the leaves of the tea plant (Camellia sinensis (L.) Kuntze). Its widespread use is due to the specific taste and energizing effect as well as the reported health benefits. Green tea leaves possess antioxidant, anti-inflammatory, antimicrobial and anticancer activity as well as cancer prevention properties and weight loss reduction [1,2]. Green tea leaves contain various biologically active substances such as polyphenols (catechins, flavonoids, theaflavins, tannins), polysaccharides, alkaloids, saponins, free fatty acids, vitamins and minerals and others. However, it is thought that green tea beneficial effects are related mainly to the catechins content [3,4,5].
Catechins are polyphenolic compounds with a characteristic structure and a varying number of phenolic hydroxyl groups [6]. Catechins comprise about 70% of the total polyphenolic content and may reach above 30% of the dry weight of green tea leaves [7,8]. It is well known that green tea catechins undergo chemical reactions such as epimerization and oxidation during the manufacturing and brewing processes [9]. Moreover, the catechin content in tea leaves vary based on different factors such as green tea variety, country of origin, environmental factors such as growing conditions and storage conditions [10,11,12]. In this regard, various catechins and their epimers are found in green tea but the most prevalent in tea leaves are (−)-epigallocatechin-3-gallate (EGCG), (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG) and (−)-epicatechin (EC). Among them, EGCG is the predominant catechin as it content may rich about 50–70% of the total catechin content. The structures of major catechins and their epimers are shown in Figure 1. Additionally, it is believed that EGCG is responsible for most of the health benefits related to green tea consumption [7,13,14,15,16,17].
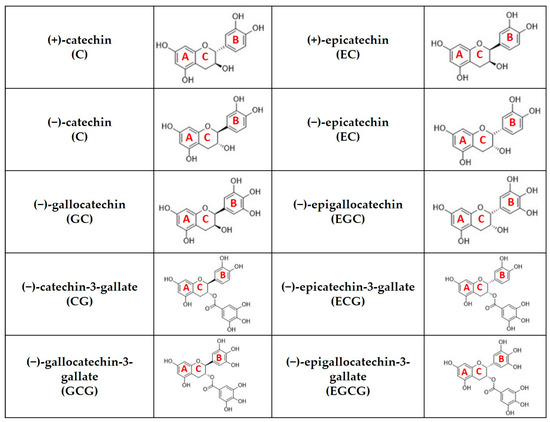
Figure 1.
Chemical structure of catechins that are commonly detected in green tea leaves (A represents A-ring, B represents B-ring, C represents C-ring).
One of the major and well-studied effects of green tea polyphenols is their antioxidant effect. Both catechins and other green tea polyphenols (quercetin, kaempferol, myricetin, rutin and others) have antioxidant activity. Since catechins predominate among the tea polyphenols, it is believed that green tea antioxidant effect is mainly due to them [14,18,19]. Catechins antioxidant potential is observed both in vitro and in vivo but it should be noted that individual catechins possess different antioxidant properties directly related to the position and number of hydroxyl groups in their molecule [1,16,20]. Physiologically, free radicals including reactive oxygen species (ROS) and reactive nitrogen species (RNS) are normal products of cellular metabolism and undergo inactivation by antioxidant enzymes such as catalase (CAT), glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD). Under certain conditions, the balance between the generation of free radicals and their inactivation may be disrupted, resulting in oxidative stress and cellular damage. Therefore, an additional intake of antioxidants may be beneficial and may have a preventive effect on certain diseases such as cardiovascular and tumor diseases [1,20,21]. However, there is evidence that long-term administration of high doses of antioxidants may result in a pro-oxidant effect and promote cell damage [22]. Thus, beneficial effects of green tea are directly related to the administered dose of catechins. At the same time, it should be considered that consumption of large quantities of green tea beverages or the administration of high doses of green tea catechins does not guarantee health benefits and may even lead to adverse effects. In the literature, hepatotoxicity linked to green tea catechins administration is well described [7,17]. In conclusion, despite green tea antioxidant potential, further research is needed to fully understand the benefits and risks associated with the consumption of green tea beverages as well as green tea catechins in different forms and doses.
This review aims to present currently available scientific data on the antioxidant and pro-oxidant activity of green tea products as well as the relationship between pro-oxidant properties of catechins and liver damage in humans.
2. Methods
In order to achieve the set objective, we collected and comprehensively analyzed the latest scientific information about antioxidant and pro-oxidant action of green tea extracts as well as green tea catechins. To gain a better understanding of the hormetic effect of green tea catechins and its connection to liver damage, we conducted a thorough literature search on the Web of Science, Scopus, PubMed and ResearchGate databases and selected a number of articles, mostly published in the last ten years. The initial search yielded over 2200 literature sources related to the topic. The following keywords and their combination were used for the research: green tea, catechins, epigallocatechin-3-gallate, health benefits, antioxidant, pro-oxidant, hormetic, anticancer, hepatotoxicity, and liver damage. After applying predefined inclusion and exclusion criteria and conducting a critical assessment of the methodological quality of the publications, 151 documents from over 90 different journals were selected for further analysis. The selection process involved eliminating duplicate information, screening titles and abstracts, and performing an in-depth full-text evaluation of potentially relevant articles. Both research articles and review articles written in English were included in the manuscript. Additionally, scientific information was retrieved from the internet databases of the European Food Safety Authority, Health Canada and Norwegian Institute of Public Health as well as the United States Pharmacopoeia.
3. Antioxidant Activity of Green Tea Leaves
Green tea is recognized as a significant source of dietary antioxidants. Notably, its main components—polyphenols, particularly catechins—are well-documented and extensively studied for their antioxidant properties, as evidenced by both in vitro and in vivo research, including clinical studies [23,24]. In addition, green tea contains carotenoids, tocopherols, ascorbic acid (vitamin C), and essential minerals such as chromium (Cr), manganese (Mn), selenium (Se), and zinc (Zn), along with various phytochemical compounds. These constituents collectively enhance the antioxidant potential of green tea polyphenols [25].
It is essential to consider that the technological process significantly influences the antioxidant potential of green tea. Green tea leaves contain a much higher catechin content compared to black tea due to the oxidation of catechins to theaflavins during the fermentation process. It is established that the higher catechin content in tea correlates with greater antioxidant activity. The concentration of polyphenolic compounds, including catechins, is influenced by the cultivation conditions of Camellia sinensis, encompassing both climatic and agrotechnical factors. It is noteworthy that the antioxidant activity of green tea infusions is positively correlated with increasing temperature and varies significantly depending on the specific type and geographic origin of the green tea leaves [6,26]. The percentage of green tea constituents influenced by the environmental and agricultural factors, including growing conditions, soil, climatic conditions, and other external factors such as light, geography, microbes, and temperature [27].
Several major mechanisms are thought to contribute to the antioxidant potential of catechins, which can be classified as direct and indirect.
3.1. Direct Antioxidant Properties
The mechanisms of direct antioxidant activity include direct scavenging of free radicals, wherein catechins interact with unstable and highly reactive species to form relatively stable phenolic oxygen radicals, as well as the chelation of metal ions such as copper and iron, which catalyze oxidative reactions [1,20,21,28]. The direct mechanisms of antioxidant activity are present in Figure 2.
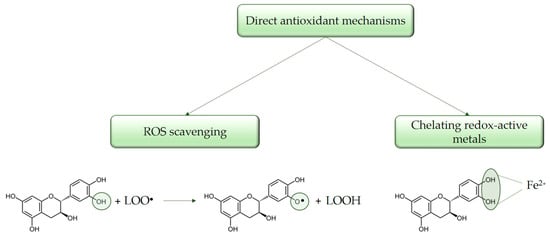
Figure 2.
Direct mechanisms of catechins antioxidant activity.
3.1.1. Direct ROS Scavenging
Catechin and its diastereoisomers share a common chemical structure characterized by phenolic hydroxyl groups, which have the capacity to stabilize free radicals [29]. This structural feature underlies their direct antioxidant activities, allowing catechins to act as free radical scavengers. The phenolic hydroxyl groups of catechins can react with ROS and RNS by donating a hydrogen atom and/or an electron, resulting in the formation of a relatively stable flavonoid radical [30,31]. It is assumed that flavonoids perform their antioxidant function through two reactions, each of which involves the transfer of one electron. In the first reaction, the free radical is reduced and a flavonoid radical (o-semiquinone) is formed. Then, the resulting flavonoid radical, depending on its structure, can bind to another oxidizing radical and donate another hydrogen atom, forming a quinone, or it can dimerize with another flavonoid radical, forming a stable dimer [32]. As it was mentioned, individual polyphenols, including catechins, exhibit distinct antioxidant potential depending on their specific structure, particularly the number and location of hydroxyl groups in their molecule. The antioxidant activity of phenolic compounds is positively correlated with the number of hydroxyl groups present in the molecule. In this regard, some variations in the interactions between individual flavonoids and ROS have been found. For example, catechin gallates show high antioxidant activity against superoxide radicals (O2•−), while luteolin and kaempferol demonstrate pronounced activity against hydroxyl radicals (OH•) [33,34,35]. Furthermore, catechins containing a catechol group exhibit lower antioxidant capacity in comparison to those with a pyrogalol group [6,35,36]. Various studies on the antioxidant activity of catechins classify them according to their effectiveness as radical scavengers as follows: EGCG > ECG > EGC > EC > C [37,38,39,40,41]. Catechins contribute to the reduction of free radicals by donating one electron from their phenolic hydroxyl group. The resulting aroxyl radicals achieve stability through resonance within the aromatic ring structure [42,43]. Upon interaction with the initial reactive species, a radical form of the antioxidant is generated. This radical is stabilized through charge delocalization, a process facilitated by the interaction between the phenolic hydroxyl groups and the π-electrons within the benzene ring [44].
