Abstract
Maratheftiko (Vitis vinifera sp.) is a prestigious grape variety native to Cyprus, but wines originating from this variety have not been examined with respect to the effect of aging on major quality determinants, such as their aromatic and polyphenolic composition. Following a previous work on the impact of prefermentation treatments on Maratheftiko wines, this work was carried out with the objective of studying the effect of barrel aging on Maratheftiko wines, produced on industrial scale with different prefermentation technologies. These technologies includes combinations of the saigneé process, cold maceration, and enzyme and tannin addition. The influence of these treatments was illustrated by determining the pigment, non-pigment polyphenols, and volatiles from two consecutive harvests. The predominant non-pigment polyphenol for the 2021 vintage was quercetin 3-O-glucuronide, accompanied by a ferulate derivative, but for the 2022 vintage, quercetin 3-O-glucuronide predominated along with caftaric acid. The principal anthocyanin in all samples examined was malvidin 3-O-glucoside, followed by its p-coumarate derivative. The primary aromatic substances determined were isoamyl alcohol, followed by 2-phenylethanol. Principal component analysis showed that there was discrimination based on prefermentation treatments; however, distinction was more pronounced based on vintage. This investigation provided heretofore unreported data and revealed novel insights into the effect of aging on Maratheftiko wines.
1. Introduction
The quality of wines is an exceptionally complicated subject, and it may be defined by numerous physicochemical attributes, including alcohol percentage, sugar concentration, acidity, and pH, but also the composition of the wines in terms of aromatic (volatile) and non-volatile compounds. These factors are major determinants of the sensory perception of wines, as well as its stability and its evolution during aging [1,2,3]. These variables can be significantly defined by the genetic background of the grape variety used and environmental conditions (rainfall, sunlight, and soil), but they may also be intentionally manipulated through the deployment of targeted winemaking techniques [4,5,6].
Polyphenolic compounds, which include anthocyanin pigments and volatile substances, are the most influential groups of wine constituents, fundamentally affecting the organoleptic character and, eventually, the overall wine quality [2]. Volatile substances may originate from inherent grape compounds, but they may also arise from must fermentation through yeast metabolism and wood-related constituents, if barrel- or wooden chip-based aging takes place [7,8]. On the other hand, polyphenols originate almost exclusively from the raw material (grapes) and occur in the skin and seeds. This category of substances embraces several subdivisions, such as simple phenolics and flavonoids, and they affect a range of sensory properties, such as pigmentation (anthocyanins), mouthfeel, and astringency (condensed tannins) [5,8].
Specifically for red wines, the aging process is usually an integral part of wine manufacturing, and it is traditionally carried out by maintaining wines in oak barrels for a period that may last from a few months to several years [9,10]. A multitude of examinations have unequivocally demonstrated the beneficial influence of barrel aging on the sensory properties of red wines, which are associated with color stabilization, the softening of “aggressive” tannins, and enrichment with aromatic compounds deriving from oak wood. An issue of prime importance is wood species but also toasting, which greatly contributes to the formation of peculiar aromatic compounds, such as furfural derivatives, and volatile phenols, such as guaiacol, eugenol, and vanillin. The entrainment of these compounds into aging wine imparts unique flavor notes, e.g., smoky, vanilla, caramel, etc., [9,11]. Furthermore, a cascade of complex reactions may alter the proanthocyanindin structure, making wine tannins less harsh and providing long-term anthocyanin stability through the formation of novel pigments and anthocyanin–tannin co-polymers [6,12].
The Cypriot vineyard has a peculiar varietal composition, embracing several native V. vinifera species, which have a high potential for the production of quality wines. However, their enological characteristics are rather poorly investigated [13]. A variety that has been specifically praised for yielding unique wines is Maratheftiko, but wine production is mainly based on empirical practices and observations, whereas analytical data on aromatic and polyphenolic composition are limited [14,15,16,17]. Recently, two investigations pertaining to the ripening course of Maratheftiko grapes [13] and the effect of pre-fermentation treatments on the volatile and polyphenolic profile of Maratheftiko wines [18] shed light into the quality attributes of this variety. On the other hand, studies on the effects of traditional oak barrel aging have never been reported for Maratheftiko wines. This being the case, the current study was undertaken with objectives of examining the influence of barrel aging on industrial Maratheftiko wines originating from different pre-fermentation treatments on their aromatic character, the composition of pigments, and other major polyphenolic constituents. Thus, this work is a continuation of a previous study [18], where the wines produced through different prefermentation technologies were aged in oak barrels. To the best of the authors’ knowledge, this is the first study on Maratheftiko wine aging in oak barrels, providing novel data of high industrial value and offering a crucial perspective.
