Foliar Selenium Biofortification in Temperate Fruit Crops: Impact on Selenium Accumulation and Nutritional Quality of Fruits and Juices
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Plot
2.2. Harvest, Storage and Juice Production
2.3. Estimation of TSS, Glucose, Fructose, Total Sugar, Malic Acid, Total Acid Content and Ph
2.4. Determination of Total Polyphenols, Total Anthocyanins and Antioxidant Activity
2.5. Determination of Elemental Content by ICP-OES
2.6. Determination of Phenolic Compounds by HPLC-DAD
2.7. Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schiavon, M.; Nardi, S.; Dalla Vecchia, F.; Ertani, A. Selenium biofortification in the 21st century: Status and challenges for healthy human nutrition. Plant Soil 2020, 453, 245–270. [Google Scholar] [CrossRef]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Filippini, T.; Wise, L.A. Environmental selenium and human health: An update. Curr. Environ. Health Rep. 2018, 5, 464–485. [Google Scholar] [CrossRef]
- De Vita, P.; Platani, C.; Fragasso, M.; Ficco, D.B.M.; Colecchia, S.A.; Del Nobile, M.A.; Padalino, L.; Di Gennaro, S.; Petrozza, A. Selenium-enriched durum wheat improves the nutritional profile of pasta without altering its organoleptic properties. Food Chem. 2017, 214, 374–382. [Google Scholar] [CrossRef]
- Górska, S.; Maksymiuk, A.; Turło, J. Selenium-containing polysaccharides structural diversity, biosynthesis, chemical modifications and biological activity. Appl. Sci. 2021, 11, 3717. [Google Scholar] [CrossRef]
- Sariñana-Navarrete, M.D.Á.; Hernández-Montiel, L.G.; Sánchez-Chavez, E.; Reyes-Perez, J.J.; Murillo-Amador, B.; Reyes-González, A.; Preciado-Rangel, P. Foliar fertilization of sodium selenite and its effects on yield and nutraceutical quality in grapevine. Rev. Fac. Agron. 2021, 38, 806–824. [Google Scholar] [CrossRef]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C. Current knowledge on Selenium biofortification to improve nutraceutical profile of food: A comprehensive review. J. Agric. Food Chem. 2020, 68, 4057–4097. [Google Scholar] [CrossRef] [PubMed]
- Preciado-Rangel, P.; Hernández-Montiel, L.G.; Valdez-Cepeda, R.D.; Cruz-Lázaro, E.D.L.; Lara-Capistrán, L.; Morales-Morales, B.; Gaucin-Delgado, J.M. Biofortification with selenium increases bioactive compounds and antioxidant capacity in tomato fruits. Terra Latinoam. 2021, 39, 1–10. [Google Scholar] [CrossRef]
