Preparation of Polyphenol-Rich Herbal Beverages from White Willow (Salix alba) Bark with Potential Alzheimer’s Disease Inhibitory Activity In Silico
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Preparation of Willow Infusions
2.4. Determination of the Total Phenolic Content
2.5. Separation and Quantification of Phenolic Constituents Content
2.6. Determination of Antioxidant Activity
2.6.1. DPPH Assay
2.6.2. FRAP Assay
2.7. HPLC Analysis
2.8. Color Measurement
2.9. Molecular Docking
2.10. Statistical Analysis
3. Results and Discussion
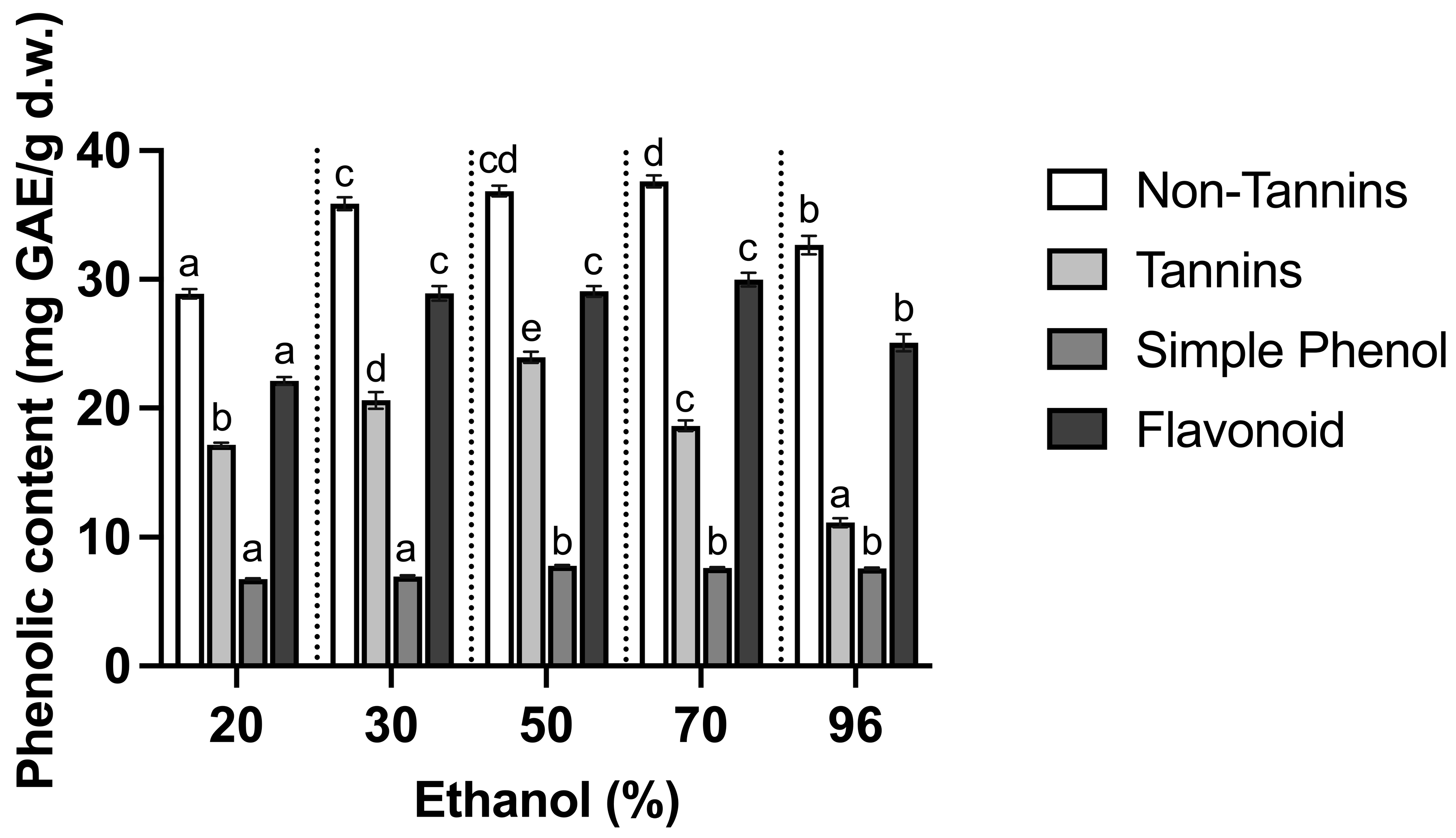
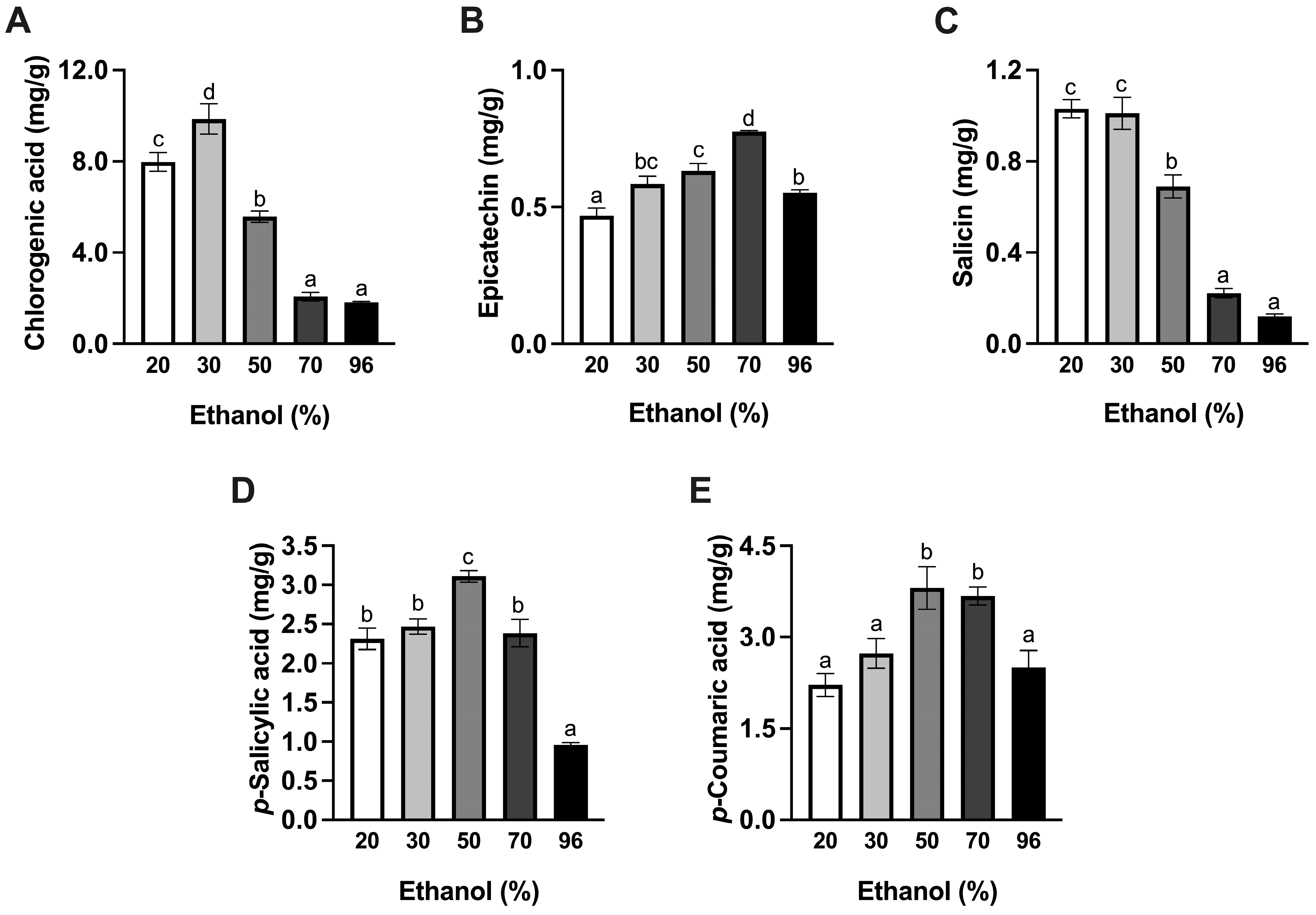
3.1. Effect of Ethanol
3.2. Effect of Temperature
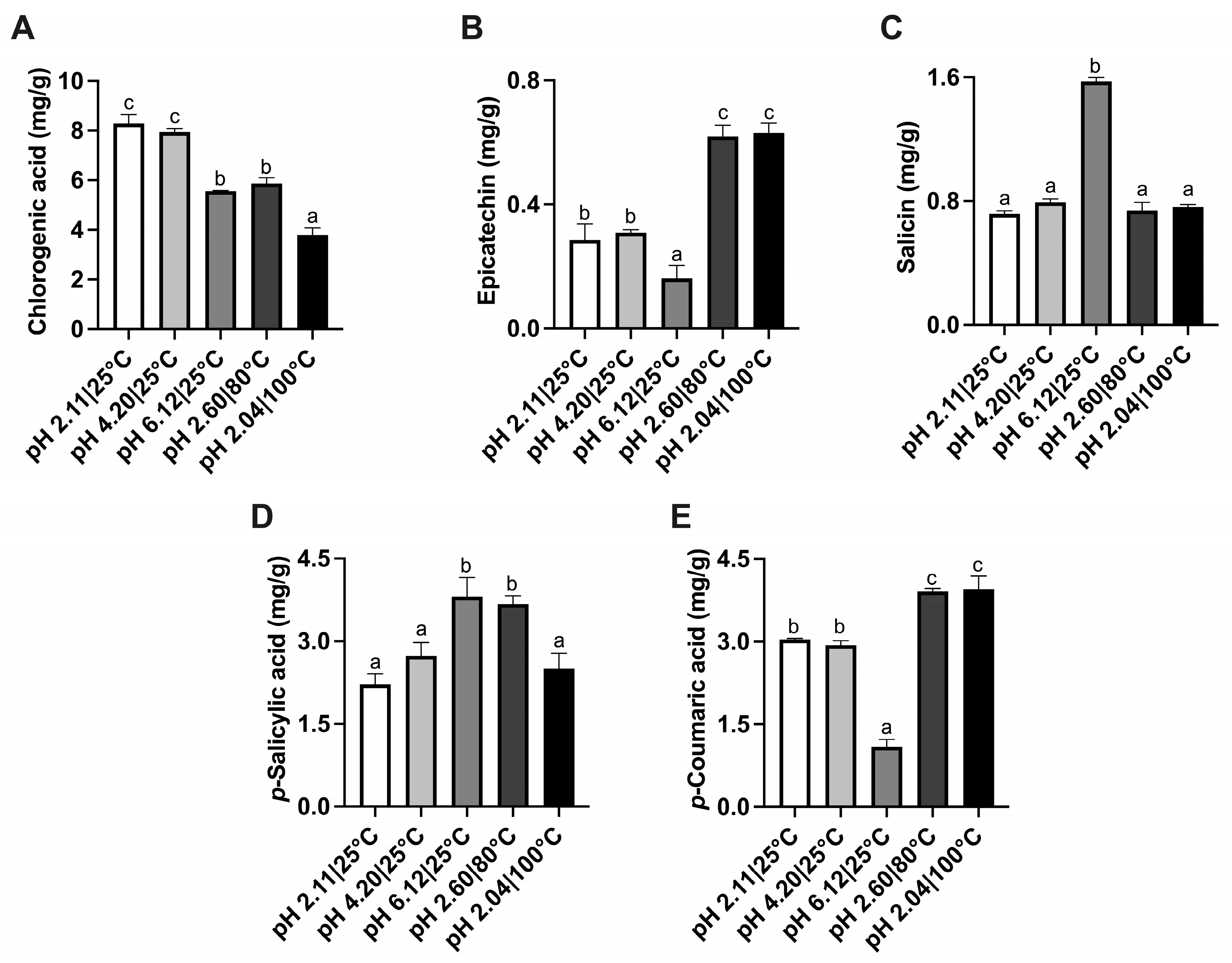
3.3. Effect of pH
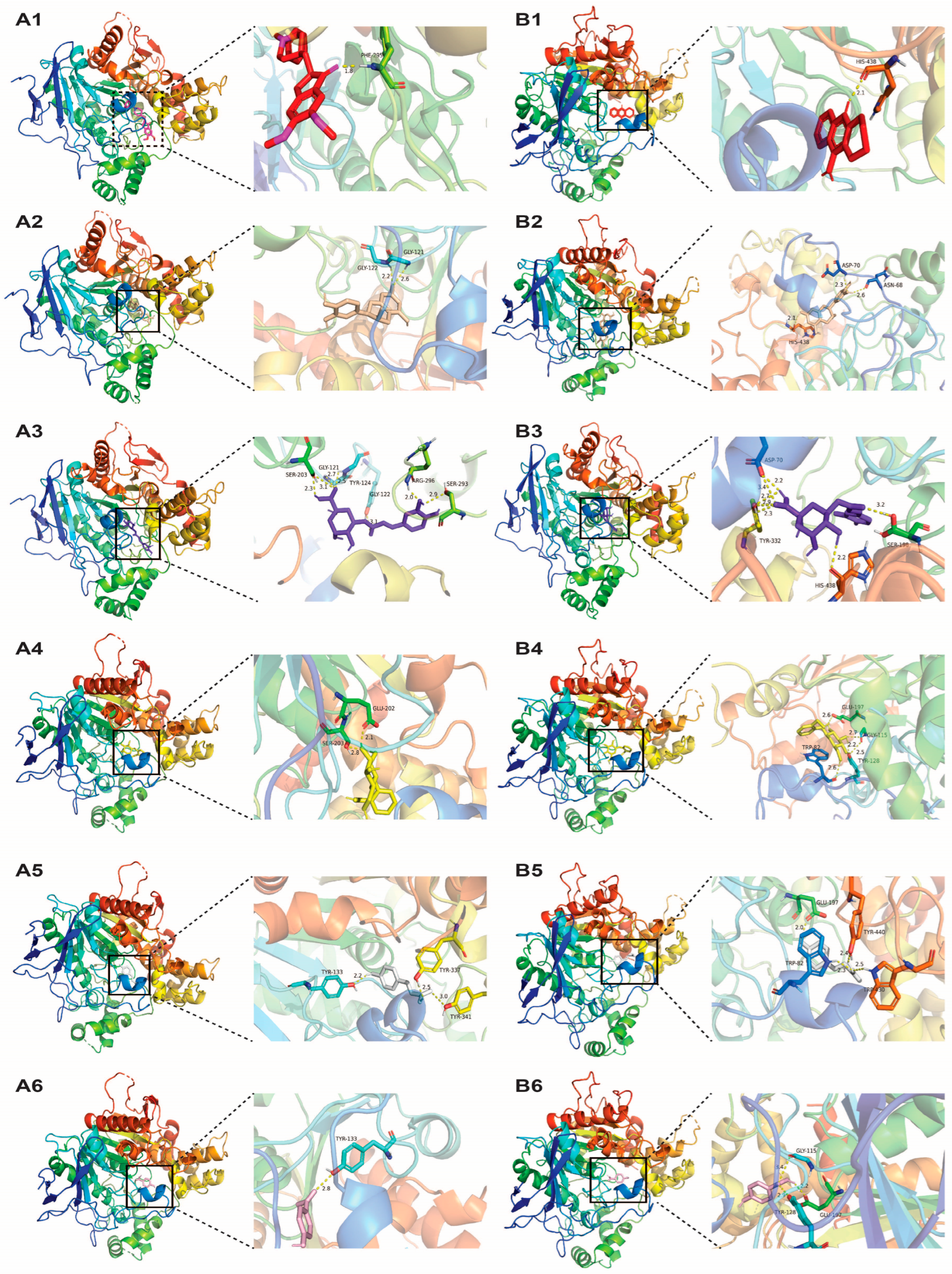
3.4. Neuroprotective Potentials of White Willow Polyphenols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, X.; Huang, Y.; Zhang, Z.; Zhong, S.; Xie, G.; Wang, L.; Xiao, H. Factors associated with health-related quality of life among family caregivers of people with Alzheimer’s disease. Psychogeriatrics 2020, 20, 398–405. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, D.-D.; Huang, S.-Y.; Fang, A.-P.; Li, H.-B.; Zhu, H.-L. Effects and mechanisms of natural products on Alzheimer’s disease. Crit. Rev. Food Sci. Nutr. 2023, 63, 3168–3188. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Macdonald, I.R.; Martin, E.; Rosenberry, T.L.; Darvesh, S. Probing the peripheral site of human butyrylcholinesterase. Biochemistry 2012, 51, 7046–7053. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Kaufer, D.I.; Hendrickson, R.; Ivanco, L.S.; Lopresti, B.; Davis, J.G.; Constantine, G.; Mathis, C.A.; Moore, R.Y.; DeKosky, S.T. Cognitive correlates of alterations in acetylcholinesterase in Alzheimer’s disease. Neurosci. Lett. 2005, 380, 127–132. [Google Scholar] [CrossRef]
- Nordberg, A.; Svensson, A.L. Cholinesterase inhibitors in the treatment of Alzheimer’s disease: A comparison of tolerability and pharmacology. Drug Saf. 1998, 19, 465–480. [Google Scholar] [CrossRef]