2. Materials and Methods
2.1. Chemicals and Reagents
Cyanin chloride (≥90%), catechin (>98%), quercetin 3-O-glucuronide (≥95%), trans-caffeic acid (≥98%), quercetin 3-O-galactoside (≥97%), quercetin (≥95%), 2-octanol, and rutin (quercetin 3-O-rutinoside) (>94%) were obtained from Sigma-Aldrich (Darmstadt, Germany). All solvents used for chromatography were of HPLC grade.
2.2. Wines
The wines destined for aging were produced industrially through three different prefermentation treatments from two consecutive vintages. A full description of all treatments and the vinification implemented have been given in an earlier investigation of ours [18]. In short, the treatment was as follows:
- Treatment C (control)—Must fermentation was accomplished with pomace contact for 11 days. Temperature was maintained at 13–17 °C.
- Treatment CE—Cryoextraction was deployed before must fermentation, at 5 °C, for 48 h, and it was followed by saigneé. The saigneé technique was applied by removing a must volume corresponding to 10% of the total volume from the mash. Then, vinification was carried out as described for treatment C.
- Treatment CEE—Cryoextraction was implemented before fermentation, at 5 °C, for 48 h, followed by saigneé and the addition of pectolytic enzymes. Then, vinification was performed as described for treatment C.
- Treatment CEET—Cryoextraction was deployed before fermentation, at 5 °C, for 48 h, followed by saigneé and the addition of pectolytic enzymes and enological tannins. Then, vinification was carried out as described for treatment C.
2.3. Barrel Aging
The wines produced with different prefermentation treatments and the control (no treatment), as described previously [18], were subjected to barrel aging. Wooden barrels were procured from Ermitage (La Charité sur Loire, France). The barrels used were manufactured with French oak (Quercus robur), were medium toasted, and had a capacity of 225 L. Wines were aged for 6 months, in vaults maintaining a temperature of 13–17 °C.
2.4. Gas Chromatography-Mass Spectrometry (GC-MS)—Sample Preparation and Analysis
The composition of aromatic (volatile) compounds was accomplished with headspace solid-phase microextraction (HS-SPME), using a previously published methodology [18], as described in detail elsewhere [19]. Briefly, each wine sample was spiked with 2-octanol (final concentration 2 mg L−1), which served as the internal standard, and a preconditioned fiber was used to perform the SPME. After completing the SPME, the fiber was inserted into the gas chromatograph injector. GC-MS analysis was performed as described previously [19], using an Agilent Technologies (Folsom, CA, USA) gas chromatograph (model 7890A), coupled with a mass selective detector (model 5975C). Analyses were carried out on an Agilent J&W DB-1 (30 m × 320 μm × 0.25 μm) capillary column, with helium carrier gas, at a flow rate of 1.5 mL min−1, in splitless mode. The identification of compounds was based on the comparison of the mass spectra acquired with mass spectra libraries NIST11 (NIST, Gaithersburg, MD, USA) and W8N08 (John Wiley & Sons, Inc., Hoboken, NJ, USA), using Agilent Technologies (Folsom, CA, USA) MSD Chemstation software (ver. E.02.00.493). Quantitation was accomplished using the GC peak regions (without correction factors) via the normalization approach, and average values were derived from at least 2 consecutive injections to compute the concentrations, which were expressed as mg of 2-octanol equivalents per L of wine.
2.5. Liquid Chromatography Determinations
The detection and identification of major polyphenols and pigments has been described in full detail in previous studies [20,21]. For all anthocyanins and non-pigment polyphenols, quantitation was accomplished using external standards. The trans-caftaric acid was determined as caffeic acid, and the p-coumaric acid and ferulic acid derivatives were determined as p-coumaric acid and ferulic acid, respectively. The quantification of anthocyanins was performed using cyanin chloride as the standard, and the results were given as cyanin equivalents.
2.6. Statistical Processing
Solid-phase microextractions were performed at least twice and GC-MS analyses at least in triplicate. Polyphenol determination was accomplished with the direct injection of wine, at least in triplicate. The values given were expressed as the mean ± standard deviation. Linear regression was performed to construct calibration curves (HPLC, GC), using SigmaPlot™ 12.5 (Systat Software Inc., San Jose, CA, USA). The normality of the data was assessed using the Shapiro–Wilk test. Significant differences were detected with the Kruskal–Wallis test, using IBM SPSS Statistics™ 29 (SPSS Inc., Chicago, IL, USA), taking into consideration the non-normal distribution of the data. Principal Component Analysis (PCA) was performed with the online web tool SRplot (http://www.bioinformatics.com.cn/srplot, accessed on 1 March 2024).