3.1.2. Metal Ion Chelation
Catechins effectively interrupt radical chain reactions and thus prevent the oxidation of cellular lipids. The antioxidant capacity of phenolic compounds is further attributed to their ability to chelate metal ions such as Fe2+, Cu2+, Ca2+ that are involved in free radical production. It is reported that iron stimulates hydroxyl radical production in the Fenton reaction (a reaction between iron and hydrogen peroxide that generates hydroxyl radicals). The presence of adjacent hydroxyl groups within the molecule functions as sites for the iron chelation. It is suggested that one of the main binding sites for metals is with the hydroxyl groups at the third and fourth positions in the B-ring of flavonoids (the so-called catechol moiety) [21,29,45]. By forming inactive complexes with these metal ions, catechins prevent their involvement in Fenton and Haber-Weiss reactions. Consequently, catechins effectively inhibit the initiation of the lipid peroxidation process [1,20]. At the same time, different studies reported that the cellular microenvironment also has a significant impact on the antioxidant activity of polyphenols. There is evidence that the behavior of flavonoids in the presence of metal ions is very complex, which is why there is conflicting information about their antioxidant activity [46].
3.2. Indirect Antioxidant Properties
Catechins may also exert indirect antioxidant effects by increasing the expression of endogenous antioxidant enzymes, inhibition of pro-oxidant enzymes activity, as well as the mechanisms responsible for the generation of oxygen free radicals [20,25,46,47,48]. The major mechanisms of catechins’ indirect antioxidant activity are shown in Figure 3.
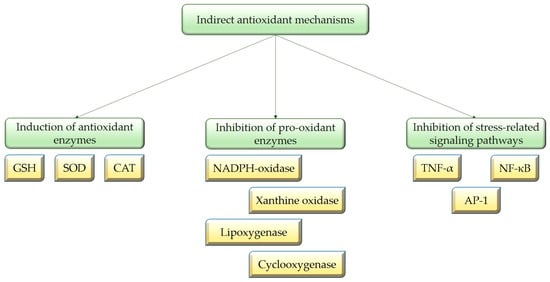
Figure 3.
Indirect mechanisms of catechins antioxidant activity.
Catechins indirectly exert their antioxidant effect by modulating important signaling pathways and transcription factors, such as the nuclear factor kappa B (NF-kB), mitogen-activated protein kinase (MAPK) and the nuclear factor erythroid 2-related factor 2 (NRF2) signaling pathways, as well as the activator protein 1 (AP-1) transcription factor, the signal transducer and activator of transcription 1 (STAT1) transcription factor, and others. Thus, catechins influence protein expression in cells and have the ability to increase the expression of endogenous antioxidant enzymes such as GSH-Px, CAT and SOD. These enzymes are crucial for scavenging ROS and their up-regulation results in simultaneous decrease in the formation of malondialdehyde (MDA), which is produced in the process of lipid peroxidation and serves as a marker for oxidative stress [24,42,49,50,51,52,53,54,55]. According to Khan et al. (1992), the administration of 0.2% catechins in the drinking water of mice led to a significant enhancement in the activities of CAT, SOD and GSH-Px [56]. In another study, two weeks of green tea consumption resulted in the induction of catalase expression in the aorta of spontaneously hypertensive rats [57]. Moreover, catechins have been demonstrated to effectively regenerate vitamin E, by complementing the functions of glutathione (GSH) involved in its recycling [6,58]. In addition, green tea increased plasma and tissue GSH levels in multiple animal studies [49,50,59]. GSH acts as a reducing substrate for glutathione peroxidase during the scavenging of H2O2. Glutathione reductase is a crucial enzyme that converts GSH from its oxidized form back to its reduced form; additionally, glutamyl cysteine synthetase is a rate-limiting enzyme in GSH synthesis. EGCG and ECG can enhance the activity of these two enzymes, thereby ensuring that GSH-Px persistently scavenges oxygen free radicals [20,25,47,49].
Tea catechins can also inhibit pro-oxidant enzymes activity, including xanthine oxidase (XO), nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase, cyclooxygenases (COX), and lipoxygenases. Therefore, catechins may inhibit mechanisms responsible for the generation of oxygen free radicals [20]. It is reported that flavonoids bind to the enzyme XO and inhibit its activity through competitive and mixed-type inhibition [60]. However, the reduced activity of the COX-2 enzyme is due to inhibition of its expression through the modulation of key regulatory molecules in cells such as the activation of PPARγ (peroxisome proliferator activated factor gamma) or the inhibition of NF-κB [61]. According to Biasi et al. (2011), EGCG has the potential to inhibit NADPH oxidase, thereby reducing the subsequent increase in ROS levels [62]. Fraga et al. (2010) suggested that EC and its metabolites have the potential to suppress the activity of NADPH oxidase, leading to a reduction in superoxide production [29]. The O-methylated metabolite of EC possesses structural similarities to apocynin, which is regarded as a classical NADPH oxidase inhibitor [29]. EC may inhibit NADPH oxidase through several mechanisms: direct binding to nitrogen oxides; modulation of calcium influx, thereby preventing NADPH oxidase activation; and regulation of upstream signaling pathways of NADPH oxidase, such as blocking the interaction of ligands with receptors that promote NADPH oxidase activity [63]. Additionally, this class of phytochemicals can inhibit the oxidant production by modulating the interaction of ligands with receptors, e.g., tumor necrosis factor alpha (TNF-α), and they can also inhibit a variety of oxidative stress-related pathways that are implicated in the processes of inflammation [61,63]. Moreover, catechins have the ability to be absorbed onto the cell membrane surface, where they provide a physical barrier against hydro soluble radicals. Inserted into the lipid bilayer, polyphenols would easily scavenge lipid soluble radicals [64]. Therefore, catechins protect membranes and their components from oxidation by offering antioxidant activity through mechanisms that are not solely reliant on free radical scavenging or metal chelation [29].
4. Pro-Oxidant Activity of Green Tea Leaves
As it was mentioned, flavonoids including catechins exhibit complex interactions with metal ions that can result in either antioxidant or pro-oxidant effects, depending on various factors such as their concentration, chemical structure, presence of metal ions, the cellular microenvironment in different tissues, and duration of application. In this regard, results from in vitro studies have shown that flavonoid–metal complexes exhibit a pro-oxidant effect, which can lead to fragmentation of the deoxyribonucleic acid (DNA) molecule. Furthermore, some authors suggest that the pro-oxidant effect may play an essential role in the antitumor activity of flavonoids. In cells rich in copper, they can induce the formation of ROS and subsequent breakage of DNA chains, which leads to tumor cells apoptosis [34,46,65,66]. There are several mechanisms related to the pro-oxidant properties of polyphenols and they are generally expressed in stimulating the oxidation of other compounds. Flavonoids including catechins may exert direct pro-oxidant action due to the increased formation of highly reactive hydroxyl radicals as well as the formation of phenoxyl radicals in the process of neutralization of ROS [65,67]. A suppression of mitochondrial respiration was also reported [6]. Additionally, there are suggestions that oxidative DNA damage may also occur when flavonoids interact with a specific sequence of the DNA molecule resulting in single-strand DNA breaks especially in the presence of reactive nitrogen species [68,69]. Despite the reported pro-oxidant effects of tea polyphenols in the presence of metal ions, most studies show that low doses of flavonoids usually chelate metal ions and suppress ROS formation [70]. Therefore, pro-oxidant properties of polyphenols should not be considered solely as negative effects. In certain cases, pro-oxidant activity can influence cell signaling and alter cellular functions. As a result, the pro-oxidant effect may contribute to some of the biological effects of catechins and other polyphenols [71,72,73].
The mechanism of anticancer activity of green tea catechins is not fully understood yet. It is assumed that several mechanisms are responsible for the antitumor effect of catechins such as the modulation of key signaling pathways (NF-kB, MAPK, EGFR, VEGF and PI3k/AKT) and protein expression in cancer cells, the regulation of enzyme activity (inhibition of tyrosine kinases), the induction of apoptosis, and others. Additionally, there is substantial evidence suggesting that the pro-oxidant activity of green tea catechins plays an important role in the inhibition of cancer cell growth as well as the induction of apoptosis [74,75,76]. Some researchers propose that low doses of polyphenols are more effective in cancer prevention, likely due to their antioxidant properties and their ability to modulate cell signaling pathways. Conversely, higher doses of polyphenols seem to exert a stronger antitumor effect, probably due to their pro-oxidant properties, which may lead to an induction of apoptosis in cancer cells [71,72,73]. However, despite the reported antitumor activity of EGCG, at present it cannot be used in the therapy of malignant diseases due to its low oral bioavailability and low stability [46,74].
Moreover, it is suggested that the bactericidal effect of EGCG is due to its ability to lower the O2 level in bacterial cells, due to the increased formation of H2O2 [77].
Nevertheless, despite the abovementioned beneficial effects of catechins in cancer and bacterial cells, pro-oxidant activity in healthy human cells may also be related to toxic effects. In this regard, hepatotoxicity associated with green tea catechins intake is reported [78].
