Abstract
Cereals have been a foundational component of human diets across different continents, with rice dominating in Asia, sorghum in Africa, wheat in Europe, and maize in America. Mexico, more accurately Mesoamerica, is recognized as the origin of maize (including pigmented maize), with its first ancestor traced back to Tehuacán, Puebla, Mexico. Pigmented maize owes its vibrant colors due to its anthocyanin (i.e., cyanidin-3-glucoside) contents, which contribute to the red, purple, or blue coloration and offer notable health benefits. The antioxidant properties of maize are crucial, given the role of oxidative stress in various diseases, and present a valuable resource for functional foods and nutraceuticals. Emerging studies underscore the prebiotic potential of anthocyanins, showing their ability to modulate gut microbiota positively. This review aims to explore the potential of pigmented maize in traditional Mexican beverage (such as pozol and tejuino) production, emphasizing the bioactive compounds (mainly anthocyanins) present and their health benefits while also considering new opportunities in the functional food industry.
1. Introduction
Cereals have historically served as significant food supplies for humanity, with rice being predominantly produced in Asia, sorghum in Africa, wheat in Europe, and maize in America. Maize is a primary food source in Latin America, the Caribbean, Asia, and Africa. This crop has a significant role in ensuring food security and fostering economic growth [1]. Mexico, along with other countries in Mesoamerica such as Belize, Colombia, Costa Rica, El Salvador, Guatemala, Honduras, Nicaragua, Panama, and the Dominican Republic, are considered the origin of maize. Studies report that the first ancestor of maize was found in Tehuacán, Puebla, Mexico [2,3]. Maize has been historically used in various applications, including food and non-food sectors. However, in modern times, its usage has expanded to include the production of bioethanol and other by-products. Consequently, there is a growing demand to enhance maize yields through the implementation of new technologies such as hybridization and genetically modified organisms (GMOs) [4]. The beneficial components found in corn have led to a reassessment of its utilization, despite its focus on serving the livestock and bioethanol sectors. Pigmented maize contains anthocyanins, colored, water-soluble pigments belonging to the phenolic group and known for their diverse health benefits. Mexico possesses the greatest diversity of native corn species (Zea mays L.), with more than 60 corn varieties classified, including pigmented maize [5]. Pigmented maize, characterized by colors such as pink, red, blue, and purple, is the result of the biochemical regulation of anthocyanins present in the husks, cobs, and silks of the maize plant [6,7]. In Mexico, maize is primarily consumed in the form of tortillas and is essential food in the diet [8]. The production of Mexican tortillas involves a thermal process called “nixtamalization”. This technique employs alkaline heat to enhance the nutritional characteristics of maize. However, in the case of pigmented corn, it unfortunately has a detrimental effect by reducing the overall phenolic compound and anthocyanin contents, thereby diminishing its antioxidant potential [9]. Pre-Hispanic corn preparations, such as “pozol and tejuino”, offer effective methods for preserving its bioactive ingredients. These traditional fermented foods from Mexico are prepared without the use of high temperatures [10], where moderate temperatures are used. Due to its remarkable flavonoid content, a specific pigmented maize, purple maize, has gained increasing interest from consumers as a natural food additive.
Emerging findings about the impact of prebiotic compounds on the gut microbiota have shown a general rise in the consumption of foods for their functional qualities; the examination of food components and their derivatives as bioactive molecules with advantageous effects on health and, particularly in this case, the inclusion of chemical anthocyanin compounds is now being evaluated as part of a holistic approach to healthcare [11]. Currently, there is a prevailing inclination to examine food components and their derivatives as bioactive molecules that possess advantageous effects on health. Pigmented maize contains dietary fibers and bioactive compounds such as anthocyanins. Both the fibers and bioactive compounds (such as anthocyanins) can be metabolized by the gut bacteria, leading to a rise in short-chain fatty acids [12,13]. These fatty acids, when utilized by other cells, have a positive effect on health. Anthocyanins, on the other hand, were previously researched solely for their antioxidant properties; nevertheless, current research has revealed that bacteria may metabolize these phenolic molecules. This is consistent with the definition of a prebiotic compound, which is selectively used as a substrate by host bacteria and provides health benefits [14]. The whole process of the modification of the natural food matrix into a functional food is described in Figure 1. This illustrates the purpose of this review, which is to create future perspectives and opportunities in the field of beverage and functional food development by integrating information on traditional pigmented corn-based beverages and understanding the significance of the bioactive components (mainly anthocyanin) present in them.