3. Results and Discussion
3.1. Composition of Non-Pigment Polyphenols
The characteristic chromatographic traces for the major polyphenols detected and quantified are shown in Figure 1. Furthermore, analytical quantitative data are given in Table 1. Irrespective of the prefermentation technology applied and the vintage, quercetin 3-O-glucuronide was the predominant metabolite. For the 2021 vintage, the ferulate derivative had comparable levels, yet for the 2022 vintage, quercetin 3-O-glucuronide was by far the most abundant polyphenol. Moreover, all samples from the 2022 vintage were richer in total polyphenols, which is in absolute agreement with the richness of the grapes used [13] and the non-aged wines [18].
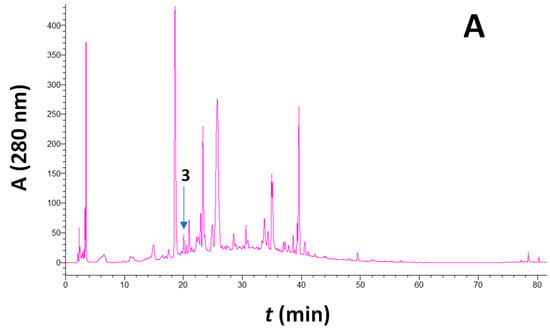
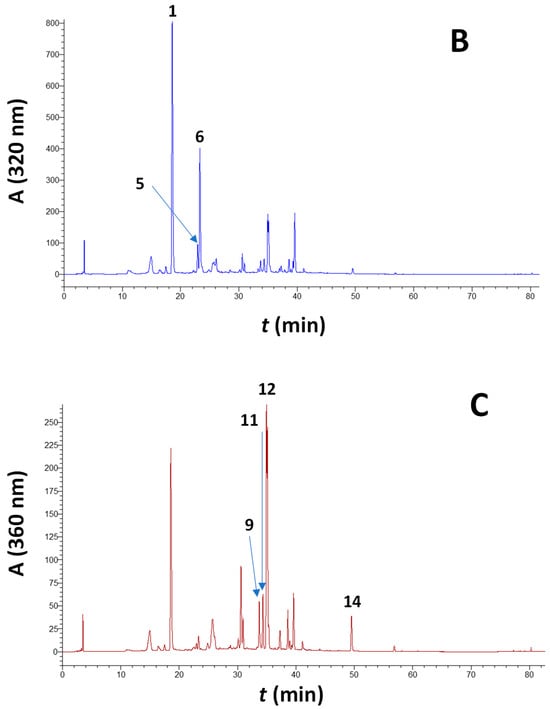
Figure 1.
Typical HPLC traces of a barrel-aged Maratheftiko wine, recorded at 280 (A), 320 (B), and 360 nm (C). Peak assignment: 1, caftaric acid; 3, catechin; 5, p-coumaric acid derivative; 6, ferulic acid derivative; 9, rutin; 11, quercetin 3-O-galactoside; 12, quercetin 3-O-glucuronide; and 14, quercetin.

Table 1.
Analytical non-pigment polyphenolic composition of the aged wines considered in this study. Treatment assignments: C, control; CE, cryoextraction and saignée; CEE, cryoextraction, saignée, and enzyme addition; CEET, cryoextraction, saignée, and enzyme and tannin addition. The values (mg L−1) presented are the average of triplicate determinations ± standard deviation.
Compared to the non-aged wines, as described in our earlier work [18], wines that underwent aging exhibited a uniformly lower polyphenol concentration. In the previous study concerning the effect of prefermentation treatments on the polyphenolic profile [18], the total polyphenol concentration of the samples considered was in the following order for the 2021 vintage: C > CEE > CEET > CE. However, the pattern after aging was CE > CEE > CEET > C. This finding suggests that wines produced with different prefermentation treatments may display differentiated aging behavior with regard to polyphenol evolution. On the other hand, the order for the vintage 2022 prior to aging was C = CE > CEET > CEE, and this remained identical after aging. This strongly indicates that, in this case, aging did not affect the relative polyphenolic richness. Thus, it could be argued that the evolution of the polyphenolic composition was not treatment-related but vintage-related.