- Ohbe, H.; Jo, T.; Matsui, H.; Fushimi, K.; Yasunaga, H. Cholinergic Crisis Caused by Cholinesterase Inhibitors: A Retrospective Nationwide Database Study. J. Med. Toxicol. 2018, 14, 237–241. [Google Scholar] [CrossRef]
- Deshpande, P.; Gogia, N.; Singh, A. Exploring the efficacy of natural products in alleviating Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1321–1329. [Google Scholar]
- Aleman, R.S.; Marcia, J.; Duque-Soto, C.; Lozano-Sánchez, J.; Montero-Fernández, I.; Ruano, J.A.; Hoskin, R.T.; Moncada, M. Effect of Microwave and Ultrasound-Assisted Extraction on the Phytochemical and In Vitro Biological Properties of Willow (Salix alba) Bark Aqueous and Ethanolic Extracts. Plants 2023, 12, 2533. [Google Scholar] [CrossRef]
- Zaiter, A.; Becker, L.; Petit, J.; Zimmer, D.; Karam, M.C.; Baudelaire, E.; Scher, J.; Dicko, A. Antioxidant and antiacetylcholinesterase activities of different granulometric classes of Salix alba (L.) bark powders. Powder Technol. 2016, 301, 649–656. [Google Scholar] [CrossRef]
- Gligorić, E.; Igić, R.; Srđenović Čonić, B.; Kladar, N.; Teofilović, B.; Grujić, N. Chemical profiling and biological activities of “green” extracts of willow species (Salix L., Salicaceae): Experimental and chemometric approaches. Sustain. Chem. Pharm. 2023, 32, 100981. [Google Scholar] [CrossRef]
- Piątczak, E.; Dybowska, M.; Płuciennik, E.; Kośla, K.; Kolniak-Ostek, J.; Kalinowska-Lis, U. Identification and accumulation of phenolic compounds in the leaves and bark of Salix alba (L.) and their biological potential. Biomolecules 2020, 10, 1391. [Google Scholar] [CrossRef]
- Maleš, I.; Pedisić, S.; Zorić, Z.; Elez-Garofulić, I.; Repajić, M.; You, L.; Vladimir-Knežević, S.; Butorac, D.; Dragović-Uzelac, V. The medicinal and aromatic plants as ingredients in functional beverage production. J. Funct. Foods 2022, 96, 105210. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Van Vuuren, S.F.; Motlhatlego, K.E.; Netshia, V. Traditionally used polyherbals in a southern African therapeutic context. J. Ethnopharmacol. 2022, 288, 114977. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Chapter 15—Extraction of Polyphenols From Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 243–259. [Google Scholar]