- Gaucin-Delgado, J.M.; Hernandez-Montiel, L.G.; Sanchez-Chavez, E.; Ortega-Ortiz, H.; Fortis-Hernandez, M.; Reyes-Pérez, J.J.; Preciado-Rangel, P. Agronomic biofortification with selenium improves the yield and nutraceutical quality in tomato under soilless conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1221–1232. [Google Scholar] [CrossRef]
- Lyons, G. Biofortification of cereals with foliar selenium and iodine could reduce hypothyroidism. Front. Plant Sci. 2018, 9, 2–8. [Google Scholar] [CrossRef]
- Lapornik, B.; Prošek, M.; Golc-Wondra, A. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 14–158. [Google Scholar] [CrossRef]
- Shah, N.; Marathe, S.J.; Croce, D.; Ciardi, D.; Longo, V.; Árvay, J.; Shamekh, S. An investigation of the antioxidant potential and bioaccumulated minerals in Tuber borchii and Tuber maculatum mycelia obtained by submerged fermentation. Arch. Microbiol. 2022, 204, 64. [Google Scholar] [CrossRef]
- Franková, H.; Musilová, J.; Árvay, J.; Šnirc, M.; Jančo, I.; Lidiková, J.; Vollmannová, A. Changes in Antioxidant Properties and Phenolics in Sweet Potatoes (Ipomoea batatas L.) Due to Heat Treatments. Molecules 2022, 27, 1884. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, R.; Proietti, P.; Nasini, L.; Del Buono, D.; Tedeschini, E.; Businelli, D. Increase in the selenium content of extra virgin olive oil: Quantitative and qualitative implications. Grasas Aceites 2014, 65, e025. [Google Scholar] [CrossRef]
- Tedeschini, E.; Proietti, P.; Timorato, V.; D’Amato, R.; Nasini, L.; Dei Buono, D.; Businelli, D.; Frenguelli, G. Selenium as stressor and antioxidant affects pollen performance in Olea europaea. Flora 2015, 215, 16. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Daneshvar Hakimi Meybodi, N.; Teixeira da Silva, J.A. Foliar application of selenium and nano-selenium affects pomegranate (Punica granatum cv. Malase Saveh) fruit yield and quality. S. Afr. J. Bot. 2019, 124, 350–358. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought Induced Changes in Growth, Osmolyte Accumulation and Antioxidant Metabolism of Three Maize Hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef]
- European Commission. Reports of the Scientific Committee for Food: Nutrient and Energy Intakes for the European Community; 31st Series; Commission of the European Communities: Luxembourg, 1993; pp. 1–255. [Google Scholar]
- Zhu, S.; Liang, Y.; An, X.; Kong, F.; Gao, D.; Yin, H. Changes in sugar content and related enzyme activities in table grape (Vitis vinifera L.) in response to foliar selenium fertilizer. J. Sci. Food Agric. 2017, 97, 4094–4410. [Google Scholar] [CrossRef]