The data available in the literature are rather poor and inconsistent to substantiate evolution patterns for the different classes of polyphenols occurring in red wines during barrel aging. Regarding hydroxycinnamates such as caftaric acid and p-coumarate derivatives, a slight decrease was observed for aging in wooden barrels, while the type of wood appeared to exert some influence on the extent of the changes. A more pronounced effect was also seen for catechin and quercetin, but the results regarding quercetin 3-O-galactoside were inconsistent [22]. The effect of wood type (acacia and oak) has also been revealed by other investigations, which showed a varied influence on hydroxycinnamates, catechin, quercetin glycosides, and quercetin [23]. Latter studies confirmed the decrease in substances including p-coumaric acid, caffeic acid, and catechin [24]. However, other examinations have demonstrated an increase in quercetin concentration during aging, a decrease in quercetin 3-O-rhamnoside (quercitrin), and fluctuations in rutin [25]. The increase in quercetin was putatively ascribed to the liberation of aglycone through quercetin glycoside hydrolysis. At this point, it should be noted that, compared to non-aged wines [13], the aged samples analyzed in this study displayed consistently higher quercetin concentrations, which might be an indication of quercetin glycoside hydrolysis.
3.2. Anthocyanin Composition
The typical anthocyanin profile of the wines examined is shown in Figure 2. In all cases, the pigment with the highest abundance was malvidin 3-O-glucoside, followed by its p-coumarate derivative and paeonidin 3-O-glucoside (Table 2). This pattern was identical to the one found for the non-aged wines [13], and matched the one reported in an earlier study on Maratheftiko wines [16]. Wines from the 2022 vintage were richer in anthocyanins compared to those from the 2021 vintage, which is in accordance with what was observed for the non-aged wines from the same vintages [13]. Furthermore, all wines tested had significantly lower anthocyanin concentrations compared to the non-aged ones, indicating the extensive disappearance of monomeric pigments.
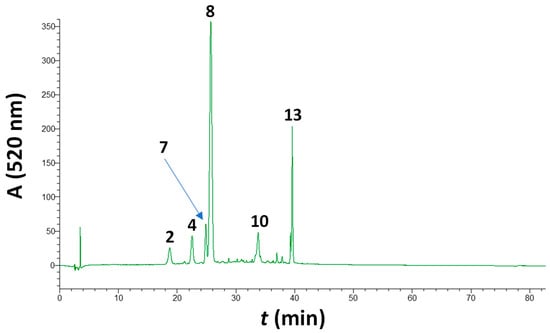
Figure 2.
Characteristic chromatogram of a barrel-aged Maratheftiko wine, illustrating its pigment profile. Peak assignment: 2, delphinidin 3-O-glucoside; 4, petunidin 3-O-glucoside; 7, paeonidin 3-O-glucoside; 8, malvidin 3-O-glucoside; 10, malvidin 3-O-glucoside acetate; and 13, malvidin 3-O-glucoside p-coumarate.

Table 2.
Analytical pigment composition of the aged wines considered in this study. Treatment assignments: C, control; CE, cryoextraction and saignée; CEE, cryoextraction, saignée, and enzyme addition; CEET, cryoextraction, saignée, and enzyme and tannin addition. The values (mg L−1) presented are the average of triplicate determinations ± standard deviation.
This outcome was anticipated, as has been reported in several investigations, demonstrating a gradual decrease in the concentration of the native grape pigments during barrel aging. Early examinations showed that a significant decrease in native grape anthocyanins may has already occurred in the first four months of aging, and after sixteen months, the total anthocyanin concentration dropped to approximately 50% of the initial value [26]. Similar results were reported in other detailed studies on aging wine pigments, where a decrease by almost 50% of the initial total anthocyanin concentration was seen after the 20th month of aging [27]. These authors also found that caffeoyl anthocyanin derivatives largely disappeared within the first four months of aging. A latter study has indicated that the course of the disappearance may indeed depend on the anthocyanin structure [24].
Considering a high amount of data, it is irrefutable that barrel aging results in a steady decline in monomeric anthocyanin concentration, giving rise to numerous novel derivative pigments [28]. However, there have also been studies supporting that no significant decline in anthocyanins occurred during oak barrel aging but during subsequent bottle maturation [29]. On the other hand, a more recent examination on a large number of red wines aged in oak barrels and bottles did confirm the gradual decrease in total monomeric anthocyanin concentration [30], while more recent data has suggested a sharper anthocyanin decline occurs [31]. This decline was also manifested at a varying extent, depending on the carrier used for storage (barrel, stainless steel tanks, and pottery altars) [32], the type of wood, the age of the barrels [33], and barrel-to-barrel variations [34].
Anthocyanins are particularly reactive molecules, and during aging, they may undergo a range of transformations [6]. Because these transformations largely depend on reactions with flavanols, the relative concentration of flavanols/anthocyanins could play a key role and may be significantly affected by the ripening stage and time of harvest [35]. Indeed, enological (condensed) tannin addition in red wines was shown to favorably affect color stability by promoting reactions with anthocyanins [36]. Apart from flavanols, the presence of several other molecules may contribute to the formation of various anthocyanin derivatives due to which native grape anthocyanins are extensively transformed into a wide spectrum of pigments [6]. By virtue of their resistance to SO2-induced bleaching and oxidative decomposition, these anthocyanin derivatives are considered pivotal in color stabilization and endurance and the improvement of the chromatic characteristics of barrel-aged red wines.