- Vuong, Q.V.; Golding, J.B.; Stathopoulos, C.E.; Nguyen, M.H.; Roach, P.D. Optimizing conditions for the extraction of catechins from green tea using hot water. J. Sep. Sci. 2011, 34, 3099–3106. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Hussien, N.N.; Marzoog, T.R.; Awad, H.A. Phenolic content, antioxidant, antimicrobial and cytotoxic activities of ethanolic extract of Salix alba. Am. J. Biochem. Biotechnol. 2013, 9, 41–46. [Google Scholar] [CrossRef]
- Gligorić, E.; Igić, R.; Suvajdžić, L.; Grujić-Letić, N. Species of the Genus Salix L.: Biochemical Screening and Molecular Docking Approach to Potential Acetylcholinesterase Inhibitors. Appl. Sci. 2019, 9, 1842. [Google Scholar] [CrossRef]
- Paterson, J.R.; Lawrence, J.R. Salicylic acid: A link between aspirin, diet and the prevention of colorectal cancer. QJM Int. J. Med. 2001, 94, 445–448. [Google Scholar] [CrossRef]
- Shen, Y.; Song, X.; Li, L.; Sun, J.; Jaiswal, Y.; Huang, J.; Liu, C.; Yang, W.; Williams, L.; Zhang, H.; et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 2019, 111, 579–587. [Google Scholar] [CrossRef]
- Gligorić, E.; Igić, R.; Teofilović, B.; Grujić-Letić, N. Phytochemical Screening of Ultrasonic Extracts of Salix Species and Molecular Docking Study of Salix-Derived Bioactive Compounds Targeting Pro-Inflammatory Cytokines. Int. J. Mol. Sci. 2023, 24, 11848. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Harbourne, N.; Marete, E.; Jacquier, J.C.; O’Riordan, D. Effect of drying methods on the phenolic constituents of meadowsweet (Filipendula ulmaria) and willow (Salix alba). LWT—Food Sci. Technol. 2009, 42, 1468–1473. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of Objective Color Measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.; Priyadarsini, K.I.; Mohan, H. Evaluating the antioxidant activity of different plant extracts and herbal formulations. Res. Chem. Intermed. 2005, 31, 145–151. [Google Scholar] [CrossRef]