- Frias, J.; Gulewicz, P.; Martínez-Villaluenga, C.; Pilarski, R.; Blazquez, E.; Jiménez, B.; Gulewicz, K.; Vidal-Valverde, C. Influence of germination with different selenium solutions on nutritional value and cytotoxicity of lupin seeds. J. Agric. Food Chem. 2009, 57, 1319–1325. [Google Scholar] [CrossRef]
- Lazo-Vélez, M.A.; Chávez-Santoscoy, A.; Serna-Saldivar, S.O. Selenium- enriched breads and their benefits in humannutrition and health as affected by agronomic, milling, and baking factors. Cereal Chem. 2015, 92, 134–144. [Google Scholar] [CrossRef]
- Guardado-Félix, D.; Serna-Saldivar, S.O.; Gutiérrez-Uribe, J.A.; Chuck-Hernández, C. Selenium in germinated chickpea (Cicer arietinum L.) increases the stability of its oil fraction. Plants 2019, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Ning, M. Antioxidant activity stability anddigestibility of protein from Se-enriched germinated brown rice. LWT Food Sci. Technol. 2021, 142, 111032. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Zhang, X.; Zhang, W.; Huang, L.; Zhang, Z.; Yuan, L.; Liu, X. Effects of foliar application of selenate and selenite at different growth stages on Selenium accumulation and speciation in potato (Solanum tuberosum L.). Food Chem. 2019, 286, 550–556. [Google Scholar] [CrossRef]
- Mahn, A. Modelling of the effect of selenium fertilization on the content of bioactive compounds in broccoli heads. Food Chem. 2017, 233, 492–499. [Google Scholar] [CrossRef]
- Mezeyová, I.; Mezey, J.; Šlosár, M.; Hegedusova, A.; Rosa, R. Selenization and its impact on quantitative and qualitative parameters of carrot juice. Food Biosci. 2024, 59, 103933. [Google Scholar] [CrossRef]
- Golian, M.; Mezeyová, I.; Andrejiová, A.; Hegedűsová, A.; Adamec, S.; Štefániková, J.; Árvay, J. Effects of selected biostimulants on qualitative and quantitative parameters of nine cultivars of the genus Capsicum spp. Open Agric. 2024, 9, 20220266. [Google Scholar] [CrossRef]
| Fruit Species | Varieties | Number of Plants | Replications | Spacing | Planting Year |
|---|---|---|---|---|---|
| Raspberries | Kwanza, Kweli, Imara, Himbotop, Polka, Sugana | 10 | 3 | 2.5 × 0.25 m | 2021 |
| Blueberries | Duke, Draper | 10 | 3 | 3.5 × 1 m | 2019 |
| Redcurrants | Junifer, Jonkheer van Teets, Rovada | 10 | 3 | 3.5 × 0.4 m | 2019 |
| Honeysuckle | Polaris | 10 | 3 | 3.5 × 1 m | 2019 |
| Apples | Evelina | 10 | 3 | 4 × 1 m | 2018 |
| Analyzed Parameter | Unit | Raspberries | Blueberries | ||
|---|---|---|---|---|---|
| Control | Se Application | Control | Se Application | ||
| Se | mg/kg | ˂LOD | 2.570 ± 0.306 | 0.715 ± 0.019 a | 1.791 ± 0.066 b |
