Formulation Design and Functional Characterization of a Novel Fermented Beverage with Antioxidant, Anti-Inflammatory and Antibacterial Properties
Abstract
1. Introduction
2. Material and Methods
2.1. Water Kefir Grains
2.2. Raw Material
2.3. Preparation of Green Tea Water Kefir
2.4. Experimental Design
R2 = 92.14%
R2 = 93.53%
R2 = 96.65%
2.5. Determination of Microbial Growth
2.6. pH and Acidity
2.7. Determination of Total Phenolic Compounds
2.8. Flavonoids Assay
2.9. Determination of Antioxidant Activity
2.9.1. 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Assay
2.9.2. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic Acid) Diammonium Salt Radical Scavenging Assay
2.9.3. Iron-Chelating Activity
2.9.4. Power Reducing Activity
2.10. Anti-Inflammatory Activity of the Optimal Formula
2.11. Consumer Acceptance Test
2.12. Antimicrobial Activity of GTWK
2.13. Statistical Analysis
3. Results and Discussion
3.1. Effect of Honey on Microbial Growth, Antioxidant Activity, and Consumer Acceptance
3.2. Study of Independent Factors on GTWK Formulation
3.2.1. Effect of Independent Factors on Microbial Growth
3.2.2. Effect of Variables on pH
3.2.3. Effect of Variables on TPC and Antioxidant Activity
3.2.4. Effect of Variables on Overall Acceptability
3.2.5. Optimization of Independent Variables and Model Validation
3.3. Antioxidant Activity Profile of the Optimized GTWK Formula
3.4. Anti-Inflammatory Effect of the Optimized GTWK Formula
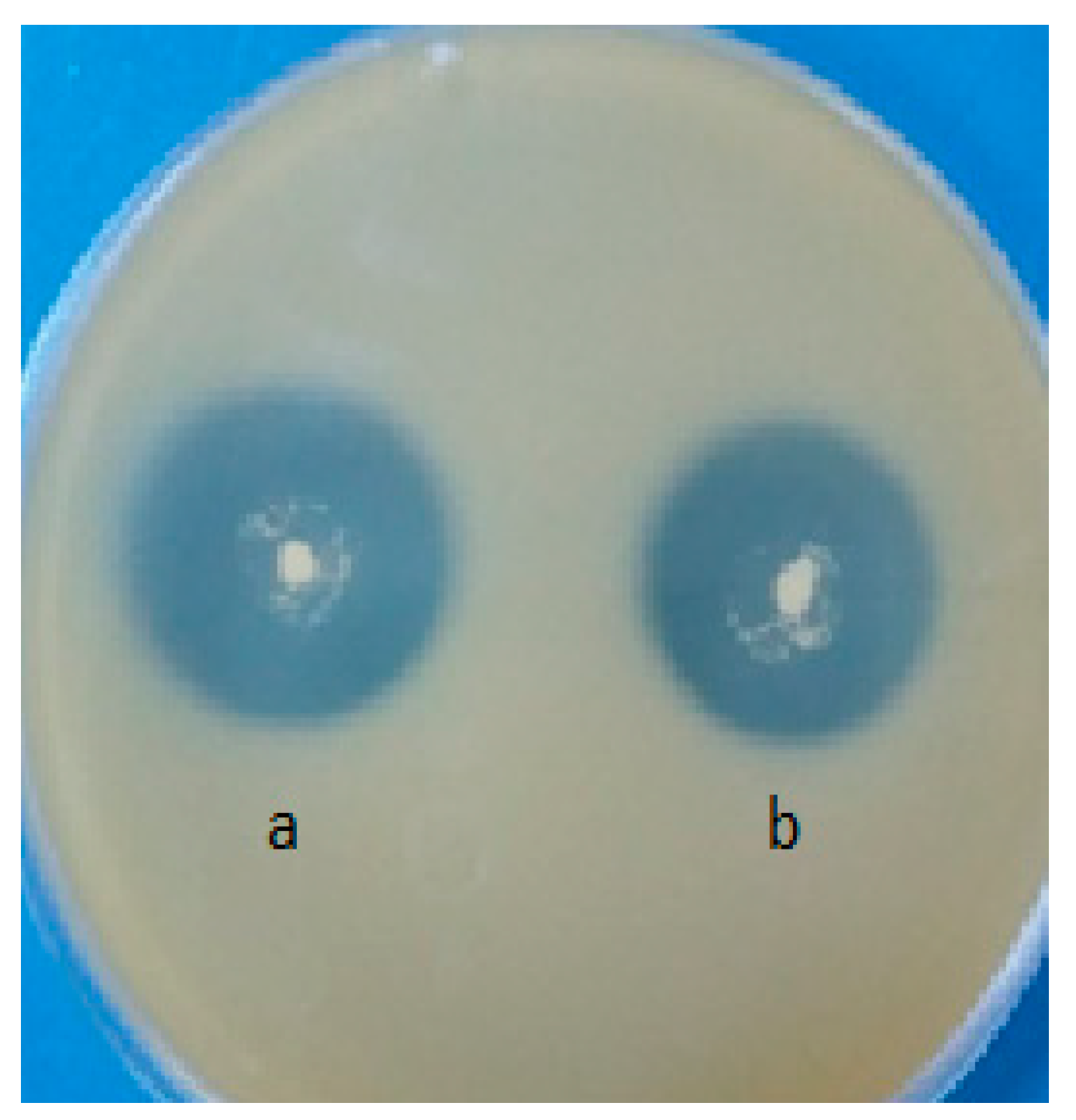
3.5. Antimicrobial Activity of the Optimized GTWK Formula
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abs | Absorbance |
| ANOVA | Analysis of variance |
| CCD | Central composite design |
| CFU | Colony forming unit |
| DPPH | 2,2-Diphenyl-1-Picrylhydrazyl |
| GAE | Galic acid equivalent |
| GTWK | Green tea water kefir beverage |
| LAB | Lactic acid bacteria |
| OA | Overall acceptability |
| PDA | Potato dextrose agar |
| RSM | Response surface methodology |
| TPC | Total phenolic compounds |
| TTA | Total titratable acidity |
References
- Guzel-Seydim, Z.B.; Gökırmaklı, Ç.; Greene, A.K. A comparison of milk kefir and water kefir: Physical, chemical, microbiological and functional properties. Trends Food Sci. Technol. 2021, 113, 42–53. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Araújo, T.H.; Schneedorf, J.M.; Ferreira, C.S.; Moraes, G.O.I.; Coimbra, R.S.; Rodrigues, M.R. A novel beer fermented by kefir enhances anti-inflammatory and anti-ulcerogenic activities found isolated in its constituents. J. Funct. Foods 2016, 21, 58–69. [Google Scholar] [CrossRef]