3.3. Profile of Volatile Constituents
A tentative identification and quantification of a total number of 16 volatiles in the wine samples studied was carried out (Table 3). The aromatic profile of the wines tested essentially consisted of five alcohols and eight esters, the predominant of which, based on concentration, was isoamyl alcohol (banana-like odor), followed by 2-phenylethanol (rose-like odor). It is to be noted that, while in two of the 2021 vintage wines isoamyl formate was the second most abundant volatile, it was consistently absent from all the samples of the 2022 vintage. This was also true for 2-methyl-1-butanol and ethyl 2-hydroxypropanoate. In general, the aromatic profiles of all aged wines were significantly diversified from those reported for the non-aged ones [13], illustrating the profound effect of aging on volatile composition.

Table 3.
Profile of the major aromatic compounds detected and tentatively identified in the aged wines examined. Treatment assignments: C, control; CE, cryoextraction and saignée; CEE, cryoextraction, saignée, and enzyme addition; CEET, cryoextraction, saignée, and enzyme and tannin addition. The values (mg L−1) presented are the average of triplicate determinations ± standard deviation.
Another important finding is the detection of furfuryl alcohol in some of the wines analyzed (Table 3), which further manifested the influence of barrel aging on the volatile composition of the wines. This particular alcohol is not a native grape constituent, nor does it originate from fermentation. Furfuryl alcohol derives from furfural, which occurs in toasted wood used for barrel-making, and it may occur at levels reaching over 3 mg L−1 [37,38], although its concentration may be greatly affected by the wood’s toasting degree and aging time [39]. Although it may impart odors described as moldy hey, its perception level is about 45 mg L−1, making it rather an insignificant contributor to wine aroma [38]. Other authors have characterized its scent as cocoa, smoky, nutty, and burnt, and they have reported a threshold of 2 mg L−1 [40], which is still much higher than the concentrations found in this study.
On the contrary, the effect of other compounds may be considered significant, given their particularly low threshold levels. For example, isoamyl acetate, which occurred at levels higher than 0.66 mg L−1 in the 2022 vintage wines, has a banana/sweet/fruity odor and a perception threshold of 30 μg L−1 [40]. Likewise, ethyl hexanoate and diethyl succinate had concentrations well above 0.20 mg L−1 in all samples analyzed and, given their low thresholds [41], could represent major flavor contributors. Furthermore, other principal constituents, such as isoamyl alcohol and 2-phenylethanol, could have a significant impact on the aromatic profile of the wines, imparting tones such as malt/whisky [42] and rose/flowery [40], respectively.
3.4. Principal Component Analysis (PCA)-Based Discrimination
PCA carried out on the experimental data elucidated the effect of various prefermentation treatments and vintage year on wine composition, highlighting distinct patterns of differentiation. The plots were constructed with SRPlot [43], and they are shown in Figure 3 and Figure 4.
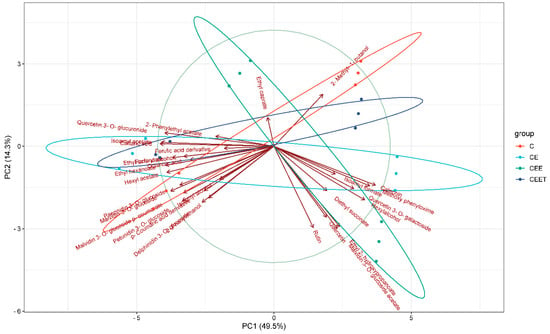
Figure 3.
PCA plot showing the grouping of wine samples according to the prefermentation treatments: C (control); CE (cryoextraction and saignée); CEE (cryoextraction, saignée, and enzyme addition); and CEET (cryoextraction, saignée, and enzyme and tannin addition).
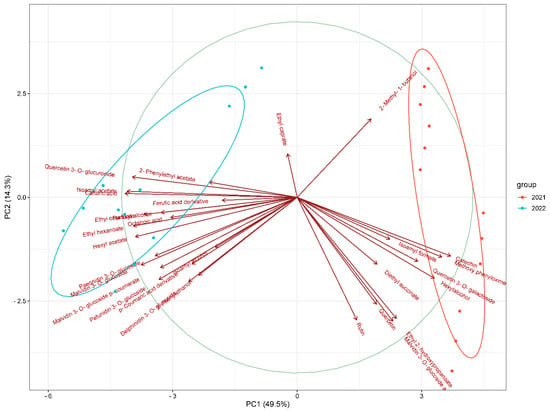
Figure 4.