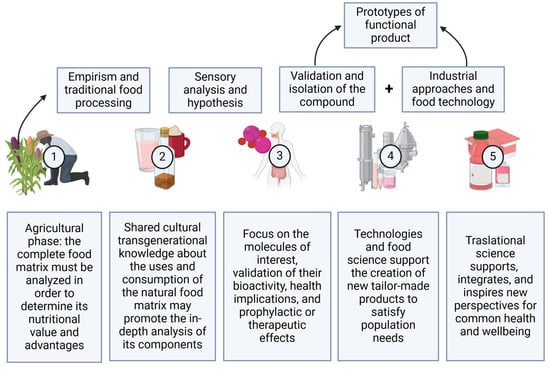
Figure 1.
Integrated creation of functional food. Created on BioRender.com (2024) (accessed on 17 July 2024).
2. Principal Antioxidant Compounds Reported in Pigmented Maize
In Mexico and all of America, one of the main cereals consumed is maize, the second most cultivated crop worldwide. Interestingly, white, and yellow maize have been the most popular for consumption and the more known [15]. However, a great number of varieties of less-known pigmented maize are produced each year, containing significant amounts of antioxidants. Specifically, they contain anthocyanins, bioactive substances responsible for the vibrant colors in vegetables. They gather in the stem, leaves, inflorescences, and in the maize cob in bracts, rachis, and kernels [16]. Anthocyanins, also called anthocyanin glycosides, are natural, water-soluble pigments from the group of flavonoids. They are present in the vacuoles of plant cells, depending on the pH, and are responsible for the red, purple, or blue color of the leaves, flowers, and fruits of blueberries, cherries, raspberries, purple cabbage, eggplant, and maize [17].
These compounds can have many natural variations according to the origin of the plant [18]. But in fact, in their basic structure, they are known as anthocyanidins (or aglycons), which contain an aromatic ring bonded to an heterocyclic ring with oxygen, which is also bonded by a carbon–carbon bond to a third aromatic ring. Aglycones have shown absorbance in the ultraviolet and visible light spectra. They exist in six types: pelargonidin, cyanidin, delphinidin, peonidin, petunidin, and malvidin [19]. Thus, anthocyanins are glycosides and acylglycosides of anthocyanidins, and their basic molecular structure is a C6-C3-C6 carbon skeleton with structures of polyhydroxy or polymethoxy derivatives of 2-phenylbenzophyryllium or flavylium [20].