- Zamberi, N.R.; Abu, N.; Mohamed, N.E.; Nordin, N.; Keong, Y.S.; Beh, B.K.; Abu Bakar Zakaria, Z.; Nik Abdul Rahman, N.M.; Banu Alitheen, N. The antimetastatic and antiangiogenesis effects of kefir water on murine breast cancer cells. Integr. Cancer Ther. 2016, 15, 53–66. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. The role of tea in human health: An update. J. Am. Coll. Nutr. 2002, 21, 1–13. [Google Scholar] [CrossRef]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, A.; Luna, M.; Rucker, N.; Wong, S.; Gomer, C.J. Pro-apoptotic and anti-inflammatory properties of the green tea constituent epigallocatechin gallate increase photodynamic therapy esponsiveness. Lasers Surg. Med. 2011, 43, 644. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reto, M.; Figueira, M.E.; Filipe, H.M.; Almeida, C.M. Chemical composition of green tea (Camellia sinensis) infusions commercialized in Portugal. Plant Foods Hum. Nutr. 2007, 62, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Serafini, M. Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Zijp, I.M.; Korver, O.; Tijburg, L.B.M. Effect of tea and other dietary factors on iron absorption. Crit. Rev. Food Sci. Nutr. 2000, 40, 371–398. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus Limon (Lemon) phenomenon-a review of the chemistry, pharmacological properties, applications in the modern pharmaceutical, food, and cosmetics industries, and biotechnological studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Ramazan, I.; Irfan, S.; Mahmood, S.; Ranjha, M.M.A.N.; Shahzad, J.K.; Jaangla, K.T.; Bin Masood, A.; Mustafa, S. A critical review on nutritional and medicinal importance of lemon. Acta Sci. Agric. 2019, 3, 95–97. [Google Scholar] [CrossRef]
- Mohan, A.; Quek, S.Y.; Gutierrez-Maddox, N.; Gao, Y.; Shu, Q. Effect of honey in improving the gut microbial balance. Food Qual. Saf. 2017, 1, 107–115. [Google Scholar] [CrossRef]
- Zahoor, F.; Sooklim, C.; Songdech, P.; Duangpakdee, O.; Soontorngun, N. Selection of potential yeast probiotics and a cell factory for xylitol or acid production from honeybee samples. Metabolites 2021, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Mežnarić, S.; Karačonji, I.B.; Crnković, G.; Lesar, A.; Pavlešić, T.; Vučković, D.; Gobin, I. Combined inhibitory effect of fir (Abies Alba Mill) honeydew honey and probiotic bacteria Lactiplantibacillus plantarum on the growth of Salmonella enterica Serotype Typhimurium. Antibiotics 2022, 11, 145. [Google Scholar] [CrossRef]
- Latha, S.; Sivaranjani, G.; Dhanasekaran, D. Response Surface Methodology: A nonconventional statistical tool to maximize the throughput of Streptomyces Species biomass and their bioactive metabolites. Crit. Rev. Microbiol. 2017, 43, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.H.; Montgomery, D.C. Response Surface Methodology Product and Process Optimization Using Designed Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Laureys, D.; De Vuyst, L. Microbial Species Diversity, Community Dynamics, and Metabolite Kinetics of Water Kefir Fermentation. Appl. Environ. Microbiol. 2014, 80, 2564–2572. [Google Scholar] [CrossRef]
- Tyl, C.; Sadler, G.D. pH and titratable acidity. In Food Analysis; Springer: Berlin/Heidelberg, Germany, 2017; pp. 389–406. [Google Scholar] [CrossRef]