- Duh, P.-D.; Yen, G.-C. Antioxidative activity of three herbal water extracts. Food Chem. 1997, 60, 639–645. [Google Scholar] [CrossRef]
- Sukweenadhi, J.; Setiawan, F.; Yunita, O.; Kartini, K.; Avanti, C. Antioxidant activity screening of seven Indonesian herbal extract. Biodiversitas 2020, 21, 2062–2067. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Das, P.R.; Eun, J.B. A comparative study of ultra-sonication and agitation extraction techniques on bioactive metabolites of green tea extract. Food Chem. 2018, 253, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Boussetta, N.; Soichi, E.; Lanoisellé, J.L.; Vorobiev, E. Valorization of oilseed residues: Extraction of polyphenols from flaxseed hulls by pulsed electric fields. Ind. Crops. Prod. 2014, 52, 347–353. [Google Scholar] [CrossRef]
- Bosso, A.; Guaita, M.; Petrozziello, M. Influence of solvents on the composition of condensed tannins in grape pomace seed extracts. Food Chem. 2016, 207, 162–169. [Google Scholar] [CrossRef]
- Köhler, A.; Förster, N.; Zander, M.; Ulrichs, C. Inter- and intraspecific diversity of Salix bark phenolic profiles—A resource for the pharmaceutical industry. Fitoterapia 2023, 170, 105660. [Google Scholar] [CrossRef] [PubMed]
- Förster, N.; Antoniadou, K.; Zander, M.; Baur, S.; Mittermeier-Kleßinger, V.K.; Dawid, C.; Ulrichs, C.; Mewis, I. Chemoprofiling as Breeding Tool for Pharmaceutical Use of Salix. Front. Plant Sci. 2021, 12, 579820. [Google Scholar] [CrossRef]
- Lohtander, T.; Arola, S.; Laaksonen, P. Biomordanting willow bark dye on cellulosic materials. Color. Technol. 2020, 136, 3–14. [Google Scholar] [CrossRef]
- Rajha, H.N.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. A comparative study of physical pretreatments for the extraction of polyphenols and proteins from vine shoots. Food Res. Int. 2014, 65, 462–468. [Google Scholar] [CrossRef]