| pH | 2.92 ± 0.01 a | 2.843 ± 0.093 a | 3.413 ± 0.015 b | 3.06 ± 0.046 a | |
| Mn | mg/kg | 16.722 ± 0.036 b | 6.501 ± 0.009 a | 8.877 ± 0.044 a | 11.616 ± 0.096 b |
| ferulic acid | mg/kg | 1.13 ± 0.03 a | 8.23 ± 0.07 b | 16.783 ± 0.040 a | 16.703 ± 0.032 a |
| AA DDPH | % | 91.433 ± 0.850 a | 91.8 ± 0.529 a | 89.433 ± 0.635 b | 79.633 ± 0.404 a |
| AA TEAC | μmolTE/g | 12.717 ± 0.117 a | 12.767 ± 0.757 a | 12.437 ± 0.092 b | 11.06 ± 0.056 a |
| AA FRAP | μmolTE/g | 8.823 ± 0.118 a | 8.853 ± 0.092 a | 9.01 ± 0.04 b | 8.893 ± 0.012 a |
| resveratrol | mg/kg | 9.33 ± 0.04 b | 8.763 ± 0.035 a | 4.103 ± 0.350 a | 3.830 ± 0.170 a |
| fructose | g/L | 49.953 ± 0.263 a | 57.77 ± 5.499 a | 50.32 ± 1.121 a | 49.733 ± 0.651 a |
| glucose | g/L | 24.497 ± 1.192 a | 28.193 ± 0.193 b | 31.677 ± 0.719 a | 32.483 ± 0.335 a |
| malic acid | g/L | 13.207 ± 0.162 a | 15.323 ± 0.289 b | 8.387 ± 0.265 a | 7.28 ± 0.14 b |
| total acid | g/L | 14.06 ± 0.233 a | 16.54 ± 0.299 b | 8.597 ± 0.015 b | 7.607 ± 0.101 a |
| Ba | mg/kg | 3.226 ± 0.008 b | 2.143 ± 0.006 a | 2.103 ± 0.008 a | 2.545 ± 0.022 b |
| Ca | mg/kg | 3852.37 ± 9.625 b | 1574.057 ± 3.215 a | 1697.437 ± 10.451 a | 3897.36 ± 46.455 b |
| Li | mg/kg | 0.020 ± 0.001 a | 0.017 ± 0.002 a | 0.040 ± 0.000 b | 0.024 ± 0.004 a |
| myricetin | mg/kg | ˂LOD | ˂LOD | 5.103 ± 0.100 a | 6.3 ± 0.750 a |
| chlorogenic acid | mg/kg | ˂LOD | ˂LOD | 323.713 ± 2.067 a | 410.43 ± 0.491 b |
| quercetin | mg/kg | ˂LOD | ˂LOD | 7.017 ± 0.226 a | 7.04 ± 0.061 a |
| TSS | Brix | 7.645 ± 0.095 a | 8.52 ± 0.108 b | 8.87 ± 0.044 b | 8.74 ± 0.061 a |
| total sugar | g/L | 76.3 ± 2.151 a | 82.567 ± 0.484 b | 93.393 ± 1.082 b | 90.2 ± 0.585 a |
| Cr | mg/kg | 0.122 ± 0.017 | ˂LOD | 0.370 ± 0.041 a | 0.405 ± 0.024 a |
| Cu | mg/kg | 7.024 ± 0.013 a | 8.591 ± 0.029 b | 8.293 ± 0.032 b | 7.158 ± 0.020 a |
| Fe | mg/kg | 25.379 ± 0.198 a | 28.343 ± 0.084 b | 58.010 ± 0.543 b | 29.530 ± 0.364 a |
| Mg | mg/kg | 984.823 ± 2.447 a | 1025.78 ± 0.719 b | 618.318 ± 2.047 b | 449.990 ± 2.434 a |
| Na | mg/kg | 150.444 ± 0.466 a | 171.543 ± 0.076 b | 219.412 ± 1.059 b | 152.059 ± 0.348 a |
| Ni | mg/kg | 4.184 ± 0.158 b | 3.335 ± 0.006 a | 2.554 ± 0.219 a | 3.778 ± 0.316 b |
| Sr | mg/kg | 29.188 ± 0.133 b | 14.320 ± 0.013 a | 15.927 ± 0.059 b | 11.268 ± 0.053 a |
| caffeic acid | mg/kg | 1.05 ± 0.03 a | 1.15 ± 0.721 a | 7.443 ± 0.031 b | 7.133 ± 0.021 a |
| cinnamic acid | mg/kg | 3.47 ± 0.01 b | 3.097 ± 0.006 a | ˂LOD | ˂LOD |
| coumaric acid | mg/kg | 6.873 ± 0.475 b | 5.32 ± 0 a | 6.927 ± 0.006 b | 4.833 ± 0.023 a |