5. Association Between the Pro-Oxidant Activity of Green Tea Catechins and Hepatotoxicity Manifestation
Traditional green tea is the second most widely consumed beverage after water, whereas the use of green tea extract (GTE) has emerged as a relatively recent phenomenon, gaining widespread popularity in the production of dietary supplements, primarily for weight loss support [79,80,81,82,83]. However, since the 1990s, green tea-derived products have been associated with potentially severe and irreversible liver damage [82,83,84,85,86]. In this regard, growing evidence suggests that green tea catechins may also act as pro-oxidants, which could underlie hepatotoxicity [87]. Based on in vitro and in vivo experiments, catechins and their gallate esters have been identified as the probable culprits of hepatotoxicity, with EGCG demonstrating the highest cytotoxic potential [79,81,82,88,89,90]. In experiments with rat liver cells, Galati et al. (2006) demonstrated that catechins, particularly EGCG, disrupt the mitochondrial membrane, leading to the collapse of the mitochondrial membrane potential, and induce the formation of ROS and deplete GSH, resulting in hepatocyte cytotoxicity [89]. As a result, cells with depleted GSH are even more susceptible to EGCG toxicity and additional ROS formation, suggesting that GSH plays a role in the detoxification of this compound [89]. EGCG has also been implicated as a potential inhibitor of cystathionine β-synthase activity—an enzyme involved in the conversion of methionine to cysteine in the process of GSH synthesis [91]. Additionally, Sang et al. (2005) found that the treatment of experimental mice with toxic doses of EGCG (200 and 400 mg/kg, i.p.) led to the formation of two cysteine conjugates of EGCG, likely arising from the formation of a quinone, which then reacts with the thiol group of cysteine as well as other molecules containing protein (such as GSH) [92]. The role of the pro-oxidative effect of green tea polyphenolic compounds in hepatotoxicity is further confirmed by Lambert et al. (2010), who, by treating CF-1 mice with high doses of EGCG, observed a dose-dependent increase in hepatic lipid peroxidation, hepatic metallothionein I/II expression, and hepatic levels of gamma H2A histone family member X (γH2AX) [84]. Similarly to other authors, they concluded that the hepatotoxic effect of high doses of EGCG seems to be linked to induction of oxidative stress in liver cells [84]. Furthermore, according to Galati et al. (2006), the inhibition of catechol-O-methyltransferase by the anti-Parkinson agent Entacapone significantly increases EGCG toxicity and ROS formation, suggesting that methylation in hepatocytes may participate in the detoxification process [89]. It is possible that the enzyme protects cells from oxidative stress and hepatotoxicity through methylation of the 4′- and 4″-hydroxyl groups of EGCG, which are potential sites for quinone formation and redox cycling [92]. Additionally, it has been reported that the reductive detoxification of EGCG may be catalyzed by NADPH:quinone oxidoreductase 1 [89].
Moreover, over 90 cases of hepatotoxicity in humans were reported over the last 25 years [86]. Particularly, in the early 2000s, 13 cases of acute liver injury were reported following the use of the weight-loss product Exolise®, which contained a high concentration of EGCG. Most reported cases presented a hepatocellular injury pattern within three months of use, with recovery occurring after discontinuation. At least seven patients experienced rapid recurrence of liver injury upon re-exposure to the product. Consequently, the product was withdrawn from the French and Spanish markets in 2003 [81,93,94]. Mazzanti et al. (2009) concluded that the reported cases of liver damage can likely be related to catechins intake, especially EGCG [81]. Although catechins exhibit limited oral bioavailability, it is proposed that under certain conditions, such as fasting or repeated dosing, their plasma concentrations may be increased resulting in hepatotoxic effect. Consistent with other authors’ findings, Mazzanti et al. (2009) assumed that the observed liver damage is due to the ability of EGCG or its metabolites to generate oxidative stress [81]. Additionally, an idiosyncratic or immune-mediated allergic response cannot be ruled out as a contributing mechanism [81]. Subsequently, other case reports indicated about liver damage due to green tea intake [95,96,97,98,99,100,101,102,103,104]. The analysis of individual clinical cases reveals elevated levels of transaminases, alkaline phosphatase, gamma-glutamyltransferase, and bilirubin [81]. Abdominal pain, and at times jaundice, may occur [84,96]. The histological examination revealed inflammatory reactions, cholestasis, occasionally steatosis, and necrosis. Later, in 2009, the multi-ingredient herbal dietary supplement Hydroxycut®, containing GTE, was also withdrawn from the U.S. market due to reported cases of hepatotoxicity [95].
The randomized Minnesota Green Tea Trial in postmenopausal women provides even more compelling evidence of the hepatotoxic effects of EGCG. It was a placebo-controlled, double-blind trial that investigated the effects of daily GTE (equal to approximately 800 mg EGCG) intake over 12 months in order to observe its influence on breast cancer biomarkers [105]. Subsequently, increases in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were observed in the GTE group compared to the placebo one. A total of 26 participants (5.1%) in the treatment group exhibited moderate or severe abnormalities in liver function tests. Furthermore, in some participants, signs of liver injury improved following the discontinuation of GTE; however, upon resumption of its consumption, they developed recurrent liver injury at an accelerated rate [106,107,108,109]. Another multi-phase clinical study with Polyphenon E® (50% EGCG), including a small number of multiple sclerosis patients, also demonstrated clear signs of hepatotoxicity. A total intake of 800 mg EGCG daily for 6 months led to the discontinuation of one participant from phase I due to abnormalities in liver function tests. The entire study was halted at phase II, as five out of seven patients in the GTE group had also developed abnormal liver function tests (including abdominal pain and elevated liver enzymes) [110]. Several clinical cases that reported liver injury associated only with green tea consumption are summarized in Table 1. It should be noted that cases of hepatotoxicity linked to the intake of multi-ingredient supplements containing green tea are not included. Additionally, cases in which the patient administered green tea products and medications simultaneously are also not mentioned. However, such a scenario is more common among the reported clinical data. At the same time, numerous studies indicate potential herb-drug interactions. According to in vitro and in vivo experiments, green tea catechins may inhibit the activity of drug-metabolizing enzymes and thus may alter the effectiveness and safety of concomitantly administered drugs [111]. Therefore, the possibility for liver damage due to herb-drug interactions should not be excluded.

Table 1.
Studies that reported cases of hepatotoxicity associated with green tea consumption.
Given its usual prolonged use, the potential link between green tea intake and liver cancer has also been investigated. The National Toxicology Institute conducted chronic toxicity and carcinogenesis studies with GTE (48.4% EGCG) in B6C3F1/N mice and Wistar Han rats. Increased incidences of hematopoietic cell proliferation and inflammation in the liver were observed in male mice receiving 300 mg/kg. In rats, elevated incidences of hepatic necrosis and hyperplasia of oval cells were seen in females at 1000 mg/kg [112]. However, the analysis of epidemiological data in humans remains inconclusive [113].
In contrast to concerns regarding hepatotoxicity, some experimental and clinical data support the potential benefits of GTE, including their role as a protective agent in drug-induced liver injury, as polyphenols possess antioxidant, anti-inflammatory, and prebiotic properties [114]. Moreover, based on a comprehensive literature analysis, Winiarska-Mieczan et al. (2024) suggest that hepatotoxicity may be induced only in the presence of pre-existing mitochondrial abnormalities, as these lead to increased mitochondrial membrane permeability to EGCG [115]. In addition, the conducted analysis of randomized controlled trials by Isomura et al. (2016) shows that the overall incidence of liver damage associated with green tea is relatively low (0.5%) [116]. Pharmacokinetic studies in healthy volunteers also indicate that due to its low bioavailability, the maximum achievable plasma concentration of EGCG reaches approximately 8 μM, rarely exceeding the concentrations typically used in in vitro studies demonstrating hepatotoxic effects (10–100 μM) [117,118]. However, it should be noted that under certain conditions, catechin levels in plasma may rise, and toxicity may be observed [81,82,119]. The bioavailability of catechins following oral administration is low: plasma levels of EGCG correspond to only 0.2–2.0% of the administered dose. Furthermore, there is uncertainty regarding the actual exposure to EGCG due to its insufficient in vitro and in vivo stability [119,120,121,122,123,124]. In this regard, Isbrucker et al. (2006 a, b) found that the area under the curve for EGCG was significantly higher in fasted dogs (205.7 ng h/mL) than in those pre-fed (19.8 ng h/mL) following two weeks of daily administration of 300 mg/kg via the diet [125,126]. Furthermore, in pre-fed dogs, 13 weeks of daily 500 mg/kg EGCG administration did not result in adverse effects, whereas in fasted dogs, the same dose led to increased serum bilirubin levels in all animals, as well as elevated ALT, AST, and γ-glutamyltransferase (γ-GT) levels in one or more dogs from the high-dose groups [125,126]. The increased risk of hepatotoxicity in fasted dogs receiving Polyphenon E® (63.3–64.8% EGCG) was also confirmed by a study from the National Cancer Institute, which was prematurely terminated due to widespread morbidity and mortality at a dose of 1000 mg/kg. Significant hepatotoxicity was observed, including increases in AST, ALT, alkaline phosphatase (ALP), total bilirubin, and triglycerides, centrilobular necrosis, and chronic active inflammation with infiltration of neutrophils and mononuclear cells in the liver, as well as brown pigment in Kupffer cells. The authors concluded that the no observed adverse effect level (NOAEL) was less than 200 mg/kg (128 mg/kg EGCG; human equivalent dose (HED) of 71 mg/kg) [127]. These observations are supported by the clinical research conducted by Chow et al. (2005) showing that fasting increases the plasma concentration of EGCG fivefold compared to ingestion with food [119]. Additionally, Chow (2001) reported that plasma EGCG concentrations rise with higher doses, likely due to metabolic saturation during first-pass metabolism in the liver [128]. The fact that most green tea-containing dietary supplements are marketed for weight loss further suggests that recipients are more likely to take them on an empty stomach, thus increasing their risk in this specific context [83,116].