- Musci, M.; Yao, S. Optimization and validation of folin-ciocalteu method for the determination of total polyphenol content of pu-erh tea. Int. J. Food Sci. Nutr. 2020, 68, 913–918. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Kodama, D.H.; Gonçalves, A.E.D.S.; Lajolo, F.M.; Genovese, M.I. Flavonoids, Total phenolics and antioxidant capacity: Comparison between commercial green tea preparations. Food Sci. Technol. 2010, 30, 1077–1082. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Rufián-Henares, J.A.; Morales, F.J. Assessing the antioxidant activity of melanoidins from coffee brews by different antioxidant methods. J. Agric. Food Chem. 2005, 53, 7832–7836. [Google Scholar] [CrossRef] [PubMed]
- Custódio, L.; Justo, T.; Silvestre, L.; Barradas, A.; Duarte, C.V.; Pereira, H.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. Microalgae of different phyla display antioxidant, metal chelating and acetylcholinesterase inhibitory activities. Food Chem. 2012, 131, 134–140. [Google Scholar] [CrossRef]
- Son, S.H.; Yang, S.J.; Jeon, H.L.; Yu, H.S.; Lee, N.K.; Park, Y.S.; Paik, H.D. Antioxidant and immunostimulatory effect of potential probiotic Lactobacillus paraplantarum SC61 isolated from korean traditional fermented food, Jangajji. Microb. Pathogenesis 2018, 125, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ahmad, A.A.; Yang, Y.; Liang, Z.; Shen, W.; Feng, M.; Shen, J.; Lan, X.; Ding, X. Lactobacillus rhamnosus CY12 enhances intestinal barrier function by regulating tight junction protein expression, oxidative stress, and inflammation response in lipopolysaccharide-induced Caco-2 cells. Int. J. Mol. Sci. 2022, 23, 11162. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.-C.; Jee, S.Y.; Lee, S.G.; Park, S.J.; Lee, J.R.; Kim, S.C. Anti-inflammatory activity of the methanol extract of moutan cortex in LPS-activated Raw264.7 cells. J. Evid. Based Complement. Altern. Med. 2007, 4, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Kadyan, S.; Rashmi, H.M.; Pradhan, D.; Kumari, A.; Chaudhari, A.; Deshwal, G.K. Effect of Lactic Acid Bacteria and Yeast fermentation on antimicrobial, antioxidative and metabolomic profile of naturally carbonated probiotic whey drink. LWT 2021, 142, 111059. [Google Scholar] [CrossRef]
- Fiorda, F.A.; de Melo Pereira, G.V.; Thomaz-Soccol, V.; Medeiros, A.P.; Rakshit, S.K.; Soccol, C.R. Development of kefir-based probiotic beverages with DNA protection and antioxidant activities using soybean hydrolyzed extract, colostrum and honey. LWT-Food Sci. Technol. 2016, 68, 690–697. [Google Scholar] [CrossRef]
- Padauleng, M.A.; Maruddin, F.; Nur Yuliati, F.; Tashi Wangdi, J.; Ihsan Andi Dagong, M. The physiochemical properties of kefir using honey concentrations. Canrea J. Food Technol. Nutr. Culin. 2021, 4, 8–16. [Google Scholar] [CrossRef]
- Laureys, D.; Aerts, M.; Vandamme, P.; De Vuyst, L. Oxygen and diverse nutrients influence the water kefir fermentation process. Food Microbiol. 2018, 73, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.G.; Faria, J.A.F.; Walter, E.H.M.; Andrade, R.R.; Cavalcanti, R.N.; Oliveira, C.A.F.; Granato, D. Processing optimization of probiotic yogurt containing glucose oxidase using response surface methodology. J. Dairy Sci. 2010, 11, 5059–5068. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Behera, S.K.; Witness Qaku, X.; Sekar, S.; Ndinteh, D.T.; Nanjundaswamy, H.M.; Ray, R.C.; Kayitesi, E. Quality enhancement of Prickly Pears (Opuntia sp.) Juice through probiotic fermentation using Lactobacillus fermentum-ATCC 9338. LWT-Food Sci. Technol. 2017, 75, 453–459. [Google Scholar] [CrossRef]