PCA plot showing the grouping of wine samples according to their vinification year (2021 and 2022).
The PCA plot in Figure 3 shows the differences in the samples based on the applied prefermentation treatments, indicating that each treatment significantly influenced the compositional profile of the wines. The plot explains 65% of the variance. A percentage of 49.5% of the variance is attributed to the first principal component (PC1), which is negatively correlated with most of the compounds considered. The second principal component (PC2) accounts for 14.3% of the variance, and most of the compounds are negatively correlated with PC2. As can be seen, the CEE group diverges substantially from both CE and CEET. This could suggest that the inclusion of enzymes in CEE led to significant changes in the composition of the wine. In contrast, CE and CEET exhibit a partial overlap, indicating compositional similarities between these two treatments. The addition of tannins (CEET sample) appears to have introduced some differentiation, but its effect is not as pronounced as that of the enzyme pretreatment. Interestingly, the control group is not clearly separated but instead overlaps with all other groups. This overlap may suggest that while the prefermentation treatments altered the composition of the wine, the main characteristics of the control wines were retained to some extent in the treated samples.
Figure 4 presents the PCA clustering based on the vinification year, demonstrating a distinct separation between samples from 2021 and 2022. This separation underscores the role of vintage conditions, including climatic variables such as temperature, rainfall, and sunlight, in shaping the compositional attributes of the wines. These differences were expected to affect the constituents of the wines, such as organic acids, phenolic compounds, and volatile aroma compounds. Within each vintage cluster, sub-clustering can be observed, supporting the view that prefermentation treatment had an impact on the wine composition, alongside the vintage effect. Overall, the above results highlight that prefermentation treatments and vintage year are key parameters in wine composition.
4. Conclusions
This work elucidated for the first time the impact of barrel aging on industrially produced Maratheftiko wines that underwent various prefermentation treatments. Aging was shown to provoke significant changes that differentiated wines in terms of both their volatile and non-volatile fraction. These differences are evidence that barrel aging may have a profound influence on Maratheftiko quality attributes. It is of interest to note that, although the aromatic profile of the wines examined was greatly influenced by aging, no significant enrichment in any barrel-derived compound was noticed. The principal component analysis showed that prefermentation treatments may differentiate the wines tested to some degree; however, a clear distinction could be made based on vintage year. This study provides some novel data regarding the effect of aging on Maratheftiko wines, which may be of great value to stakeholders in future examinations. It is proposed that additional research may include other aging techniques (e.g., the use of wooden chips), to further clarify the contribution of such treatments to the production of high-quality wines.
Author Contributions
Conceptualization, K.R. and D.P.M.; methodology, K.R., V.A. and D.P.M.; validation, T.C., V.A. and D.P.M.; formal analysis, K.R., T.C., A.T., V.A. and D.P.M.; investigation, K.R., A.T. and D.P.M.; writing—original draft preparation, D.P.M. and S.I.L.; writing—review and editing, D.P.M. and S.I.L.; visualization, D.P.M.; supervision, D.P.M. and S.I.L.; project administration, K.R., D.P.M. and S.I.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
Artemis Toulaki works for the company Wineconsulting. The other authors declare no conflicts of interest.
References
- Merkytė, V.; Longo, E.; Windisch, G.; Boselli, E. Phenolic compounds as markers of wine quality and authenticity. Foods 2020, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Pittari, E.; Moio, L.; Piombino, P. Interactions between polyphenols and volatile compounds in wine: A literature review on physicochemical and sensory insights. Appl. Sci. 2021, 11, 1157. [Google Scholar] [CrossRef]