Concurrent consumption of green tea products and hepatotoxic foods or medications may increase the risk of morbidity [113,129]. Moreover, it is evident that adverse events caused by multi-component herbal mixtures containing green tea have a shorter latency period and result in significantly more severe liver damage, including documented cases of liver transplantation [130,131,132]. However, clinical cases have been reported without the concomitant use of medications/herbs, suggesting that the independent use of green tea products still remains suspicious [81,133,134].
The production techniques employed in the manufacturing of GTE not only serve to remove undesirable concomitant substances but may also result in significantly higher concentrations of catechins compared to traditional green tea beverages, to the extent that they may be considered as pure substances [81,135,136,137]. Extracts derived from tea varieties with higher levels of methylated EGCG may also lead to increased systemic exposure to EGCG [137]. Simultaneously, the concentration of extracts may elevate the levels of potential hepatotoxic contaminants. GTE produced through extraction with organic solvents may contain residues of these solvents (such as chloroform, dichloromethane, ethyl acetate, or others) in the final product. However, further controlled studies are needed to establish their relationship with hepatotoxicity [82]. Pesticides may also be present in the final product during the tea infusion process [82,138]. According to Malnick et al. (2022), a significant issue is the intentional adulteration of herbal products, as well as contamination with heavy metals (mercury, lead, arsenic, and copper) [82,114]. Additionally, tea substances may be contaminated with pyrrolizidine alkaloids during the co-harvesting with other plants containing these compounds (e.g., senecionine-N-oxide, retrorsine-N-oxide, intermedine, lycopsamine). These are secondary plant metabolites known to be hepatotoxic (associated with the development of hepatic sinusoidal obstructive syndrome), genotoxic, and carcinogenic. On the other hand, according to Oketch-Rabah et al. (2020), the characteristics of liver damage associated with GTE are hepatocellular, which contrasts with the signal transduction oxidative stress damage induced by pyrrolizidine alkaloids [82]. Therefore, the relationship between hepatotoxicity and all the aforementioned risk factors concerning the contamination of green tea products should be the subject of future and much more thorough investigations.
Most cases of liver damage have occurred in women, raising the question of the role of sex in determining whether green tea exposure leads to liver damage. In this context, Goodin et al. (2006) have shown that female mice of the Swiss Webster strain are more susceptible to EGCG-induced toxicity compared to males [139]. They suggest that EGCG may accumulate in female mice liver to a greater extent than in male mice due to a decreased ability for its elimination [139]. On the other hand, in chronic toxicity and carcinogenesis studies with GTE (48.4% EGCG) conducted by the National Toxicology Institute, increased incidences of hematopoietic cell proliferation and liver inflammation were observed in male specimens. Additionally, a lower NOAEL for hepatic effects was identified in male mice (100 mg/kg, compared to 300 mg/kg in females) [112]. As noted by other authors, it is important to consider that women are more susceptible to liver damage, and it is also known that there is a higher prevalence of supplement use among women [140]. Therefore, these findings do not conclusively indicate that a specific sex or population is more susceptible to hepatotoxicity induced by GTE [141,142].
There is also a genetic and immune-related hypothesis regarding the green tea-induced hepatotoxicity. Research on diversity outbred mice, a genetically heterogeneous population exposed to EGCG (50 mg/kg; daily for three days), indicates good toleration by most rodents, but a small subset (16%) developed severe hepatotoxicity. This suggests that a small but significant portion of the mouse population is susceptible to liver damage when exposed to EGCG at levels that are non-toxic to the majority [143]. If genetic factors influence some sensitivity in humans too, this could offer an explanation for the rarely observed severe drug-induced liver injury with jaundice. Additional evidence supporting the hypothesis that genetic predisposition plays a substantial role in GTE-induced liver damage was provided by a recent study on PD-1 knockout mice, designed to model idiosyncratic drug-induced liver injury. This study demonstrated that EGCG could induce immune-mediated liver damage. PD-1 knockout mice treated with a higher dose of GTE (500 mg/kg) showed a delayed increase in ALT, which was not observed in similarly treated wild-type mice [82]. In this context, Hoofnagle et al. (2021) established a strong association between green tea-related liver damage and the HLA-B35:01* allele, which is present in 5–15% of the American population [144]. Within their study, 72% of patients were carriers of at least one copy of the HLA-B35:01* allele, further indicating that liver damage following green tea consumption may have an idiosyncratic and immune-mediated nature [144]. The literature also suggests that a significant portion of patients with hepatitis following green tea consumption are from Spanish-speaking communities. However, the hypothesis of ethnic-genetic predisposition is not widely accepted due to the issue of obesity and the high consumption of weight-loss products within this population [81,145]. This observation is supported by the European Food Safety Authority (EFSA), which noted that liver effects were more pronounced in individuals with a high body mass index, a notable finding since GTE are commonly used in dietary supplements for weight control [113].
6. Discussion
Green tea beneficial effects are well known and have been observed in a number of in vitro studies. The putative mechanisms of action of catechins have been described. However, their effects in humans are still not fully understood. Moreover, the circumstances under which tea catechins exert antioxidant and pro-oxidant effects remain unclear, as well as the relationship between the reported toxic effects of catechins and their pro-oxidant properties.
The literature review indicates that among the numerous clinical cases of GTE-associated hepatotoxicity, few are well-documented with comprehensive information regarding dosage, and the patient’s medical history, including concurrent use of other medications. Often, questions regarding the full qualitative and quantitative composition of the product, the duration of intake, and the temporal relationship between consumption and the onset of damage remain unanswered [113]. Frequently, these cases involve patients with chronic liver diseases (alcoholism or viral hepatitis), whose clinical and biochemical characteristics may overlap with GTE-induced toxicity [114]. Medications used to treat these conditions may also contribute to the herbal toxicity. This issue significantly complicates the assessment of the causal relationship between liver damage and green tea-products intake, and should be considered by experts in the field. Nevertheless, it can be noted that the characteristics of liver damage observed in all reviewed cases were hepatocellular or hepatitis in nature [82].
According to Teschke and Xuan (2019), the risk of hepatotoxicity due to GTE consumption is real, with exposure potentially leading to liver damage, including severe hepatic injury [146]. Additionally, the relatively rare reports of liver damage with jaundice, which suggest greater severity, and the numerous factors influencing this outcome, contribute to the unpredictability of the pathology in question. Genetic variability and bioavailability may explain susceptibility to the wide range of doses reported in cases of liver damage induced by GTE and suggest that it could also be idiosyncratic in susceptible individuals. Against the backdrop of thousands of studies supporting the health benefits of systemic green tea consumption, the authors argue that the benefit-risk ratio is negative. However, they maintain that green tea consumption in beverage form should not be restricted [146].
In order to enhance the reliability and conclusiveness of the obtained data, experimental and/or clinical conditions should have all additional aggravating risk factors are ruled out (e.g., production errors, toxicokinetics, genetic factors, polypharmacy, etc.), or at least their influence should be quantifiably measured. Unfortunately, in practice, such idealistic conditions are difficult to achieve. On the one hand, these are plant-based products whose proper production is a critical factor influencing qualitative variability in the final product. In this regard, according to EFSA (2018), the content of the hepatotoxic agent EGCG may vary between 1600 and 20,320 mg per 100 g of dried leaves—a 13-fold difference [113].
Other unresolved issues are related to the risk of interactions between the bioactive substances of multi-component dietary supplements, as well as the inadvertent or deliberate contamination and adulteration of these products. Therefore, the qualitative aspects of herbal products should be taken into account when assessing their safety and potential adverse effects. Given the more lenient regulatory framework concerning natural products, non-clinical and clinical safety and efficacy assessments are not required before the market launch of herbal products. This means that safety concerns are not identified until post-marketing surveillance [81]. Considering that, according to the FDA, adverse events related to dietary supplement consumption are significantly lower than those associated with drugs (under 10%); one must question the true scale of the problem and whether green tea-based weight loss products will follow the fate of numerous other products from this category being withdrawn [147]. Furthermore, a specific process for evaluating the causal relationship in herbal-induced liver damage has not been developed, although significant efforts are being made in this regard through scientific organizations such as the Drug-Induced Liver Injury Network or the validated Roussel Uclaf Causality Assessment Method [148,149].
Following the high-profile cases of hepatotoxicity, regulatory statements have emerged over the years from various national and international health and scientific structures. The United States Pharmacopeial Convention has conducted a systematic analysis of available preclinical and clinical data and has proposed the inclusion of warning labels on powdered decaffeinated GTE [82]. In 2017, Health Canada, also expressing concern, strengthened its warning regarding the hepatotoxicity risk associated with GTE in its monographs [150]. In 2018, based on clinical research, the EFSA announced that the intake of GTE as a dietary supplement providing EGCG amounts equal to or above 800 mg per day increases serum transaminases, indicating liver damage. Nevertheless, according to the panel, it is not possible to identify a dose of EGCG from GTE that can be considered safe, as in one case, the intake of 375 mg of 80% ethanol extract led to hepatotoxicity [113]. Norway also reviewed potential safety concerns related to GTE consumption and concluded that a daily intake exceeding 0.4 mg EGCG/kg body weight as a bolus could cause adverse biological effects. Furthermore, it was noted that there is an increased susceptibility to toxicity when GTE or green tea infusion is consumed following fasting [151].
7. Conclusions
In the scientific databases available, a number of review and research articles report the antioxidant activity of green tea leaves and especially green tea catechins. Antioxidants intake has been linked to an undeniable health benefit. At the same time, pro-oxidant properties may be related to both beneficial and toxic effects of catechins. It is suggested that the low levels of free radicals formed due to the pro-oxidant activity of polyphenols may lead to an increase in the levels of endogenous antioxidants, resulting in an overall cytoprotection. On the other hand, pro-oxidant properties of polyphenols may lead to cell damage. Therefore, the question remains: how much antioxidant supplementation is too much? The answer to this question is still unclear.