In maize, the number of anthocyanins depends on the genotype, plant organ, and environmental conditions where the plant grows. Pigmented maize has a high content of anthocyanins compared to white maize, with 322.7 and 196.7 µg of anthocyanins/g of sample, respectively. Researchers also identified the presence of cyanidin 3-glucoside and cyanidin 3-succinylglucoside through high-performance liquid chromatography (HPLC) [21]. The content of monomeric anthocyanins in purple maize varied between 290 and 1333 mg per 100 g of dry matter, measured in cyanidin 3-glucoside equivalents, while the total phenolic content ranged from 950 to 3516 mg per 100 g of dry matter, measured in gallic acid equivalents. Furthermore, cyanidin-3-glucoside, pelargonidin-3-glucoside, peonidin-3-glucoside, cyanidin-3-maloylglucoside, pelargonidin-3-maloylglucoside, and peonidin-3-maloylglucoside were reported, and among them, 35.6–54.0% of the anthocyanins were acylated [22]. de la Parra et al. [23] identified the same profile in blue maize: peonidin-3-glucoside, delphinidin-3-glucosidic, petunidin-3-glucoside, malvidin-3-rutinoside, pelargonidin 3-glucoside, cyanidin 3,5-diglucoside, and pelargonin 3,5-diglucoside. Additionally, blue, pink, purple, red, and multicolored corn all have a complex mix of anthocyanins, with 18–27 compounds. Cyanidin derivatives make up 70% of all identified anthocyanins, making them the most common type. Fernandez-Aulis et al. [24] reported the presence of cyanidin-3-(6′malonyl) glucoside in “Cacahuacintle” maize husk, where the maize is white, whereas the husks and cobs have an intense purple color, which can be considered a competitive source of anthocyanins with the available commercial source. The Mixteco variety (blue maize) contains approximately 19.02 to 66.92 mg of cyanidin compounds, including cyanidin-3-(3′,6′-dimalonil-glucoside), pelargonidin-3-glucoside dimalonate, pelargonidin-3-(sinapoyl-glucoside)-5-glucoside, pelargonidin 3-(3′,6′-dimalonil-glucoside), pelargonidin 3-(6′-malonilglucoside)-5-(6′-acetylglucoside), pelargonidin 3-glucoside-5-(6′-acetylglucoside), pelargonidin 3-(6′-malonilglucoside), and pelargonidin 3,5-diacetylglucoside3 [25]. The main anthocyanins found in colored corn from other parts of Latin America, like Peru, were cyanidin-3-dimalonylglucoside, cyanidin-3-glucoside, pelargonid-in-3-glucoside, peonidin-3-glucoside, and their malonated forms [26].
In summary, we can observe that cyanidin-3-glucoside is one of the most important anthocyanin components in maize grains [27]. Purple maize cobs and leaves represented a more complete flavonoid profile than the grains and were excellent sources of anthocyanins ((Epi)catechin(4–8)-cyanidin-3,5-diglucoside) and flavonols, especially cyanidin, peonidin, and pelargonidin derivatives, as well as quercetin and kaempferol derivatives [23,26,28].
In purple corn, anthocyanins are synthesized from phenylalanine. Phenylalanine ammonia lyase (PAL) first deaminates phenylalanine into cinnamic acid, which subsequently transforms into 4-coumaroyl, the main precursor of anthocyanins [29]. Furthermore, the color of anthocyanins depends on the number and orientation of hydroxyl and methoxyl groups in the molecule. Increased hydroxylation (OH) shifts colors towards blue tones, while increased methoxylation shifts (OCH3) produce colorations [17,30]. It seems that the biosynthetic pathway of anthocyanins is mostly through the interaction of regulatory genes and plant hormones, such as jasmonic acid (JA) and abscisic acid (ABA), which play an essential role in activating the production of anthocyanins in plants [7]. Moreover, the tissue-specific expression of regulatory genes determines the distribution of pigmented maize anthocyanins in different tissues. Booster1 (B1) and Plant color1 (Pl1) are the bHLH and MYB regulatory factors, respectively, most often associated with regulation in plant tissues [27,31]. Structural genes directly encode enzymes required in the anthocyanin biosynthetic pathway, such as phenylalanine ammonia lyase, chalcone synthase, chalcone isomerase, flavanone 3-hydroxylase, flavonoid 3′-hydroxylase, dihydroflavonol-4-reductase, leucoanthocyanidin dioxygenase, and anthocyanidin 3-O-glucosyltransferase [32,33]. Different parameters can be used to improve the attainment of anthocyanins from pigmented maize, for example, maceration, pH, and extraction with sonication, microwave, or assisted enzymes [34]. Phenolic acids and flavonoids are common phenolic compounds in maize kernels; anthocyanins are derived from the different degrees of hydroxylation and methoxylation of the flavin skeleton (i.e., 2-phenylbenzopyran) [35]. Simple or acylated anthocyanins are mainly found in the aleurone layer of the maize endosperm or pericarp and can greatly affect the color of the kernel. Anthocyanins in the acylated form have a positive effect on maintaining in vitro stability [36].