- Chew, K.; Khoo, M.; Ng, S.; Thoo, Y.Y.; Aida, W.W.; Ho, C.W. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. Int. Food Res. J. 2011, 18, 1427. [Google Scholar]
- Dorta, E.; Lobo, M.G.; Gonzalez, M. Reutilization of mango byproducts: Study of the effect of extraction solvent and temperature on their antioxidant properties. J. Food Sci. 2012, 77, C80–C88. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Kemppainen, K.; Siika-aho, M.; Pattathil, S.; Giovando, S.; Kruus, K. Spruce bark as an industrial source of condensed tannins and non-cellulosic sugars. Ind. Crops. Prod. 2014, 52, 158–168. [Google Scholar] [CrossRef]
- Harbourne, N.; Jacquier, J.C.; O’Riordan, D. Optimisation of the aqueous extraction conditions of phenols from meadowsweet (Filipendula ulmaria L.) for incorporation into beverages. Food Chem. 2009, 116, 722–727. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, Y. Effect of pH on cream particle formation and solids extraction yield of black tea. Food Chem. 2001, 74, 155–160. [Google Scholar] [CrossRef]
- Adje, F.; Lozano, Y.F.; Lozano, P.; Adima, A.; Chemat, F.; Gaydou, E.M. Optimization of anthocyanin, flavonol and phenolic acid extractions from Delonix regia tree flowers using ultrasound-assisted water extraction. Ind. Crops. Prod. 2010, 32, 439–444. [Google Scholar] [CrossRef]
- Rusak, G.; Komes, D.; Likić, S.; Horžić, D.; Kovač, M. Phenolic content and antioxidative capacity of green and white tea extracts depending on extraction conditions and the solvent used. Food Chem. 2008, 110, 852–858. [Google Scholar] [CrossRef]
- Zimmermann, B.F.; Gleichenhagen, M. The effect of ascorbic acid, citric acid and low pH on the extraction of green tea: How to get most out of it. Food Chem. 2011, 124, 1543–1548. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. An Overview of the Phenolics of Canola and Rapeseed—Chemical, Sensory and Nutritional Significance. J. Am. Oil Chem. Soc. 1992, 69, 917–924. [Google Scholar] [CrossRef]