| 4-OH benzoic acid | mg/kg | 12.845 ± 0.205 b | 10.497 ± 0.165 a | 35.05 ± 0.411 b | 22.413 ± 0.071 a |
| TPC | mg/kg | 1176.053 ± 25.302 a | 1449.08 ± 20.787 b | 1842.053 ± 35.600 b | 1595.627 ± 35.696 a |
| TAC | mg/kg | 0.803 ± 0.006 a | 0.61 ± 0.01 b | 8.497 ± 0.083 b | 6.337 ± 0.031 a |
| K | mg/kg | 5354.833 ± 2.046 b | 5038.893 ± 3.878 a | 4226.373 ± 11.327 b | 3833.81 ± 13.981 a |
| Zn | mg/kg | 29.046 ± 0.299 b | 7.936 ± 0.625 a | 8.371 ± 0.254 a | 13.790 ± 1.073 b |
| Al | mg/kg | 19.759 ± 0.070 a | 29.527 ± 1.074 b | 50.383 ± 1.119 b | 40.482 ± 0.927 a |
| rutin | mg/kg | 7.193 ± 0.235 b | 4.743 ± 0.035 a | 9.14 ± 0.221 b | 6.56 ± 0.305 a |
| Redcurrants | Honeysuckle | Apples | |||||
|---|---|---|---|---|---|---|---|
| Analyzed Parameter | Unit | Control | Se Application | Control | Se Application | Control | Se Application |
| Se | mg/kg | ˂LOD | 8.975 ± 0.31 b | ˂LOD | 3.781 ± 0.592 | ˂LOD | 1.227 ± 0.021 |
| pH | 2.943 ± 0.015 a | 2.92 ± 0.026 a | 3.167 ± 0.058 a | 3.247 ± 0.115 a | 3.59 ± 0.01 a | 3.6 ± 0.1 a | |
| Mn | mg/kg | 7.758 ± 0.041 a | 8.175 ± 0.044 b | 9.048 ± 0.037 a | 9.560 ± 0.064 b | 5.018 ± 0.042 a | 4.988 ± 0.033 a |
| ferulic acid | mg/kg | 1.17 ± 0.01 a | 1.107 ± 0.115 b | 1.107 ± 0.006 a | 8.307 ± 0.596 b | 2.86 ± 0.017 a | 5.633 ± 0.035 b |
| AA DDPH | % | 75.233 ± 0.060 a | 79.033 ± 0.503 b | 89 ± 0.7 a | 89.733 ± 0.321 a | 60.9 ± 0.361 a | 65.067 ± 0.252 b |
| AA TEAC | μmolTE/g | 10.44 ± 0.085 a | 10.973 ± 0.070 b | 12.373 ± 0.102 a | 12.477 ± 0.047 a | 8.423 ± 0.047 a | 9.007 ± 0.035 b |
| AA FRAP | μmolTE/g | 7.07 ± 0.078 a | 6.96 ± 0.02 a | 9.227 ± 0.057 a | 9.177 ± 0.057 a | 4.7 ± 0.07 a | 4.927 ± 0.098 b |
| resveratrol | mg/kg | 3.797 ± 0.030 a | 8 ± 0.044 b | 1.183 ± 0.006 a | 1.213 ± 0.907 a | 3.793 ± 0.006 a | 3.53 ± 0.236 a |
| fructose | g/L | 42.953 ± 0.620 a | 45.313 ± 0.277 b | 53.153 ± 0.409 b | 49.233 ± 0.503 a | 90.31 ± 0.737 b | 80.457 ± 0.405 a |
| glucose | g/L | 21.313 ± 0.407 a | 24.923 ± 0.076 b | 38.383 ± 0.333 b | 35.64 ± 0.472 a | 10.04 ± 0.183 b | 9.557 ± 0.083 a |
| malic acid | g/L | 17.297 ± 0.115 b | 15.483 ± 0.344 a | 14.113 ± 0.215 a | 15.99 ± 0.221 b | 2.76 ± 0.145 a | 3.553 ± 0.051 b |
| total acid | g/L | 17.973 ± 0.081 b | 16.27 ± 0.236 a | 15.323 ± 0.582 a | 15.033 ± 0.153 a | 3.427 ± 0.215 a | 4.333 ± 0.497 b |
| Ba | mg/kg | 2.008 ± 0.007 b | 1.406 ± 0.012 a | 4.993 ± 0.020 a | 5.108 ± 0.024 b | 1.516 ± 0.013 a | 1.780 ± 0.018 b |
| Ca | mg/kg | 2047.043 ± 12.821 b | 1574.05 ± 20.669 a | 1946.153 ± 8.646 a | 1990.273 ± 28.373 a | 1143.68 ± 0.654 a | 1198.76 ± 4.606 b |