We consider the EFSA recommendation about dietary supplementation with GTE particularly valuable. According to it, daily EGCG intake should not exceed 800 mg, as higher doses have been associated with increased serum transaminase levels, indicative of liver damage. Patients should be informed that moderate consumption of green tea infusion (1–2 cups per day) is generally considered safe, provided product quality and other risk factors are accounted for. However, the possibility of an adverse reaction cannot be excluded especially in sensitive patients. Therefore, any side effect during green tea intake should be reported to a doctor or community pharmacist as soon as possible. Moreover, patients should be advised to avoid the concomitant use of green tea preparations and drugs as well as to avoid green tea consumption on an empty stomach. Furthermore, individuals with liver vulnerability should only use such products after consulting a healthcare provider or pharmacist. Additionally, all patients should be advised to choose products with clearly stated doses of catechins and that are compliant with good manufacturing practices.
In conclusion, at present, the antioxidant and pro-oxidant activity of polyphenols, including catechins, are not fully understood. Therefore, further in-depth studies of the hormetic effect of green tea catechins as well as other antioxidants in humans are necessary. This will lead to a better understanding of the circumstances responsible for the occurrence of antioxidant or pro-oxidant effects. Consequently, the range of doses at which the antioxidant effect is predominantly manifested can be more precisely determined for green tea catechins as well as other natural antioxidants.
Author Contributions
Conceptualization, M.R.-I. and S.S.; methodology, N.H.; software, N.H.; validation, M.R.-I., S.S. and N.H.; formal analysis, S.S.; investigation, M.R.-I.; resources, S.S. and N.H.; data curation, S.S.; writing—original draft preparation, S.S. and N.H.; writing—review and editing, M.R.-I.; visualization, K.D.G.; supervision, M.R.-I.; project administration, K.D.G.; funding acquisition, K.D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed by the European Union—Next Generation EU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project № BG-RRP-2.004-0009-C02.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EGCG | (−)-epigallocatechin-3-gallate |
| EGC | (−)-epigallocatechin |
| ECG | (−)-epicatechin-3-gallate |
| EC | (−)-epicatechin |
| C | Catechin |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| CAT | Catalase |
| GSH-Px | Glutathione peroxidase |
| SOD | Superoxide dismutase |
| NF-kB | Nuclear factor kappa B |
| MAPK | Mitogen-activated protein kinase |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| AP-1 | Activator protein 1 |
| STAT1 | Signal transducer and activator of transcription 1 |
| MDA | Malondialdehyde |
| GSH | Glutathione |
| XO | Xanthine oxidase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| COX | Cyclooxygenase |
| PPARγ | Peroxisome proliferator activated factor gamma |
| TNF alpha | Tumor necrosis factor alpha |
| DNA | Deoxyribonucleic acid |
| GTE | Green tea extract |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| γH2AX | gamma H2A histone family member X |
| γGT | γ-glutamyltransferase |
| ALP | Alkaline phosphatase |
| NOAEL | No observed adverse effect level |
| HED | Human equivalent dose |
| EFSA | European Food Safety Authority |
References
- Truong, V.L.; Jeong, W.S. Cellular Defensive Mechanisms of Tea Polyphenols: Structure-Activity Relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Sirisena, S.; Ng, K. Phytochemical profile of differently processed tea: A review. J. Food Sci. 2022, 87, 1925–1942. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with Green tea polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Tang, G.Y.; Meng, X.; Gan, R.Y.; Zhao, C.-N.; Liu, Q.; Feng, Y.-B.; Li, S.; Wei, X.-L.; Atanasov, A.G.; Corke, H.; et al. Health functions and related molecular mechanisms of tea components: An update review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef]
- Bag, S.; Mondal, A.; Majumder, A.; Banik, A. Tea and its phytochemicals: Hidden health benefits & modulation of signaling cascade by phytochemicals. Food Chem. 2021, 371, 131098. [Google Scholar] [CrossRef]
- LIczbiński, P.; Bukowska, B. Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations. Ind. Crop. Prod. 2022, 175, 114265. [Google Scholar] [CrossRef]
- Wang, H.; Helliwell, K. Epimerisation of catechins in green tea infusions. Food Chem. 2000, 70, 337–344. [Google Scholar] [CrossRef]
- Krahe, J.; Krahe, M.A.; Naumovski, N. The Implications of Post-Harvest Storage Time and Temperature on the Phytochemical Composition and Quality of Japanese-Styled Green Tea Grown in Australia: A Food Loss and Waste Recovery Opportunity. Beverages 2021, 7, 25. [Google Scholar] [CrossRef]
- Wakamatsu, M.; Yamanouchi, H.; Sahara, H.; Iwanaga, T.; Kuroda, R.; Yamamoto, A.; Minami, Y.; Sekijima, M.; Yamada, K.; Kajiya, K. Catechin and caffeine contents in green tea at different harvest periods and their metabolism in miniature swine. Food Sci. Nutr. 2019, 7, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Deka, H.; Barman, T.; Dutta, J.; Devi, A.; Tamuly, P.; Paul, R.K.; Karak, T. Catechin and caffeine content of tea (Camellia sinensis L.) leaf significantly differ with seasonal variation: A study on popular cultivars in North East India. J. Food Compos. Anal. 2021, 96, 103684. [Google Scholar] [CrossRef]
- Zheng, X.-Q.; Zhang, X.-H.; Gao, H.-Q.; Huang, L.-Y.; Ye, J.-J.; Ye, J.-H.; Lu, J.-L.; Ma, S.-C.; Liang, Y.-R. Green Tea Catechins and Skin Health. Antioxidants 2024, 13, 1506. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea polyphenols in promotion of human health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Mancini, E.; Beglinger, C.; Drewe, J.; Zanchi, D.; Lang, U.E.; Borgwardt, S. Green tea effects on cognition, mood and human brain function: A systematic review. Phytomedicine 2017, 34, 26–37. [Google Scholar] [CrossRef]
- Rojano-Ortega, D. Regular, but not acute, green tea supplementation increases total antioxidant status and reduces exercise-induced oxidative stress: A systematic review. Nutr. Res. 2021, 94, 34–43. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Al Shabrmi, F.M.; Allemailem, K.S.; Aly, S.M.; Khan, M.A. Implications of green tea and its constituents in the prevention of cancer via the modulation of cell signalling pathway. BioMed Res. Int. 2015, 2015, 925640. [Google Scholar] [CrossRef]
- Nain, C.W.; Mignolet, E.; Herent, M.-F.; Quetin-Leclercq, J.; Debier, C.; Page, M.M.; Larondelle, Y. The Catechins Profile of Green Tea Extracts Affects the Antioxidant Activity and Degradation of Catechins in DHA-Rich Oil. Antioxidants 2022, 11, 1844. [Google Scholar] [CrossRef]
- Maiti, S.; Nazmeen, A.; Medda, N.; Patra, R.; Ghosh, T.K. Flavonoids green tea against oxidant stress and inflammation with related human diseases. Clin. Nutr. Exp. 2019, 24, 1–14. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fasipe, B.; Laher, I. Potential harms of supplementation with high doses of antioxidants in athletes. J. Exerc. Sci. Fit. 2022, 20, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Kukuła-Koch, W.; Czop, M.; Helon, P.; Gumbarewicz, E. The Role of Extracting Solvents in the Recovery of Polyphenols from Green Tea and Its Antiradical Activity Supported by Principal Component Analysis. Molecules 2020, 25, 2173. [Google Scholar] [CrossRef]
- Sheng, Y.; Sun, Y.; Tang, Y.; Yu, Y.; Wang, J.; Zheng, F.; Li, Y.; Sun, Y. Catechins: Protective mechanism of antioxidant stress in atherosclerosis. Front Pharmacol. 2023, 14, 1144878. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Yanagimoto, K.; Ochi, H.; Lee, K.G.; Shibamoto, T. Antioxidative activities of volatile extracts from green tea, oolong tea, and black tea. J. Agric. Food Chem. 2003, 51, 7396–7401. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, Z.; Wang, Y.; Guo, C.; Cai, X.; Zhang, Y.; Liu, L.; Xue, H.; Tang, J. Tea polyphenols alleviate hydrogen peroxide-induced oxidative stress damage through the Mst/Nrf2 axis and the Keap1/Nrf2/HO-1 pathway in murine RAW264.7 cells. Exp. Ther. Med. 2021, 22, 1473. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Deng, G.; Zhang, Y.-C. Multiple free radical scavenging reactions of flavonoids. Dye. Pigment. 2022, 198, 109877. [Google Scholar] [CrossRef]