- M’hir, S.; Mejri, A.; Atrous, H.; Ayed, L. Optimization of parameters using response surface methodology to develop a novel kefir-like functional beverage from cheese whey enriched with myrtle juice. J. Chem. 2021, 2021, 2984470. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Luang-In, V.; Deeseenthum, S. Exopolysaccharide-producing isolates from thai milk kefir and their antioxidant activities. Food Sci. Technol. 2016, 73, 592–601. [Google Scholar] [CrossRef]
- De Oliveira, C.B.; Fiorda-Mello, F.; De Melo Pereira, G.V.; Thomaz-Soccol, V.; Rakshit, S.K.; De Carvalho, J.C.; Soccol, C.R. In Vitro probiotic properties and DNA protection activity of yeast and Lactic Acid Bacteria isolated from a honey-based kefir beverage. Foods 2019, 8, 485. [Google Scholar] [CrossRef] [PubMed]
- Muzolf, M.; Szymusiak, H.; Gliszczyńska-Świgło, A.; Rietjens, I.M.C.M.; Tyrakowska, B. pH-dependent radical scavenging capacity of green tea catechins. J. Agric. Food Chem. 2008, 56, 816–823. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Fischer, A.; Legler, A.D.S.; Oner, M.E.; Wolken, H.F.; Köpsel, M.; Ozogul, Y.; Özyurt, G.; De Biase, D.; Ozogul, F. Physical, chemical, and sensory properties of water kefir produced from Aronia melanocarpa juice and pomace. Food Chem. X 2023, 18, 100683. [Google Scholar] [CrossRef] [PubMed]
- Varga, L. Effect of Acacia (Robinia pseudo-acacia L.) Honey on the characteristic microflora of yogurt during refrigerated storage. Int. J. Food Microbiol. 2006, 108, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Sabokbar, N.; Khodaiyan, F. Total phenolic content and antioxidant activities of pomegranate juice and whey based novel beverage fermented by kefir grains. J. Food Sci. Technol. 2016, 53, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Mortadza, S.A.S.; Alitheen, N.B. Kefir and its biological activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.Y.; Jeong, Y.; Kang, C.H. Antioxidant activity and probiotic properties of Lactic Acid Bacteria. Fermentation 2022, 8, 29. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, L.; Tian, J.; Wang, X.; Zhang, X.; Fang, Y.; Li, W. Structural characterization, rheological properties and protection of oxidative damage of an exopolysaccharide from Leuconostoc citreum 1.2461 Fermented in Soybean Whey. Foods 2022, 11, 2283. [Google Scholar] [CrossRef]
- Krishnamoorthi, R.; Srinivash, M.; Mahalingam, P.U.; Malaikozhundan, B.; Suganya, P.; Gurushankar, K. Antimicrobial, anti-biofilm, antioxidant and cytotoxic effects of bacteriocin by Lactococcus lactis Strain CH3 isolated from fermented dairy products-An in vitro and in silico approach. Int. J. Biol. Macromol. 2022, 220, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Mei, X. Antioxidant activity of an exopolysaccharide purified from Lactococcus lactis subsp. lactis 12. Carbohydr. Polym. 2010, 80, 908–914. [Google Scholar] [CrossRef]