- Sealy-Fisher, V.J.; Pizzi, A. Increased pine tannins extraction and wood adhesives development by phlobaphenes minimization. Holz Roh Werkst. 1992, 50, 212–220. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Typek, R. The influence of pH on the thermal stability of 5-O-caffeoylquinic acids in aqueous solutions. Eur. Food Res. Technol. 2011, 233, 223–232. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the Stability of Plant Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Nekhoroshev, S.V.; Nekhorosheva, A.V.; Sabutova, A.B.; Botirov, E.K.; Drenin, A.A.; Slepchenko, G.B.; Gornikov, N.V. Hydrolytic Stability of Aqueous Solutions of Salicin. Pharm. Chem. J. 2020, 54, 857–860. [Google Scholar] [CrossRef]
- Cheung, J.; Gary, E.N.; Shiomi, K.; Rosenberry, T.L. Structures of human acetylcholinesterase bound to dihydrotanshinone I and territrem B show peripheral site flexibility. ACS Med. Chem. Lett. 2013, 4, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, M.K.; Choudhury, S. Tea polyphenols as multi-target therapeutics for Alzheimer’s disease: An in silico study. Med. Hypotheses 2019, 125, 94–99. [Google Scholar] [CrossRef]



| Ethanol (%) | TPC (mg GAE/g d.w.) | DPPH (mg AAE/g d.w.) | FRAP (mmol TE/g d.w.) | Color | ||
|---|---|---|---|---|---|---|
| Lightness (L*) | Chroma (C*) | Hue Angle (H°) | ||||
| 20 | 44.25 ± 0.50 a | 75.78 ± 1.07 b | 284.05 ± 4.76 a | 18.03 ± 0.09 b | 16.49 ± 0.17 b | 52.73 ± 0.21 c |
| 30 | 54.53 ± 0.64 b | 85.94 ± 0.64 c | 334.59 ± 0.76 b | 18.00 ± 0.22 b | 17.75 ± 0.36 c | 48.50 ± 1.06 b |
| 50 | 61.06 ± 0.43 c | 103.45 ± 0.77 d | 402.49 ± 4.27 c | 16.76 ± 0.21 a | 15.29 ± 0.14 a | 43.89 ± 1.66 a |
| 70 | 54.48 ± 1.26 b | 85.76 ± 0.20 c | 341.62 ± 1.79 b | 19.27 ± 0.19 c | 19.08 ± 0.22 d | 53.64 ± 0.64 c |
| 96 | 44.71 ± 0.28 a | 73.07 ± 0.82 a | 278.32 ± 1.88 a | 23.97 ± 0.39 d | 21.03 ± 0.26 e | 74.99 ± 0.88 d |
| Temperature (°C) | TPC (mg GAE/g d.w.) | DPPH (mg AAE/g d.w.) | FRAP (mmol TE/g d.w.) | Color | ||
|---|---|---|---|---|---|---|