- Basalekou, M.; Tataridis, P.; Georgakis, K.; Tsintonis, C. Measuring wine quality and typicity. Beverages 2023, 9, 41. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Mattii, G.B. A review: Soil management, sustainable strategies and approaches to improve the quality of modern viticulture. Agronomy 2021, 11, 2359. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine polyphenol content and its influence on wine quality and properties: A review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Zhang, X.K.; Jeffery, D.W.; Li, D.M.; Lan, Y.B.; Zhao, X.; Duan, C.Q. Red wine coloration: A review of pigmented molecules, reactions, and applications. Comp. Rev. Food Sci. Food Saf. 2022, 21, 3834–3866. [Google Scholar] [CrossRef]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 2. Chemical and sensory analysis. Am. J. Enol. Vitic. 2014, 65, 25–42. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Mateus, N.; de Freitas, V. Sensorial properties of red wine polyphenols: Astringency and bitterness. Crit. Rev. Food Sci. Nutr. 2017, 57, 937–948. [Google Scholar] [CrossRef]
- Tao, Y.; García, J.F.; Sun, D.-W. Advances in wine aging technologies for enhancing wine quality and accelerating wine aging process. Crit. Rev. Food Sci. Nutr. 2014, 54, 817–835. [Google Scholar] [CrossRef]
- Ma, T.; Wang, J.; Wang, H.; Zhao, Q.; Zhang, F.; Ge, Q.; Li, C.; Gamboa, G.G.; Fang, Y.; Sun, X. Wine aging and artificial simulated wine aging: Technologies, applications, challenges, and perspectives. Food Res. Inter. 2022, 153, 110953. [Google Scholar] [CrossRef]
- Carpena, M.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Wine aging technology: Fundamental role of wood barrels. Foods 2020, 9, 1160. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Duan, C.-Q. Astringency, bitterness and color changes in dry red wines before and during oak barrel aging: An updated phenolic perspective review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1840–1867. [Google Scholar] [CrossRef] [PubMed]
- Roufas, K.; Chatzimitakos, T.; Athanasiadis, V.; Lalas, S.I.; Makris, D.P. Changes in polyphenols and anthocyanin pigments during ripening of Vitis vinifera cv Maratheftiko: A two-year study. Beverages 2023, 9, 39. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Kotanidis, A.; Dianellou, M.; Gekas, V. Phenolic content and antioxidant capacity of Cypriot wines. Czech J. Food Sci. 2015, 33, 126–136. [Google Scholar] [CrossRef]
- Copper, A.; Johnson, T.; Danner, L.; Bastian, S.; Collins, C. Preliminary sensory and chemical profiling of Cypriot wines made from indigenous grape varieties Xynisteri, Maratheftiko and Giannoudhi and acceptability to Australian consumers. OENO One 2019, 2, 229–248. [Google Scholar]
- Tsiakkas, O.; Escott, C.; Loira, I.; Morata, A.; Rauhut, D.; Suárez-Lepe, J.A. Determination of anthocyanin and volatile profile of wines from varieties Yiannoudi and Maratheftiko from the island of Cyprus. Beverages 2020, 6, 4. [Google Scholar] [CrossRef]
- Copper, A.W.; Collins, C.; Bastian, S.E.; Johnson, T.E.; Capone, D.L. Preliminary investigation of potent thiols in Cypriot wines made from indigenous grape varieties Xynisteri, Maratheftiko and Giannoudhi. OENO One 2021, 55, 223–234. [Google Scholar] [CrossRef]
- Roufas, K.; Athanasiadis, V.; Chatzimitakos, T.; Lalas, S.I.; Toulaki, A.; Makris, D.P. Impact of various prefermentation treatments on the pigment, polyphenol, and volatile composition of industrial red wines made from Vitis vinifera cv Maratheftiko. Beverages 2024, 10, 39. [Google Scholar] [CrossRef]
- Toulaki, A.K.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Roufas, K.; Mantanis, G.I.; Dourtoglou, V.G.; Lalas, S.I. Investigation of xinomavro red wine aging with various wood chips using pulsed electric field. Beverages 2024, 10, 13. [Google Scholar] [CrossRef]
- Makris, D.P.; Psarra, E.; Kallithraka, S.; Kefalas, P. The effect of polyphenolic composition as related to antioxidant capacity in white wines. Food Res. Int. 2003, 36, 805–814. [Google Scholar] [CrossRef]
- Makris, D.; Kefalas, P. Characterization of polyphenolic phytochemicals in red grape pomace. Int. J. Waste Resour. 2013, 3, 126. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Hernández, T.; Cadahía, E.; Dueñas, M.; Estrella, I. Phenolic compounds in a Spanish red wine aged in barrels made of Spanish, French and American oak wood. Eur. Food Res. Technol. 2003, 216, 150–156. [Google Scholar] [CrossRef]
- Sanz, M.; de Simón, B.F.; Esteruelas, E.; Muñoz, Á.M.; Cadahía, E.; Hernández, M.T.; Estrella, I.; Martinez, J. Polyphenols in red wine aged in acacia (Robinia pseudoacacia) and oak (Quercus petraea) wood barrels. Anal. Chim. Acta 2012, 732, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Laqui-Estaña, J.; López-Solís, R.; Peña-Neira, Á.; Medel-Marabolí, M.; Obreque-Slier, E. Wines in contact with oak wood: The impact of the variety (Carménère and Cabernet Sauvignon), format (barrels, chips and staves), and aging time on the phenolic composition. J. Sci. Food Agric. 2019, 99, 436–448. [Google Scholar] [CrossRef]