| Li | mg/kg | 0.078 ± 0.007 a | 0.044 ± 0.004 a | 0.012 ± 0.002 a | 0.017 ± 0.003 a | 0.045 ± 0.004 b | 0.032 ± 0.002 a |
| myricetin | mg/kg | ˂LOD | 4.297 ± 0.115 | ˂LOD | ˂LOD | ˂LOD | ˂LOD |
| chlorogenic acid | mg/kg | ˂LOD | ˂LOD | ˂LOD | ˂LOD | 43.45 ± 0.308 a | 48.793 ± 0.051 b |
| quercetin | mg/kg | ˂LOD | ˂LOD | ˂LOD | ˂LOD | ˂LOD | ˂LOD |
| TSS | Brix | 6.593 ± 0.095 a | 7.217 ± 0.075 b | 10.773 ± 0.125 b | 10.193 ± 0.021 a | 11.737 ± 0.152 b | 10.643 ± 0.049 a |
| total sugar | g/L | 74.71 ± 0.159 a | 80.477 ± 0.341 b | 115.44 ± 1.002 b | 110.473 ± 1.131 a | 110.193 ± 1.042 b | 100.453 ± 1.279 a |
| Cr | mg/kg | 0.653 ± 0.022 b | 0.252 ± 0.034 a | 0.404 ± 0.532 b | 0.197 ± 0.499 a | 3.300 ± 0.009 b | 0.944 ± 0.031 a |
| Cu | mg/kg | 11.767 ± 0.044 b | 7.437 ± 0.047 a | 11.235 ± 0.421 a | 12.228 ± 0.025 b | 8.834 ± 0.032 b | 8.024 ± 0.023 a |
| Fe | mg/kg | 37.361 ± 0.080 b | 28.060 ± 0.138 a | 33.417 ± 0.211 a | 34.652 ± 0.424 b | 35.414 ± 0.253 b | 26.002 ± 0.198 a |
| Mg | mg/kg | 746.321 ± 0.493 b | 667.480 ± 1.414 a | 700.420 ± 0.887 b | 671.668 ± 3.336 a | 581.595 ± 0.900 a | 625.427 ± 2.520 b |
| Na | mg/kg | 258.507 ± 1.244 b | 117.004 ± 0.654 a | 211.202 ± 0.288 b | 178.99 ± 0.418 a | 155.083 ± 0.440 a | 179.016 ± 0.573 b |
| Ni | mg/kg | 11.938 ± 0.495 b | 2.827 ± 0.118 a | 1.724 ± 0.912 a | 2.122 ± 0.079 b | 3.748 ± 0.115 b | 1.453 ± 0.162 a |
| Sr | mg/kg | 15.627 ± 0.0156 b | 9.603 ± 0.021 a | 13.315 ± 0.050 a | 15.489 ± 0.083 b | 10.778 ± 0.027 a | 11.644 ± 0.088 b |
| caffeic acid | mg/kg | 1.157 ± 0.006 b | 1.033 ± 0.006 a | 7.017 ± 0.201 a | 6.687 ± 0.085 a | 4.667 ± 0.057 | ˂LOD |
| cinnamic acid | mg/kg | ˂LOD | ˂LOD | 9.883 ± 0.015 b | 8.19 ± 0.066 a | ˂LOD | ˂LOD |
| coumaric acid | mg/kg | ˂LOD | ˂LOD | 6.993 ± 0.655 a | 6.063 ± 0.071 a | ˂LOD | ˂LOD |
| 4-OH benzoic acid | mg/kg | 37.707 ± 0.503 b | 4.14 ± 0.075 a | ˂LOD | ˂LOD | 5.163 ± 0.061 a | 6.597 ± 0.180 b |
| TPC | mg/kg | 875.907 ± 19.031 b | 711.053 ± 27.368 a | 3245.587 ± 10.881 b | 3163.733 ± 29.595 a | ˂LOD | ˂LOD |
| TAC | mg/kg | 1.237 ± 0.031 a | 1.387 ± 0.015 b | 11.85 ± 0.062 b | 11.457 ± 0.060 a | ˂LOD | ˂LOD |
| K | mg/kg | 9949.86 ± 16.973 b | 8134.497 ± 26.975 a | 9727.08 ± 1.652 b | 9436.3 ± 12.636 a | 4545.91 ± 3.557 a | 5673.103 ± 8.661 b |
| Zn | mg/kg | 11.958 ± 0.570 b | 8.084 ± 0.594 a | 10.322 ± 0.229 b | 8.999 ± 0.255 a | 7.408 ± 0.756 b | 4.377 ± 0.192 a |
| Al | mg/kg | 38.522 ± 0.253 b | 34.876 ± 0.554 a | 36.030 ± 0.641 b | 34.468 ± 0.556 a | 50.441 ± 0.138 b | 43.551 ± 1.116 a |