| Lightness (L*) | Chroma (C*) | Hue Angle (H°) | ||||
| 25 | 38.35 ± 0.37 a | 57.01 ± 0.74 a | 229.63 ± 1.27 a | 20.75 ± 0.08 d | 17.64 ± 0.57 a | 65.51 ± 0.62 d |
| 40 | 41.99 ± 0.51 b | 63.00 ± 0.54 b | 233.97 ± 2.55 a | 18.50 ± 0.46 c | 14.80 ± 1.78 a | 58.19 ± 1.06 c |
| 60 | 44.13 ± 0.52 c | 67.96 ± 1.55 c | 247.59 ± 2.93 b | 16.74 ± 0.20 b | 14.56 ± 0.04 a | 50.35 ± 1.29 b |
| 80 | 46.53 ± 0.20 d | 72.89 ± 0.59 d | 246.68 ± 1.93 b | 15.58 ± 0.16 a | 13.85 ± 0.21 b | 43.97 ± 0.17 a |
| pH | Temperature (°C) | TPC (mg GAE/g d.w.) | DPPH (mg AAE/g d.w.) | FRAP (mmol TE/g d.w.) | Color | ||
|---|---|---|---|---|---|---|---|
| Lightness (L*) | Chroma (C*) | Hue Angle (H°) | |||||
| 2.11 | 25 | 41.21 ± 0.18 b | 61.92 ± 1.83 b | 232.25 ± 0.86 a | 27.34 ± 0.19 d | 23.95 ± 0.26 d | 80.70 ± 1.05 d |
| 4.20 | 25 | 40.26 ± 0.64 b | 58.18 ± 2.13 b | 231.68 ± 2.04 a | 24.40 ± 0.34 c | 23.80 ± 0.26 d | 73.05 ± 0.74 c |
| 6.12 | 25 | 38.01 ± 0.59 a | 52.38 ± 2.95 a | 229.11 ± 1.27 a | 18.41 ± 0.26 a | 19.17 ± 0.51 a | 52.19 ± 0.54 a |
| 2.60 | 80 | 46.70 ± 0.58 c | 72.25 ± 0.75 c | 245.79 ± 1.57 b | 24.01 ± 0.34 c | 21.23 ± 0.72 c | 59.87 ± 0.26 b |
| 2.04 | 100 | 48.02 ± 1.26 c | 76.43 ± 1.39 c | 257.11 ± 1.86 c | 21.59 ± 0.68 b | 18.10 ± 0.84 b | 51.95 ± 1.63 a |
| Receptor (PDB ID) | Ligand | Binding Energy (kcal/mol) | Hydrogen Bond | Hydrophobic Interaction | Interaction | |
|---|---|---|---|---|---|---|
| Amino Acid | Distance (Å) | |||||
| AChE (4EY7) | Donepezil | −12.3 | Phe-295 | 1.79 | Trp-86, Trp-286, Tyr-337, Phe-338, Tyr-341 | Trp-86, Tyr-341, Trp-286 |
| Epicatechin | −10 | Gly-121 | 2.60 | Tyr-124, Trp-286, Phe-297, Tyr-341 | Tyr-341 | |
| Gly-122 | 2.19 | |||||
| Chlorogenic acid | −9.8 | Gly-121 | 2.72 | Trp-286, Phe-338, Tyr-341 | His-447 | |
| Gly-122 | 3.10 | |||||
| Ser-203 | 2.31, 3.12 | |||||
| Ser-293 | 2.93 | |||||
| Arg-296 | 1.99 | |||||
| Tyr-124 | 2.52 | |||||
| Salicin | −8.3 | Glu-202 | 2.09 | Phe-338, Tyr-341 | Tyr-337 | |
| Ser-203 | 2.80 | |||||
| p-coumaric acid | −7.4 | Tyr-133 | 2.21 | Trp-86 | Trp-86 | |