- Tumilaar, S.G.; Hardianto, A.; Dohi, H.; Kurnia, D. A Comprehensive Review of Free Radicals, Oxidative Stress, and Antioxidants: Overview, Clinical Applications, Global Perspectives, Future Directions, and Mechanisms of Antioxidant Activity of Flavonoid Compounds. J. Chem. 2024, 2024, 594386. [Google Scholar] [CrossRef]
- Naróg, D.; Sobkowiak, A. Electrochemistry of Flavonoids. Molecules 2023, 28, 7618. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhao, B.; Shen, S.; Hou, J.; Hu, J.; Xin, W. ESR study on the structure-antioxidant activity relationship of tea catechins and their epimers. Biochim. Biophys. Acta 1999, 1427, 13–23. [Google Scholar] [CrossRef]
- Simunkova, M.; Barbierikova, Z.; Jomova, K.; Hudecova, L.; Lauro, P.; Alwasel, S.H.; Alhazza, I.; Rhodes, C.J.; Valko, M. Antioxidant vs. Prooxidant Properties of the Flavonoid, Kaempferol, in the Presence of Cu(II) Ions: A ROS-Scavenging Activity, Fenton Reaction and DNA Damage Study. Int. J. Mol. Sci. 2021, 22, 1619. [Google Scholar] [CrossRef]
- Rice-evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Intra, J.; Kuo, S.M. Physiological levels of tea catechins increase cellular lipid antioxidant activity of vitamin C and vitamin E in human intestinal Caco-2 cells. Chem. Biol. Interact. 2007, 169, 91–99. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef]
- Zaveri, N.T. Green tea and its polyphenolic catechins: Medicinal uses in cancer and noncancer applications. Life Sci. 2006, 78, 2073–2080. [Google Scholar] [CrossRef]
- Azman, N.A.M.; Peiró, S.; Fajarí, L.; Julià, L.; Almajano, M.P. Radical scavenging of white tea and its flavonoid constituents by electron paramagnetic resonance (EPR) spectroscopy. J. Agric. Food Chem. 2014, 62, 5743–5748. [Google Scholar] [CrossRef]
- Fujisawa, S.; Kadoma, Y. Comparative study of the alkyl and peroxy radical scavenging activities of polyphenols. Chemosphere 2006, 62, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.-Y.; Sang, L.-X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–354. [Google Scholar] [CrossRef]
- Parr, A.J.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A. Understanding the Prooxidant Action of Plant Polyphenols in the Cellular Microenvironment of Malignant Cells: Role of Copper and Therapeutic Implications. Front. Pharmacol. 2022, 13, 929853. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Birch, E.J.; Everett, D.W. Green tea catechins suppress xanthine oxidase activity in dairy products: An improved HPLC analysis. J. Food Compost. Anal. 2016, 48, 120–127. [Google Scholar] [CrossRef]
- Zuo, X.; Tian, C.; Zhao, N.; Ren, W.; Meng, Y.; Jin, X.; Zhang, Y.; Ding, S.; Ying, C.; Ye, X. Tea polyphenols alleviate high fat and high glucose-induced endothelial hyperpermeability by attenuating ROS production via NADPH oxidase pathway. BMC Res. Notes 2014, 7, 120. [Google Scholar] [CrossRef]
- Frei, B.; Higdon, J.V. Antioxidant activity of tea polyphenols in vivo: Evidence from animal studies. J. Nutr. 2003, 133, 3275S–3284S. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Dingda, D.; Wang, L.; Gao, F. The primary studies of epigallocatechin-3-gallate in improving brain injury induced by chronic high-altitude natural environment in rats by 7.0T high-field MR imaging. Arch Biochem. Biophys. 2025, 764, 110224. [Google Scholar] [CrossRef]
- Ahmad, A.; Singhal, U.; Hossain, M.M.; Islam, N.; Rizvi, I. The role of the endogenous antioxidant enzymes and malondialdehyde in essential hypertension. J. Clin. Diagn. Res. JCDR 2013, 7, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramiro, I.; Martín, M.A.; Ramos, S.; Bravo, L.; Goya, L. Comparative effects of dietary flavanols on antioxidant defences and their response to oxidant-induced stress on Caco2 cells. Eur. J. Nutr. 2011, 50, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Velalar, C.N.; Ruan, R. Effects of epigallocatechin-3-gallate on mitochondrial integrity and antioxidative enzyme activity in the aging process of human fibroblast. Free Radic. Biol. Med. 2008, 44, 1032–1041. [Google Scholar] [CrossRef]
- Suhail, M.; Rehan, M.; Tarique, M.; Tabrez, S.; Husain, A.; Zughaibi, T.A. Targeting a transcription factor NF-κB by green tea catechins using in silico and in vitro studies in pancreatic cancer. Front. Nutr. 2023, 9, 1078642. [Google Scholar] [CrossRef]
- Kim, J.M.; Heo, H.J. The roles of catechins in regulation of systemic inflammation. Food Sci. Biotechnol. 2022, 31, 957–970. [Google Scholar] [CrossRef]
- Khan, S.G.; Katiyar, S.K.; Agarwal, R.; Mukhtar, H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: Possible role in cancer chemoprevention. Cancer Res. 1992, 52, 4050–4052. [Google Scholar]
- Negishi, H.; Xu, J.W.; Ikeda, K.; Njelekela, M.; Nara, Y.; Yamori, Y. Black and green tea polyphenols attenuate blood pressure increases in stroke-prone spontaneously hypertensive rats. J. Nutr. 2004, 134, 38–42. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Huang, Y.; Tsang, D.; Chen, Z.Y. Regeneration of alpha-tocopherol in human low-density lipoprotein by green tea catechin. J. Agric. Food Chem. 1999, 47, 2020–2025. [Google Scholar] [CrossRef]
- Hsu, Y.-W.; Chen, W.-K.; Tsai, C.-F. Senescence-Mediated Redox Imbalance in Liver and Kidney: Antioxidant Rejuvenating Potential of Green Tea Extract. Int. J. Environ. Res. Public Health 2022, 19, 260. [Google Scholar] [CrossRef]
- Xue, H.; Xu, M.; Gong, D.; Zhang, G. Mechanism of flavonoids inhibiting xanthine oxidase and alleviating hyperuricemia from structure–activity relationship and animal experiments: A review. Food Front. 2023, 4, 1643–1665. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Biasi, F.; Astegiano, M.; Maina, M.; Leonarduzzi, G.; Poli, G. Polyphenol Supplementation as a Complementary Medicinal Approach to Treating Inflammatory Bowel Disease. Curr. Med. Chem. 2011, 18, 4851–4865. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Oteiza, P.I. Dietary flavonoids: Role of (−)-epicatechin and related procyanidins in cell signaling. Free Radic. Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.V.; Keen, C.L.; Schmitz, H.H.; Fraga, C.G.; Oteiza, P.I. Flavan-3-ols and procyanidins protect liposomes against lipid oxidation and disruption of the bilayer structure. Free Radic Biol. Med. 2003, 34, 84–92. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. PTR 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Arif, H.; Rehmani, N.; Farhan, M.; Ahmad, A.; Hadi, S.M. Mobilization of Copper ions by Flavonoids in Human Peripheral Lymphocytes Leads to Oxidative DNA Breakage: A Structure Activity Study. Int. J. Mol. Sci. 2015, 16, 26754–26769. [Google Scholar] [CrossRef]
- Caro, A.A.; Davis, A.; Fobare, S.; Horan, N.; Ryan, C.; Schwab, C. Antioxidant and pro-oxidant mechanisms of (+) catechin in microsomal CYP2E1-dependent oxidative stress. Toxicol. Vitr. 2019, 54, 1–9. [Google Scholar] [CrossRef]
- Atrahimovich, D.; Avni, D.; Khatib, S. Flavonoids-Macromolecules Interactions in Human Diseases with Focus on Alzheimer, Atherosclerosis and Cancer. Antioxidants 2021, 10, 423. [Google Scholar] [CrossRef]
- Kejík, Z.; Kaplánek, R.; Masařík, M.; Babula, P.; Matkowski, A.; Filipenský, P.; Veselá, K.; Gburek, J.; Sýkora, D.; Martásek, P.; et al. Iron Complexes of Flavonoids-Antioxidant Capacity and Beyond. Int. J. Mol. Sci. 2021, 22, 646. [Google Scholar] [CrossRef]
- Griendling, K.K.; Touyz, R.M.; Zweier, J.L.; Dikalov, S.; Chilian, W.; Chen, Y.R.; Harrison, D.G.; Bhatnagar, A.; American Heart Association Council on Basic Cardiovascular Sciences. Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System: A Scientific Statement From the American Heart Association. Circ. Res. 2016, 119, e39–e75. [Google Scholar] [CrossRef]
- Isacescu, E.; Chiroi, P.; Zanoaga, O.; Nutu, A.; Budisan, L.; Pirlog, R.; Atanasov, A.G.; Berindan-Neagoe, I. Melanoma Cellular Signaling Transduction Pathways Targeted by Polyphenols Action Mechanisms. Antioxidants 2023, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jin, P.; Guan, Y.; Luo, M.; Wang, Y.; He, B.; Li, B.; He, K.; Cao, J.; Huang, C.; et al. Exploiting Polyphenol-Mediated Redox Reorientation in Cancer Therapy. Pharmaceuticals 2022, 15, 1540. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Martino, E.; Ilisso, C.P.; Bagarolo, M.L.; Porcelli, M.; Cacciapuoti, G. Pro-oxidant and pro-apoptotic activity of polyphenol extract from Annurca apple and its underlying mechanisms in human breast cancer cells. Int. J. Oncol. 2017, 51, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-W.; Muthu, M.; Pushparaj, S.S.C.; Gopal, J. Anticancer Therapeutic Effects of Green Tea Catechins (GTCs) When Integrated with Antioxidant Natural Components. Molecules 2023, 28, 2151. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, Z.; Han, Y.; Wang, J.; Wang, Y.; Chen, X.; Shao, Y.; Cheng, Y.; Zhou, W.; Lu, X.; et al. A review on anti-cancer effect of green tea catechins. J. Funct. Foods 2020, 74, 104172. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pezzani, R.; Redaelli, M.; Zorzan, M.; Imran, M.; Ahmed Khalil, A.; Salehi, B.; Sharopov, F.; Cho, W.C.; Sharifi-Rad, J. Preclinical Activities of Epigallocatechin Gallate in Signaling Pathways in Cancer. Molecules 2020, 25, 467. [Google Scholar] [CrossRef]