Other compounds such as butylated hydroxyanisole, vitamin E, catechin, and quercetin are also reported to exist in maize. Maize contains many secondary metabolites such as carotenoids, phenolic compounds, phenolic acids, and flavonoids, as common phenolic compounds in maize kernels, which exist in free, esterified (covalently bound with other molecules), and insoluble bound forms [37]. Maize contains between 0.03 and 0.33% tocopherols [38], naturally occurring vitamin E forms with significant functions in preventing oxidative stress and the oxidation of other lipid components [39].
3. Bioactive Compounds in Maize and Health Effects
In recent years, antioxidants have become a crucial focus in biomedical research for their significant health benefits. As the incidence of several non-communicable diseases increases, the discovery of epigenetic modifications due to lifestyle patterns and nutrition affecting aging and life quality has become more and more evident. From this perspective, antioxidant compounds have been analyzed from several angles. The first and most important should be that as we continue to live, human beings oxidize, as in several biological processes, the mitochondria produce a large quantity of endogenous reactive oxygen species (ROS) [40]. As several endogenous or exogenous processes lead to the production of different amounts of ROS, and their overproduction may have detrimental effects on nucleic acids, proteins, and lipids, the existence of antioxidant molecules is crucial for wellbeing and to protect the different tissues, as in the last years, oxidative stress and all its consequences have been linked to immunological, neurological, and cardiovascular disorders [41,42]. Evidently, several antioxidant enzymatic systems already exist in organisms (i.e., superoxide dismutase, catalase, glutathione reductase, and peroxidase), but the second approach to the study of antioxidants is to understand how external antioxidant molecules can act in the body, ameliorating enzymatic antioxidant processes or even directly regulating them. In this pathway, research on glutathione, arginine, uric acid, tocopherols, carotenes, lipoic acids, flavonoids, polyphenols, and phytoestrogens has been successful [43]. It is, however, very interesting to note how all these complex antioxidants may be found in several vegetable sources. In 2023, Ranilla et al. [44] tested the variability in the free and dietary fiber-bound phenolic and carotenoid compound metabolites of three maize types (white, red, and orange) from the Peruvian Andean variety Cabanita and its different maturity stages (milk–S1, dough–S2, and mature–S3). In fact, they found that the pigmented (red and orange) maize varieties contained flavonoids such as luteolin derivatives and anthocyanins. Orange maize had a higher 2,2-Diphenyl-1-picrylhydrazyl (DPPH) hydrophilic antioxidant capacity than the others. Together with the red types, the more pigmented ones had higher bound ferulic acid and total phenolic contents (free + bound) than the white maize. The orange maize had the highest total carotenoid contents and contained β-cryptoxanthin and zeaxanthin isomers. Although no in vivo or other in vitro tests in eukaryotic cells were performed in the project, all Cabanita maize types had similar health-relevant functionality according to the variables they tested, which significantly decreased with grain development and should eventually be tested in other preclinical tests [44].
In a 2015 study, Urias-Lugo and his team demonstrated that non-acidic extracts from native blue maize at concentrations ranging from 4.31 to 7.23 mg/mL inhibited half of the growth of untransformed NIH3T3 cells compared to controls. Through testing nine compounds, they realized that CyMalGlu extract showed the strongest correlation with the reduction in cell viability in Caco2, HepG2, MCF7, and PC3 cells, although the highest contents of anthocyanins were not significantly better as cancer cell inhibitors [45]. However, in 2019, another study revealed a strong link between the total anthocyanin content and the DPPH assay. This demonstrated that, despite the vast differences in total phenolic and anthocyanin levels among the tested corns, the phytochemical levels could validate the bioactivity of the colored maize [46].
Anthocyanin potential has been analyzed for several biological functions. As nowadays obesity and metabolic disorders are a public health problem worldwide [47], the action of the different maize extracts on metabolic traits has been analyzed. In 2019, twenty-two Peruvian maize samples corresponding to the varieties Arequipeño, Cabanita, Kculli, Granada, and Coruca were analyzed, and their total phenolic contents (TPCs), anthocyanin contents, and capacity were tested, showing strong α-glucosidase, α-amylase, and lipase inhibition in vitro. It is also intriguing that the more pigmented samples exhibited high lipase and α-glucosidase inhibition potential [48]. In 2018, anthocyanins from commercially available purple maize were found to be key players in the enhancement of insulin secretion and hepatic glucose uptake in cultured cells from the pancreas and hepatocytes through the activation of free fatty acid receptor-1 and glucokinase [49].