- Ramalho, J.B.; Soares, M.B.; Spiazzi, C.C.; Bicca, D.F.; Soares, V.M.; Pereira, J.G.; da Silva, W.P.; Sehn, C.P.; Cibin, F.W.S. In vitro probiotic and antioxidant potential of Lactococcus lactis Subsp. Cremoris LL95 and its effect in mice behaviour. Nutrients 2019, 11, 901. [Google Scholar] [CrossRef] [PubMed]
- Fakruddin, M.; Hossain, M.N.; Ahmed, M.M. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complement. Altern. Med. 2017, 17, 64. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Vincentini, O.; Cantatore, V.; Cavoski, I.; Gobbetti, M. Fermented Portulaca oleracea L. juice: A novel functional beverage with potential ameliorating effects on the intestinal inflammation and epithelial injury. Nutrients 2019, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Cabral, B.D.; Larrosa-Pérez, M.; Gallegos-Infante, J.A.; Moreno-Jiménez, M.R.; González-Laredo, R.F.; Rutiaga-Quiñones, J.G.; Gamboa-Gómez, C.I.; Rocha-Guzmán, N.E. Oak kombucha protects against oxidative stress and inflammatory processes. Chem. Biol. Interact. 2017, 272, 1–9. [Google Scholar] [CrossRef] [PubMed]
- SaeidiFard, N.; Djafarian, K.; Shab-Bidar, S. Fermented foods and inflammation: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2020, 35, 30–39. [Google Scholar] [CrossRef]
- Vinderola, G.; Perdigón, G.; Duarte, J.; Farnworth, E.; Matar, C. Effects of the oral administration of the exopolysaccharide produced by Lactobacillus kefiranofaciens on the gut mucosal immunity. Cytokine 2006, 36, 254–260. [Google Scholar] [CrossRef]
- Carasi, P.; Racedo, S.M.; Jacquot, C.; Romanin, D.E.; Serradell, M.A.; Urdaci, M.C. Impact of kefir derived Lactobacillus kefiri on the mucosal immune response and gut microbiota. J. Immunol. Res. 2015, 2015, 361604. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chen, H.L.; Fan, H.C.; Tung, Y.T.; Kuo, C.W.; Tu, M.Y.; Chen, C.M. Anti-inflammatory, antioxidant, and antifibrotic effects of kefir peptides on salt-induced renal vascular damage and dysfunction in aged stroke-prone spontaneously hypertensive rats. Antioxidants 2020, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Sirirat, D.; Jelena, P. Bacterial inhibition and antioxidant activity of kefir produced from thai jasmine rice milk. Biotechnology 2010, 9, 332–337. [Google Scholar] [CrossRef]
- de Lima, M.D.S.F.; da Silva, R.A.; da Silva, M.F.; da Silva, P.A.B.; Costa, R.M.P.B.; Teixeira, J.A.C.; Porto, A.L.F.; Cavalcanti, M.T.H. Brazilian kefir-fermented sheep’s milk, a source of antimicrobial and antioxidant peptides. Probiotics Antimicrob. Proteins 2018, 10, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Alakomi, H.L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef]