- Mita, S.R.; Muhtar, N.I.; Kusuma, S.A.F.; Sriwidodo, S.; Hendrawan, R.P. Catechins as Antimicrobial Agents and Their Contribution to Cosmetics. Cosmetics 2025, 12, 11. [Google Scholar] [CrossRef]
- Acosta, L.; Byham-Gray, L.; Kurzer, M.; Samavat, H. Hepatotoxicity with High-Dose Green Tea Extract: Effect of Catechol-O-Methyltransferase and Uridine 5′-Diphospho-glucuronosyltransferase 1A4 Genotypes. J. Dietary Suppl. 2023, 20, 850–869. [Google Scholar] [CrossRef]
- Seeff, L.B.; Bonkovsky, H.L.; Navarro, V.J.; Wang, G. Herbal Products and the Liver: A Review of Adverse Effects and Mechanisms. Gastroenterology 2015, 148, 517–532.e3. [Google Scholar] [CrossRef]
- Kao, Y.H.; Chang, H.H.; Lee, M.J.; Chen, C.L. Tea, obesity, and diabetes. Mol. Nutr. Food Res. 2006, 50, 188–210. [Google Scholar] [CrossRef]
- Mazzanti, G.; Menniti-Ippolito, F.; Moro, P.A.; Cassetti, F.; Raschetti, R.; Santuccio, C.; Mastrangelo, S. Hepatotoxicity from Green Tea: A Review of the Literature and Two Unpublished Cases. Eur. J. Clin. Pharmacol. 2009, 65, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Oketch-Rabah, H.A.; Roe, A.L.; Rider, C.V.; Bonkovsky, H.L.; Giancaspro, G.I.; Navarro, V.; Paine, M.F.; Betz, J.M.; Marles, R.J.; Casper, S.; et al. United States Pharmacopeia (USP) comprehensive review of the hepatotoxicity of green tea extracts. Toxicol. Rep. 2020, 7, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.J. Liver injury from herbal and dietary supplements. Hepatology 2017, 65, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys 2010, 501, 65–72. [Google Scholar] [CrossRef]
- Ballotin, V.R.; Bigarella, L.G.; Brandão, A.B.d.M.; Balbinot, R.A.; Balbinot, S.S.; Soldera, J. Herb-induced liver injury: Systematic review and meta-analysis. World J. Clin. Cases 2021, 9, 5490–5513. [Google Scholar] [CrossRef]
- Lin, J.K.S.; Tujios, S.R. Hidden Dangers: Herbal and Dietary Supplement Induced Hepatotoxicity. Livers 2023, 3, 618–636. [Google Scholar] [CrossRef]
- Zheng, E.X.; Navarro, V.J. Liver Injury from Herbal, Dietary, and Weight Loss Supplements: A Review. J. Clin. Transl. Hepatol. 2015, 28, 93–98. [Google Scholar] [CrossRef]
- Chen, C.; Shen, G.; Hebbar, V.; Hu, R.; Owuor, E.D.; Kong, A.-N.T. Epigallocatechin-3-gallate-induced stress signals in HT-29 human colon adenocarcinoma cells. Carcinogenesis 2003, 24, 1369–1378. [Google Scholar] [CrossRef]
- Galati, G.; Lin, A.; Sultan, A.M.; O’Brien, P.J. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic. Biol. Med. 2006, 40, 570–580. [Google Scholar] [CrossRef]
- Bun, S.S.; Bun, H.; Guédon, D.; Rosier, C.; Ollivier, E. Effect of green tea extracts on liver functions in Wistar rats. Food Chem. Toxicol. 2006, 44, 1108–1113. [Google Scholar] [CrossRef]
- Zuhra, K.; Petrosino, M.; Gupta, B.; Panagaki, T.; Cecconi, M.; Myrianthopoulos, V.; Schneiter, R.; Mikros, E.; Majtan, T.; Szabo, C. Epigallocatechin gallate is a potent inhibitor of cystathionine beta-synthase: Structure-activity relationship and mechanism of action. Nitric Oxide 2022, 128, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Lambert, J.D.; Hong, J.; Tian, S.; Lee, M.J.; Stark, R.E.; Ho, C.T.; Yang, C.S. Synthesis and structure identification of thiol conjugates of (-)-epigallocatechin gallate and their urinary levels in mice. Chem. Res. Toxicol. 2005, 18, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Stickel, F.; Kessebohm, K.; Weimann, R.; Seitz, H.K. Review of liver injury associated with dietary supplements. Liver Int. 2011, 31, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Vial, T.; Bernard, G.; Lewden, B.; Dumortier, J.; Descotes, J. Hépatite Aiguë Imputable à l’Exolise®(Camellia sinensis). Gastroenterol. Clin. Biol. 2003, 27, 1166–1167. [Google Scholar]
- Fong, T.L.; Klontz, K.C.; Canas-Coto, A.; Casper, S.J.; Durazo, F.A.; Davern, T.J.; Hayashi, P.; Lee, W.M.; Seeff, L.B. Hepatotoxicity due to hydroxycut: A case series. Am. J. Gastroenterol. 2010, 105, 1561–1566. [Google Scholar] [CrossRef]
- Stevens, T.; Qadri, A.; Zein, N.N. Two patients with acute liver injury associated with use of the herbal weight-loss supplement hydroxycut. Ann. Intern. Med. 2005, 142, 477–478. [Google Scholar] [CrossRef]
- Jimenez-Saenz, M.; Martinez-Sanchez, M.C. Acute hepatitis associated with the use of green tea infusions. J. Hepatol. 2006, 44, 616–617. [Google Scholar] [CrossRef]
- Gloro, R.; Hourmand-Ollivier, I.; Mosquet, B.; Mosquet, L.; Rousselot, P.; Salamé, E.; Piquet, M.A.; Dao, T. Fulminant hepatitis during self-medication with hydroalcoholic extract of green tea. Eur. J. Gastroenterol. Hepatol. 2005, 17, 1135–1137. [Google Scholar] [CrossRef]
- Federico, A.; Tiso, A.; Loguercio, C. A case of hepatotoxicity caused by green tea. Free Radic. Biol. Med. 2007, 43, 474. [Google Scholar] [CrossRef]
- Arzenton, E.; Magro, L.; Paon, V.; Capra, F.; Apostoli, P.; Guzzo, F.; Conforti, A.; Leone, R. Acute epatitis caused by green tea infusion: A case report. Adv. Pharmacoepidemiol. Drug Saf. 2014, 3, 170. [Google Scholar] [CrossRef]
- Amariles, P.; Angulo, N.; Agudelo-Agudelo, J.; Gaviria, G. Hepatitis asociada a infusiones acuosas de té verde: A propósito de un caso [Hepatitis associated with aqueous green tea infusions: A case study]. Farm. Hosp. 2009, 33, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Javaid, A.; Bonkovsky, H.L. Hepatotoxicity due to extracts of Chinese green tea (Camellia sinensis): A growing concern. J. Hepatol. 2006, 45, 334–335. [Google Scholar] [CrossRef] [PubMed]
- Molinari, M. Acute liver failure induced by green tea extracts: Case report and review of the literature. Liver Transpl. 2006, 12, 1892–1895. [Google Scholar] [CrossRef] [PubMed]
- Bjornsson, E.; Olsson, R. Serious adverse liver reactions associated with herbal weight-loss supplements. J. Hepatol. 2007, 47, 295–297. [Google Scholar] [CrossRef]
- Samavat, H. The Minnesota Green Tea Trial (MGTT), a randomized controlled trial of the efficacy of green tea extract on biomarkers of breast cancer risk: Study rationale, design, methods, and participant characteristics. Cancer Causes Control 2015, 26, 1405–1419. [Google Scholar] [CrossRef]
- Yu, Z. Effect of green tea supplements on liver enzyme elevation: Results from a randomized intervention study in the United States. Cancer Prev. Res. Phila. 2017, 10, 571–579. [Google Scholar] [CrossRef]
- Dostal, A.M. The safety of green tea extract supplementation in postmenopausal women at risk for breast cancer: Results of the Minnesota Green Tea Trial. Food Chem. Toxicol. 2015, 83, 26–35. [Google Scholar] [CrossRef]
- Dostal, A.M. Green tea extract and catechol-O-methyltransferase genotype modify the post-prandial serum insulin response in a randomised trial of overweight and obese post-menopausal women. J. Hum. Nutr. Diet. 2017, 30, 166–176. [Google Scholar] [CrossRef]
- Dostal, A.M. Green tea extract and Catechol-O-Methyltransferase genotype modify fasting serum insulin and plasma adiponectin concentrations in a randomized controlled trial of overweight and obese postmenopausal women. J. Nutr. 2016, 146, 38–45. [Google Scholar] [CrossRef]
- Lovera, J. Polyphenon E, non-futile at neuroprotection in multiple sclerosis but unpredictably hepatotoxic: Phase I single group and phase II randomized placebo-controlled studies. J. Neurol. Sci. 2015, 358, 46–52. [Google Scholar] [CrossRef]
- Radeva-Ilieva, M.; Stoeva, S.; Hvarchanova, N.; Georgiev, K.D. Green Tea: Current Knowledge and Issues. Foods 2025, 14, 745. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program (NTP). Toxicology studies of green tea extract in F344/NTac rats and B6C3F1/N mice and Toxicology and carcinogenesis studies of green tea extract in Wistar Han [Crl: Wi (Han)] rats and B6C3F1/N mice. Toxicol. Program Tech. Rep. Ser. 2016, 585, NTP-TR-585. [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific Opinion on the safety of green tea catechins. EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food). EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef] [PubMed]