| rutin | mg/kg | 8.807 ± 0.006 a | 7.447 ± 0.306 b | 130.29 ± 0.215 b | 115.65 ± 0.478 a | ˂LOD | 1.17 ± 0.026 |
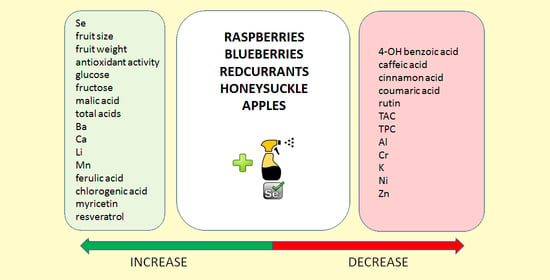
| Parameter | Raspberries | Blueberries | Redcurrants | Honeysuckle | Apples |
|---|---|---|---|---|---|
| Se | ↑ | ↑ | ↑ | ↑ | ↑ |
| pH | ↔ | ↑ | ↔ | ↔ | ↔ |
| Mn | ↓ | ↑ | ↑ | ↑ | ↔ |
| Ferulic acid | ↑ | ↔ | ↑ | ↑ | ↑ |
| AA (DPPH) | ↔ | ↓ | ↑ | ↔ | ↑ |
| AA (TEAC) | ↔ | ↓ | ↑ | ↔ | ↑ |
| AA (FRAP) | ↔ | ↓ | ↔ | ↔ | ↑ |
| Resveratrol | ↓ | ↔ | ↑ | ↔ | ↔ |
| Fructose | ↔ | ↔ | ↑ | ↓ | ↓ |
| Glucose | ↑ | ↔ | ↑ | ↓ | ↓ |
| Malic Acid | ↑ | ↓ | ↓ | ↑ | ↑ |
| Total Acid | ↑ | ↓ | ↓ | ↔ | ↑ |
| Ba | ↓ | ↑ | ↓ | ↑ | ↑ |
| Ca | ↓ | ↑ | ↓ | ↔ | ↑ |
| Li | ↔ | ↓ | ↔ | ↔ | ↓ |
| Myricetin | <LOD | ↔ | ↑ | <LOD | <LOD |
| Chlorogenic Acid | <LOD | ↑ | <LOD | <LOD | ↑ |
| Quercetin | <LOD | ↔ | <LOD | <LOD | <LOD |
| TSS | ↑ | ↓ | ↑ | ↓ | ↓ |
| Total Sugar | ↑ | ↓ | ↑ | ↓ | ↓ |
| Cr | ↑ | ↔ | ↓ | ↓ | ↓ |
| Cu | ↑ | ↓ | ↓ | ↑ | ↓ |
| Fe | ↑ | ↓ | ↓ | ↑ | ↓ |
| Mg | ↑ | ↓ | ↓ | ↓ | ↑ |
| Na | ↑ | ↓ | ↓ | ↓ | ↑ |
| Ni | ↓ | ↑ | ↓ | ↑ | ↓ |
| Sr | ↓ | ↓ | ↓ | ↑ | ↑ |
| Caffeic Acid | ↔ | ↓ | ↓ | ↔ | ↓ |
| Cinnamic Acid | ↓ | <LOD | <LOD | ↓ | <LOD |
| Coumaric Acid | ↓ | ↓ | <LOD | ↔ | <LOD |
| 4-OH Benzoic Acid | ↓ | ↓ | ↓ | <LOD | ↑ |
| TPC | ↑ | ↓ | ↓ | ↓ | <LOD |
| TAC | ↓ | ↓ | ↑ | ↓ | <LOD |
| K | ↓ | ↓ | ↓ | ↓ | ↑ |
| Zn | ↓ | ↑ | ↓ | ↓ | ↓ |
| Al | ↑ | ↓ | ↓ | ↓ | ↓ |
| Rutin | ↓ | ↓ | ↓ | ↓ | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezey, J.; Mezeyová, I.; Selnekovič, A.; Bajčan, D. Foliar Selenium Biofortification in Temperate Fruit Crops: Impact on Selenium Accumulation and Nutritional Quality of Fruits and Juices. Beverages 2025, 11, 53. https://doi.org/10.3390/beverages11020053
Mezey J, Mezeyová I, Selnekovič A, Bajčan D. Foliar Selenium Biofortification in Temperate Fruit Crops: Impact on Selenium Accumulation and Nutritional Quality of Fruits and Juices. Beverages. 2025; 11(2):53. https://doi.org/10.3390/beverages11020053
Chicago/Turabian StyleMezey, Ján, Ivana Mezeyová, Adrián Selnekovič, and Daniel Bajčan. 2025. "Foliar Selenium Biofortification in Temperate Fruit Crops: Impact on Selenium Accumulation and Nutritional Quality of Fruits and Juices" Beverages 11, no. 2: 53. https://doi.org/10.3390/beverages11020053
APA StyleMezey, J., Mezeyová, I., Selnekovič, A., & Bajčan, D. (2025). Foliar Selenium Biofortification in Temperate Fruit Crops: Impact on Selenium Accumulation and Nutritional Quality of Fruits and Juices. Beverages, 11(2), 53. https://doi.org/10.3390/beverages11020053