- Wang, L.H.; Wang, M.S.; Zeng, X.A.; Xu, X.M.; Brennan, C.S. Membrane and genomic DNA dual-targeting of citrus flavonoid naringenin against Staphylococcus aureus. Integr. Biol. 2017, 9, 820–829. [Google Scholar] [CrossRef] [PubMed]



| Variable | Parameter (%w/v) | Ranges and Level | ||||
|---|---|---|---|---|---|---|
| −1.41 | −1 | 0 | 1 | 1.41 | ||
| X1 | Honey | 1.7157 | 10 | 30 | 50 | 58.28 |
| X2 | Lemon juice | 0.171 | 1 | 3 | 5 | 5.82 |
| Parameter | Control | GTWK | ||||
|---|---|---|---|---|---|---|
| Fermentation Time (h) | 0 | 24 | 48 | 0 | 24 | 48 |
| LAB count (logUFC/mL) | 6.32 ± 0.12 | 6.5 ± 0.14 | 6.7 ± 0.32 a | 6.35 ± 0.24 | 7.38 ± 0.18 | 7.66 ± 0.32 b |
| Yeasts count (log UFC/mL) | 6.45 ± 0.11 | 6.83 ± 0.22 | 7.04±0.08 a | 6.28±0.18 | 6.33±0.25 | 6.87±0.17 b |
| pH | 6.312 ± 0.02 | 5.21 ± 0.15 | 4.25 ± 0.06 a | 6.2 ± 0.24 | 5.32 ± 0.12 | 4.66 ± 0.04 b |
| Titrable acidity (%) | 1.03 ± 0.44 | 1.27 ± 0.19 | 1.46 ± 0.11 a | 1.05 ± 0.76 | 1.11 ± 0.01 | 1.25 ± 0.22 b |
| Total phenolic content (mg eq GAE/mL) | 40.21 ± 0.39 | 41.15 ± 0.01 | 41.97 ± 0.05 a | 42.35 ± 0.12 | 43.21 ± 0.1 | 43.92 ± 0.03 b |
| % DPPH· scavenging activity | 88.713 ± 0.07 | 89.012 ± 0.61 | 90.065 ± 0.02 a | 90.52 ± 0.76 | 93.33 ± 0.14 | 94.27 ± 0.32 b |
| % ABTS·+ scavenging activity | 49.19 ± 014 | 52.11 ± 0.23 | 55.84 ± 0.05 a | 58.5 ± 0.12 | 63.02 ± 0.62 | 69.2 ± 0.4 b |
| % Iron-chelating activity | 45.022 ± 0.19 | 46.08 ± 0.03 | 47.73 ± 0.14 a | 47.92 ± 0.15 | 51.16 ± 0.43 | 56.94 ± 0.66 b |
| Run | X1 (%w/v Honey) | X2 (%w/v Lemon Juice) | Y1 | Y2 | Y3 | Y4 | Y5 | Y6 |
|---|---|---|---|---|---|---|---|---|
| 1 | 50 | 1 | 7.24 ± 0.12 | 7.11 ± 0.03 | 4.52 ± 0.11 | 37.2 ± 0.14 | 94.58 ± 0.11 | 6.74 ± 0.25 |
| 2 | 1.717 | 3 | 3.12 ± 0.07 | 4.24 ± 0.13 | 4.51 ± 0.06 | 23.31 ± 0.25 | 88.9 ± 0.065 | 3.21 ± 0.13 |
| 3 | 58.284 | 3 | 5.05 ± 0.04 | 6.86 ± 0.15 | 4.06 ± 0.09 | 45.56 ± 0.44 | 94.22 ± 0.09 | 5.56 ± 0.07 |
| 4 | 50 | 5 | 3.2 ± 0.16 | 5.98 ± 0.03 | 3.31 ± 0.10 | 47.7 ± 0.22 | 95.03 ± 0.1 | 5.33 ± 0.14 |
| 5 | 10 | 5 | 5.02 ± 0.02 | 5.01 ± 0.01 | 4.13 ± 0.22 | 38.18 ± 0.05 | 91.17 ± 0.22 | 4.69 ± 0.11 |
| 6 | 30 | 5.82 | 5.01 ± 0.3 | 6.23 ± 0.01 | 4.02 ± 0.09 | 43.14 ± 0.09 | 94.29 ± 0.09 | 6.15 ± 0.09 |
| 7 | 10 | 1 | 5.06 ± 0.07 | 5.28 ± 0.01 | 4.7 ± 0.26 | 30.05 ± 0.06 | 89.33 ± 0.26 | 4.46 ± 0.19 |
| 8 | 30 | 3 | 5.92 ± 0.09 | 6.48 ± 0.02 | 4.62 ± 0.17 | 41.68 ± 0.3 | 94.51 ± 0.17 | 6.69 ± 0.07 |
| 9 | 30 | 3 | 6.08 ± 0.07 | 6.59 ± 0.010 | 4.67 ± 0.15 | 43.17 ± 0.15 | 94.9 ± 0.15 | 7.42 ± 0.19 |
| 10 | 30 | 3 | 5.93 ± 0.02 | 6.15 ± 0.07 | 4.48 ± 0.34 | 45.02 ± 0.28 | 93.41 ± 0.34 | 7.01 ± 0.13 |
| 11 | 30 | 3 | 5.9 ± 0.18 | 6.95 ± 0.02 | 4.5 ± 0.21 | 41.73 ± 0.25 | 94.327 ± 0.21 | 6.28 ± 0.21 |
| 12 | 30 | 0.1715 | 7.36 ± 0.07 | 6.90 ± 0.01 | 4.55 ± 0.19 | 29.3 ± 0.29 | 92.12 ± 0.19 | 8.31 ± 0.35 |
| 13 | 30 | 3 | 6.05 ± 0.01 | 6.74 ± 0.004 | 4.6 ± 0.24 | 40.89 ± 0.36 | 93.89 ± 0.24 | 6.56 ± 0.47 |
| Source Title | Y1 | Y2 | Y3 | Y4 | Y5 | Y6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coeff | Signif. | Coeffi | Signif | Coeffi | Signif | Coeffi | Signif | Coeff | Signif | Coeff | Signif | |