- Malnick, S.; Maor, Y.; Neuman, M.G. Green Tea Consumption Is Increasing but There Are Significant Hepatic Side Effects. GastroHep 2022, 2307486, 5. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Jachimowicz-Rogowska, K.; Kwiecień, M.; Borsuk-Stanulewicz, M.; Tomczyk-Warunek, A.; Stamirowska-Krzaczek, E.; Purwin, C.; Stryjecka, M.; Tomaszewska, M. Regular Consumption of Green Tea as an Element of Diet Therapy in Drug-Induced Liver Injury (DILI). Nutrients 2024, 16, 2837. [Google Scholar] [CrossRef]
- Isomura, T.; Suzuki, S.; Origasa, H.; Hosono, A.; Suzuki, M.; Sawada, T.; Terao, S.; Muto, Y.; Koga, T. Liver-related safety assessment of green tea extracts in humans: A systematic review of randomized controlled trials. Eur. J. Clin. Nutr. 2016, 70, 1221–1229. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Li, X.M.; Liang, J.P.; Xiang, L.P.; Wang, K.R.; Shi, Y.L.; Yang, R.; Shi, M.; Ye, J.H.; Lu, J.L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef]
- Schönthal, A.H. Adverse effects of concentrated green tea extracts. Mol. Nutr. Food Res. 2011, 55, 874–885. [Google Scholar] [CrossRef]
- Chow, H.H.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Ranger-Moore, J.; Chew, W.M.; Celaya, C.A.; Rodney, S.R.; Hara, Y.; Alberts, D.S. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clin. Cancer Res. 2005, 11, 4627–4633. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Zhang, J.; Cui, H.; Ni, D.; Jiang, H. Dual effects of ascorbic acid on the stability of EGCG by the oxidation product dehydroascorbic acid promoting the oxidation and inhibiting the hydrolysis pathway. Food Chem. 2021, 337, 127639. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.; Jiang, X. Reaction Kinetics of Degradation and Epimerization of Epigallocatechin Gallate (EGCG) in Aqueous System over a Wide Temperature Range. J. Agric. Food Chem. 2008, 56, 2694–2701. [Google Scholar] [CrossRef] [PubMed]
- Ananingsih, V.K.; Sharma, A.; Zhou, W. Green tea catechins during food processing and storage: A review on stability and detection. Int. Food Res. 2013, 50, 469–479. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Boostani, S.; Babazadeh, A.; Rehman, A.; Rezaei, A.; Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Opportunities and challenges for the nanodelivery of green tea catechins in functional foods. Food Res Int. 2021, 142, 110186. [Google Scholar] [CrossRef] [PubMed]
- Naumovski, N.; Blades, B.L.; Roach, P.D. Food Inhibits the Oral Bioavailability of the Major Green Tea Antioxidant Epigallocatechin Gallate in Humans. Antioxidants 2015, 4, 373–393. [Google Scholar] [CrossRef]
- Isbrucker, R. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 1: Genotoxicity. Food Chem. Toxicol. 2006, 44, 626–635. [Google Scholar] [CrossRef]
- Isbruker, R.A.; Edwards, J.A.; Wolz, E.; Davidovich, A.; Bausch, J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: Dermal, acute and short-term toxicity studies. Food Chem. Toxicol. 2006, 44, 636–650. [Google Scholar] [CrossRef]
- Kapetanovic, I.M. Exposure and toxicity of green tea polyphenols in fasted and non-fasted dogs. Toxicology 2009, 260, 28–36. [Google Scholar] [CrossRef]
- Chow, H.H. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol. Biomark. Prev. 2001, 10, 53–58. [Google Scholar]
- Salminen, W.F.; Yang, X.; Shi, Q.; Greenhaw, J.; Davis, K.; Ali, A.A. Green tea extract can potentiate acetaminophen-induced hepatotoxicity in mice. Food Chem. Toxicol. 2012, 50, 1439–1446. [Google Scholar] [CrossRef]
- Yellapu, R.K.; Mittal, V.; Grewal, P.; Fiel, M.; Schiano, T. Acute liver failure caused by ‘fat burners’ and dietary supplements: A case report and literature review. Can. J. Gastroenterol. 2011, 25, 157–160. [Google Scholar] [CrossRef]
- Dela Cruz, A.C.; Patel, T.T.; Maynard, E.; Shah, M.; Lee, E.Y.; Angulo, P. Fulminant liver failure secondary to “Saba appetite control and energy” weight loss supplement. Gastroenterology 2014, 146, 1002. [Google Scholar] [CrossRef]
- Whitsett, M.; Halegoua-De Marzio, D.; Rossi, S. SlimQuick™-associated hepatotoxicity resulting in fulminant liver failure and orthotopic liver transplantation. ACG Case Rep. J. 2014, 1, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, G.; Di Sotto, A.; Vitalone, A. Hepatotoxicity of green tea: An update. Arch. Toxicol. 2015, 89, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Petitpain, N.; Kalt, P.; Ancel, D.; Petit-Laurent, F.; Trechot, P.; Barraud, H.; Bronowicki, J.P. Probable hepatoxicity from epigallocatecol gallate used for phytotherapy. Gastroenterol. Clin. Biol. 2004, 28, 404–406. [Google Scholar] [CrossRef]
- Juneja, L.R.; Kapoor, M.P.; Okubo, T.; Rao, T. Green Tea: History, Processing Techniques, Principles, Traditions, Features and Attractions. In Green Tea Polyphenols, Nutraceuticals of Modern Life; Juneja, L.R., Kapoor, M.P., Okubo, T., Rao, T., Eds.; CRS Press: Boca Raton, FL, USA; Taylor & Francis Group: New York, NY, USA, 2013; pp. 1–18. [Google Scholar]
- Vuong, Q.V.; Tan, S.P.; Stathopoulos, C.E.; Roach, P.D. Improved extraction of green tea components from teabags using the microwave oven. J. Food Compos. Anal. 2012, 27, 95–101. [Google Scholar] [CrossRef]
- Sano, M. Novel antiallergic catechin derivatives isolated from oolong tea. J. Agric. Food Chem. 1999, 47, 1906–1910. [Google Scholar] [CrossRef]
- Hayward, D.G.; Wong, J.W.; Park, H.Y. Determinations for pesticides on Black, Green, Oolong, and White Teas by gas chromatography triple-quadrupole mass spectrometry. J. Agric. Food Chem. 2015, 63, 8116–8124. [Google Scholar] [CrossRef]
- Goodin, M.G.; Bray, B.J.; Rosengreen, R.J. Sex- and straindependent effects of epigallocatechin gallate (EGCG) and epicatechin gallate (ECG) in the mouse. Food Chem. Toxicol. 2006, 44, 1496–1504. [Google Scholar] [CrossRef]
- Chalasani, N.P.; Hayashi, P.H.; Bonkovsky, H.L.; Navarro, V.J.; Lee, W.M.; Fontana, R.J. ACG Clinical Guideline: The diagnosis and management of idiosyncratic drug-induced liver injury. Am. J. Gastroenterol. 2014, 109, 950–966. [Google Scholar] [CrossRef]
- Jimenez-Saenz, M.; Martinez-Sanchez, C. Green tea extracts and acute liver failure: The need for caution in their use and diagnostic assessment. Liver Transpl. 2007, 13, 1067. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Lu, X.; Min, H.; Wu, Q.Q.; Shi, X.T.; Bian, K.Q.; Zou, X.P. Green tea and liver cancer risk: A meta-analysis of prospective cohort studies in Asian populations. Nutrition 2016, 32, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Church, R.J. Sensitivity to hepatotoxicity due to epigallocatechin gallate is affected by genetic background in diversity outbred mice. Food Chem. Toxicol. 2015, 76, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hoofnagle, J.H.; Bonkovsky, H.L.; Phillips, E.J.; Li, Y.J.; Ahmad, J.; Barnhart, H.; Durazo, F.; Fontana, R.J.; Gu, J.; Khan, I.; et al. Hla-b*35:01 and green tea-induced liver injury. Hepatology 2021, 73, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Bonkovsky, H.L. Hepatotoxicity associated with supplements containing Chinese green tea (Camellia sinensis). Ann. Intern. Med. 2006, 144, 68–71. [Google Scholar] [CrossRef]
- Teschke, R.; Xuan, T. Suspected herb induced liver injury by green tea extracts: Critical review and case 2 analysis applying RUCAM for causality assessment. Jpn. J. Gastroenterol. Hepatol. 2019, 1, 1–16. [Google Scholar]
- Pascale, B. Dietary supplements: Knowledge and adverse event reporting among American Medical Society for Sports Medicine physicians. Clin. J. Sport. Med. 2016, 26, 139–144. [Google Scholar] [CrossRef]
- Hoofnagle, J.H. Drug induced liver injury network (DILIN). Hepatology 2004, 40, 773. [Google Scholar] [CrossRef]
- Danan, G.; Teschke, R. Drug-Induced Liver Injury: Why is the Roussel Uclaf Causality Assessment Method (RUCAM) Still Used 25 Years After Its Launch? Drug Saf. 2018, 41, 735–743. [Google Scholar] [CrossRef]
- Health Canada, Green Tea Extract-Containing Natural Health Products—Rare Risk of Serious Liver Injury. 2017. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/safety-reviews/green-tea-extract-containing-natural-health-products-assessing-potential-risk-liver-injury.html (accessed on 9 February 2025).
- Dekant, W.; Fujii, K.; Shibata, E.; Morita, O.; Shimotoyodome, A. Safety assessment of green tea based beverages and dried green tea extracts as nutritional supplements. Toxicol. Lett. 2017, 277, 104–108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).