| Tyr-341 | 3.01 | |||||
| Tyr-337 | 2.53 | |||||
| p-salicylic acid | −6.5 | Tyr-133 | 2.81 | Trp-86, Tyr-337 | Trp-86 | |
| BuChE (4BDS) | Tacrine | −8.5 | His-438 | 2.11 | Trp-82, Ala-328, Tyr-332, Trp-430 | Trp-82 |
| Chlorogenic acid | −8.7 | Asp-70 | 2.23, 3.41 | Phe-329 | Trp-231 | |
| Tyr-332 | 2.71, 2.90, 2.32 | |||||
| His-438 | 2.22 | |||||
| Ser-198 | 3.19 | |||||
| Epicatechin | −8.4 | Asn-68 | 2.57 | - | Trp-82 | |
| Asp-70 | 2.33 | |||||
| His-438 | 2.11 | |||||
| Salicin | −7.4 | Trp-82 | 2.58 | Ala-328 | - | |
| Gly-115 | 2.49, 2.72 | |||||
| Tyr-128 | 2.19 | |||||
| Glu-197 | 2.61 | |||||
| p-coumaric acid | −6.9 | Trp-82 | 2.27 | Trp-82 | Trp-82 | |
| Glu-197 | 2.01 | |||||
| Trp-430 | 2.50 | |||||
| Tyr-440 | 2.44 | |||||
| p-salicylic acid | −6.1 | Tyr-128 | 2.92 | - | Trp-82 | |
| Gly-115 | 3.42 | |||||
| Glu-197 | 2.19 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Jacquier, J.-C.; Harbourne, N. Preparation of Polyphenol-Rich Herbal Beverages from White Willow (Salix alba) Bark with Potential Alzheimer’s Disease Inhibitory Activity In Silico. Beverages 2024, 10, 75. https://doi.org/10.3390/beverages10030075
Zheng L, Jacquier J-C, Harbourne N. Preparation of Polyphenol-Rich Herbal Beverages from White Willow (Salix alba) Bark with Potential Alzheimer’s Disease Inhibitory Activity In Silico. Beverages. 2024; 10(3):75. https://doi.org/10.3390/beverages10030075
Chicago/Turabian StyleZheng, Liwen, Jean-Christophe Jacquier, and Niamh Harbourne. 2024. "Preparation of Polyphenol-Rich Herbal Beverages from White Willow (Salix alba) Bark with Potential Alzheimer’s Disease Inhibitory Activity In Silico" Beverages 10, no. 3: 75. https://doi.org/10.3390/beverages10030075
APA StyleZheng, L., Jacquier, J.-C., & Harbourne, N. (2024). Preparation of Polyphenol-Rich Herbal Beverages from White Willow (Salix alba) Bark with Potential Alzheimer’s Disease Inhibitory Activity In Silico. Beverages, 10(3), 75. https://doi.org/10.3390/beverages10030075