| Model | ||||||||||||
| a0 | 6.393 | *** | 6.586 | *** | 4.574 | *** | 42.5 | *** | 94.207 | *** | 7.58 | *** |
| Linear | ||||||||||||
| a1 | 0.449 | 0.027 | 0.808 | *** | −0.132 | 0.005 | 6.017 | *** | 2.08 | *** | 0.807 | 0.005 |
| a2 | −0.789 | 0.002 | −0.294 | 0.023 | −0.2437 | *** | 4.775 | 0.002 | 0.668 | 0.007 | −0.574 | 0.023 |
| Quadratic | ||||||||||||
| a11 | −1.022 | 0.001 | −0.562 | 0.001 | −0.1383 | 0.005 | −3.29 | 0.018 | −1.287 | *** | −1.371 | *** |
| a22 | −0.177 | 0.342 | −0.064 | 0.575 | −0.1383 | 0.005 | −2.4 | 0.06 | −0.464 | 0.045 | 0.054 | 0.807 |
| Interaction | ||||||||||||
| a12 | −0.906 | 0.005 | −0.215 | 0.176 | −0.015 | 0.753 | 0.59 | 0.687 | −0.349 | 0.207 | −0.45 | 0.152 |
| R2 | 92.14% | 93.50% | 93.54% | 91.20% | 95.65% | 90.98% | ||||||
| Adj-R2 | 86.53% | 88.90% | 88.92% | 84.92% | 94.26% | 84.55% | ||||||
| Source | Sum of Squares | Degree of Freedom | Mean of Squares | F-Value | p-Value |
|---|---|---|---|---|---|
| Y1 (log CFU/mL) | |||||
| Model | 17.1504 | 5 | 3.4301 | 16.42 | 0.001 |
| Linear | 6.5901 | 2 | 3.295 | 15.77 | 0.003 |
| lack of fit | 1.1864 | 3 | 0.3954 | 5.73 | 0.063 |
| Pure error | 0.2762 | 4 | 0.069 | ||
| Total | 18.6131 | 12 | |||
| Y2 (log CFU/mL) | |||||
| Model | 8.3042 | 5 | 1.6608 | 20.25 | *** |
| Linear | 5.9199 | 2 | 2.9599 | 36.09 | *** |
| Lack of fit | 0.2121 | 3 | 0.0701 | 0.78 | 0.563 |
| Pure error | 0.3619 | 4 | 0.0907 | ||
| Total | 8.8783 | 12 | |||
| Y3 | |||||
| Model | 0.8507 | 5 | 0.1701 | 20.26 | *** |
| Linear | 0.6145 | 2 | 0.3072 | 36.59 | *** |
| Lack of fit | 0.0324 | 3 | 0.0108 | 1.64 | 0.314 |
| Pure error | 0.0263 | 4 | |||
| Total | 0.9095 | 12 | |||
| Y4 (mg GAE/mL) | |||||
| Model | 576.3831 | 5 | 115.27 | 14.51 | 0.001 |
| Linear | 472.0681 | 2 | 236.03 | 29.72 | *** |
| Lack of fit | 44.9411 | 3 | 14.981 | 5.62 | 0.064 |
| Pure error | 10.6572 | 4 | 2.664 | ||
| Total | 631.9811 | 12 | |||
| Y5 | |||||
| Model | 50.8092 | 5 | 10.162 | 40.39 | *** |
| Linear | 38.1832 | 2 | 19.092 | 75.88 | *** |
| Lack of fit | 0.4389 | 3 | 0.1463 | 0.44 | 0.735 |
| Pure error | 1.3222 | 4 | 0.3305 | ||
| Total | 52.5703 | 12 | |||
| Y6 | |||||
| Model | 20.5792 | 5 | 4.115 | 16.63 | 0.001 |
| Linear | 7.1141 | 2 | 3.557 | 14.37 | 0.003 |
| Lack of fit | 0.9647 | 3 | 0.3216 | 1.67 | 0.308 |
| Pure error | 0.7683 | 4 | 0.1921 | ||
| Total | 22.312 | 12 | |||
| Formulation | Mouthfeel | Color | Aroma | Sweetness | Acidity | Overall Acceptability |
|---|---|---|---|---|---|---|
| F1 | 7 ± 0.02 a | 6 ± 0.12 a | 7.5 ± 0.2 a | 8 ± 0.06 a | 6 ± 0.09 a | 6.74 ± 0.25 a |
| F2 | 7 ± 0.11 a | 7.5 ± 0.14 b | 5 ± 0.03 b | 4 ± 0.042 b | 3 ± 0.02 b | 3.21 ± 0.13 b |
| F3 | 7 ± 0.03 a | 8 ± 0.02 c | 8.5 ± 0.14 c | 7.5 ± 0.05 c | 4.9 ± 0.13 c | 5.56 ± 0.07 c |
| F4 | 7 ± 0.061 a | 7 ± 0.15 d | 8 ± 0.01 d | 5 ± 0.11 d | 2.5 ± 0.11 d | 5.33 ± 0.14 d |
| F5 | 7 ± 0.015 a | 7 ± 0.01 d | 6 ± 0.11 e | 5.5 ± 0.13 e | 2.63 ± 0.04 e | 4.69 ± 0.11 e |
| F6 | 7 ± 0.17 a | 6.5 ± 0.04 e | 7.5 ± 0.09 a | 6.5 ± 0.02 f | 6.7 ± 0.08 f | 6.15 ± 0.09 f |