- Fang, F.; Li, J.-M.; Zhang, P.; Tang, K.; Wang, W.; Pan, Q.-H.; Huang, W.-D. Effects of grape variety, harvest date, fermentation vessel and wine ageing on flavonoid concentration in red wines. Food Res. Int. 2008, 41, 53–60. [Google Scholar] [CrossRef]
- Boido, E.; Alcalde-Eon, C.; Carrau, F.; Dellacassa, E.; Rivas-Gonzalo, J.C. Aging effect on the pigment composition and color of Vitis vinifera L. Cv. Tannat wines. Contribution of the main pigment families to wine color. J. Agric. Food Chem. 2006, 54, 6692–6704. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Changes in the detailed pigment composition of red wine during maturity and ageing: A comprehensive study. Anal. Chim. Acta 2006, 563, 238–254. [Google Scholar] [CrossRef]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and their variation in red wines II. Anthocyanin derived pigments and their color evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef]
- Guadalupe, Z.; Ayestarán, B. Changes in the color components and phenolic content of red wines from Vitis vinifera L. Cv.“Tempranillo” during vinification and aging. Eur. Food Res. Technol. 2008, 228, 29–38. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.; Du Toit, W. Evolution of phenolic composition during barrel and bottle aging. SA J. Enol. Vitic. 2020, 41, 233–237. [Google Scholar] [CrossRef]
- Uysal, R.S.; Issa-Issa, H.; Sendra, E.; Carbonell-Barrachina, Á.A. Changes in anthocyanin pigments, trans-resveratrol, and colorimetric characteristics of Fondillón wine and other “Monastrell” wines during the aging period. Eur. Food Res. Technol. 2023, 249, 1821–1831. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Tang, K.; Rao, Z.; Chen, J. Effects of different aging methods on the phenolic compounds and antioxidant activity of red wine. Fermentation 2022, 8, 592. [Google Scholar] [CrossRef]
- Gambuti, A.; Capuano, R.; Lisanti, M.T.; Strollo, D.; Moio, L. Effect of aging in new oak, one-year-used oak, chestnut barrels and bottle on color, phenolics and gustative profile of three monovarietal red wines. Eur. Food Res. Technol. 2010, 231, 455–465. [Google Scholar] [CrossRef]
- Pfahl, L.; Catarino, S.; Fontes, N.; Graça, A.; Ricardo-da-Silva, J. Effect of barrel-to-barrel variation on color and phenolic composition of a red wine. Foods 2021, 10, 1669. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-San José, M.L. Evolution of flavanols, anthocyanins, and their derivatives during the aging of red wines elaborated from grapes harvested at different stages of ripening. J. Agric. Food Chem. 2004, 52, 1181–1189. [Google Scholar] [CrossRef]
- García-Estévez, I.; Alcalde-Eon, C.; Puente, V.; Escribano-Bailón, M.T. Enological tannin effect on red wine color and pigment composition and relevance of the yeast fermentation products. Molecules 2017, 22, 2046. [Google Scholar] [CrossRef]
- Del Fresno, J.M.; Morata, A.; Loira, I.; Escott, C.; Suarez Lepe, J.A. Evolution of the phenolic fraction and aromatic profile of red wines aged in oak barrels. ACS Omega 2020, 5, 7235–7243. [Google Scholar] [CrossRef]
- Casassa, L.F.; Ceja, G.M.; Vega-Osorno, A.; du Fresne, F.; Llodrá, D. Detailed chemical composition of Cabernet Sauvignon wines aged in French oak barrels coopered with three different stave bending techniques. Food Chem. 2021, 340, 127573. [Google Scholar] [CrossRef]
- Dumitriu, G.-D.; de Lerma, N.L.; Zamfir, C.-I.; Cotea, V.V.; Peinado, R.A. Volatile and phenolic composition of red wines subjected to aging in oak cask of different toast degree during two periods of time. LWT 2017, 86, 643–651. [Google Scholar] [CrossRef]
- del Barrio Galán, R.; Bueno-Herrera, M.; de la Cuesta, P.L.; Pérez-Magariño, S. Volatile composition of Spanish red wines: Effect of origin and aging time. Eur. Food Res. Technol. 2022, 248, 1903–1916. [Google Scholar] [CrossRef]
- Moreira, N.; Lopes, P.; Ferreira, H.; Cabral, M.; de Pinho, P.G. Influence of packaging and aging on the red wine volatile composition and sensory attributes. Food Pack. Shelf Life 2016, 8, 14–23. [Google Scholar] [CrossRef]
- Vidal, R.B.P.; Boulton, R.B.; Olivieri, A.C.; Buscema, F. Aging of Malbec Wines from Mendoza and California: Evolution of Volatile Composition. Am. J. Enol. Vitic. 2023, 74, 0740019. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).