| F7 | 7 ± 0.08 a | 7 ± 0.71 d | 6 ± 0.01 e | 4 ± 0.15 b | 3 ± 0.02 b | 4.46 ± 0.19 g |
| F8 | 7 ± 0.01 a | 7 ± 0.22 d | 6.5 ± 0.013 f | 7 ± 0.08 g | 5 ± 0.14 c | 6.69 ± 0.07 a |
| F9 | 7 ± 0.22 a | 7 ± 0.16 d | 6.5 ± 0.02 f | 7 ± 0.034 g | 6 ± 0.05 a | 7.42 ± 0.19 h |
| F10 | 7 ± 0.31 a | 7 ± 0.02 d | 6 ± 0.11 e | 7 ± 0.01 g | 5.0 ± 0.03 c | 7.01 ± 0.13 i |
| F11 | 7 ± 0.05 a | 7 ± 0.01 d | 6 ± 0.02 e | 7 ± 0.05 g | 3.9 ± 0.01 j | 6.28 ± 0.21 j |
| F12 | 7 ± 0.01 a | 6 ± 0.36 a | 7 ± 0.19 a | 6.5 ± 0.11 f | 7 ± 0.02 h | 8.31 ± 0.35 k |
| F13 | 7 ± 0.013 a | 6.5 ± 0.01 e | 6.5 ± 0.01 f | 7 ± 0.07 g | 4 ± 0.015 j | 6.56 ± 0.47 a |
| Response Variables | Experimental Value | Predicted Value |
|---|---|---|
| LAB count (log CFU/mL) | 7.61 ± 0.11 | 7.147 |
| Yeasts count (log CFU/mL) | 7.35 ± 0.07 | 7.114 |
| TPC (mg GAE/mL) | 40.17 ± 0.08 | 43.93 |
| % DPPH· scavenging activity | 95.81 ± 0.32 | 93.56 |
| OA score | 7.29 ± 0.06 | 7.319 |
| Fermentation Time (h) | % ABTS·+ Scavenging Activity | % Iron-Chelating Activity | Reducing Power (μmol HCL-Cystein/L) |
|---|---|---|---|
| 0 | 65.01 ± 0.02 | 58.03 ± 0.03 | 259.99 ± 02.25 |
| 24 | 67.34 ± 0.03 | 60.21 ± 0.04 | 311.87 ± 3.22 |
| 48 | 73.02 ± 0.11 | 61.51 ± 0.04 | 326.87 ± 9.62 |
| p-value | <0.001 | <0.001 | <0.001 |
| Microorganism | Crude Sample of GTWK (Inhibition Halos in mm) | Neutralized Sample of GTWK (Inhibition Halos in mm) | Chloramphenicol (Inhibition Halos in mm) |
|---|---|---|---|
| Echerichia coli ATCC 11229 | 14 ± 0.013 a | 13 ± 0.021 a | 14 ± 0.047 a |
| Staphylococcus aureus subsp. aureus ATCC 6538 | 13 ± 0.004 b | 13 ± 0.003 b | 13 ± 0.054 b |
| Shigella sonnei ATCC 25931 | n.d. | n.d. | 11 ± 0.013 |
| Salmonella thyphimirium ATCC 14028 | 12 ± 0.06 a | n.d. | 16 ± 0.019 b |
| Pseudomonas aeruginosa ATCC 27853 | n.d. | n.d. | 11 ± 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdi, A.; Gatri, E.; Filannino, P.; M’Hir, S.; Ayed, L. Formulation Design and Functional Characterization of a Novel Fermented Beverage with Antioxidant, Anti-Inflammatory and Antibacterial Properties. Beverages 2025, 11, 27. https://doi.org/10.3390/beverages11010027
Abdi A, Gatri E, Filannino P, M’Hir S, Ayed L. Formulation Design and Functional Characterization of a Novel Fermented Beverage with Antioxidant, Anti-Inflammatory and Antibacterial Properties. Beverages. 2025; 11(1):27. https://doi.org/10.3390/beverages11010027
Chicago/Turabian StyleAbdi, Ameni, Emna Gatri, Pasquale Filannino, Sana M’Hir, and Lamia Ayed. 2025. "Formulation Design and Functional Characterization of a Novel Fermented Beverage with Antioxidant, Anti-Inflammatory and Antibacterial Properties" Beverages 11, no. 1: 27. https://doi.org/10.3390/beverages11010027
APA StyleAbdi, A., Gatri, E., Filannino, P., M’Hir, S., & Ayed, L. (2025). Formulation Design and Functional Characterization of a Novel Fermented Beverage with Antioxidant, Anti-Inflammatory and Antibacterial Properties. Beverages, 11(1), 27. https://doi.org/10.3390/beverages11010027