Anthocyanins are known for their neuroprotective and antiproliferative effects, in addition to their metabolic benefits. These compounds help reduce oxidative stress and inflammation in neuronal cells, which can potentially prevent or slow down the progression of neurodegenerative diseases [50]. Research indicates that anthocyanins can protect neurons from oxidative damage and neurotoxicity, making them promising for treating conditions like Alzheimer’s and Parkinson’s diseases [50]. Additionally, anthocyanins have demonstrated antiproliferative effects in various cancer cell lines by inhibiting cell growth and inducing apoptosis. For example, anthocyanins from blueberries significantly inhibit the proliferation of breast cancer cells (MCF-7) and colorectal cancer cells (HCT-116), underscoring their protentional as therapeutic agents in cancer treatment [51,52]. The antioxidant properties of anthocyanins further enhance their protective effects against chronic diseases, highlighting the importance of including anthocyanin-rich foods in the diet for overall health and disease prevention.
Several other components, such as the lipids in the food matrix, have also demonstrated anti-inflammatory and antioxidant potential. Omega 9, a type of oleic acid from blue corn, is a beneficial nutrient for several pathologies with chronic proinflammatory traits, such as obesity and other chronic diseases. This is an interesting functionality by itself but also in relation to linoleic acids (omega 6), which promote the metabolic pathways of prostaglandin, thromboxane, and proinflammatory leukotriene synthesis [53]. This anti-inflammatory relationship between omegas has been analyzed in several articles [54,55].
Interestingly, maize has been described as a food matrix containing the highest content of bioavailable micronutrients in grain, specifically α-tocopherol [56], which is a naturally occurring vitamin E that protects against oxidative stress and the oxidation of other components [39]. Since 1981, there has been information about the localization of tocopherols, which are associated with germs (up to 80%) and with endosperms (up to 27%) [57]. Also, when the genotype of maize is richer in oil, it has been related to a higher amount of α- and γ-tocopherols. Red maize, for example, was the variety with the highest content of major tocopherol and tocotrienol isomers when analyzed by in vitro digestion and chemical methods from varieties of Big Flint Maize, regular Pop Maize, and Red Maize, which correlates with the pigmentation. As a labile vitamin, all the components decreased when cooked at high temperatures [57].
While the antioxidant properties of maize have been widely described, some other bioactive peptides obtained by the cleavage of maize proteins have also demonstrated antioxidant and immune-protective effects. Furthermore, they have the potential to neutralize liposoluble free radicals, like peroxyl radicals, which are produced during the oxidation of unsaturated fatty acids [58,59]; hydrophobic amino acids such as leucine, valine, alanine, and proline have an especially impressive capacity for this. The bioactive peptide zein, a prolamin like those in the gluten complex, has been shown to diminish several cytokines such as tumor necrosis factor alpha and interleukins 1 and 6, resulting in the inhibition of the expression of intercellular adhesion molecule 1 and monocyte chemotactic protein 1 linked to inflammation and the initiation of cardiovascular and metabolic pathologies, including atherosclerosis. Also, a decrease in the cyclooxygenase-2 pathway, and reductions in the production of proinflammatory leukotrienes, prostaglandins, and thromboxanes, has been observed. Other pathologies with metabolic endotoxemia generated by the LPS in Gram-negative bacteria of the intestinal dysbiosis microbiota, such as type 2 diabetes mellitus, Alzheimer’s disease, multiple sclerosis, and liver and kidney disease, are important therapeutic targets for these bioactive substances of maize [60,61].
4. Gut Microbiota Modulation by Vegetal Anthocyanins
The relationship between food compounds and the intestinal microbiota is fundamental to understand how our diet directly affects intestinal and overall health. Anthocyanins are candidates for the development of nutritional therapies aimed at preventing gastrointestinal diseases and with great potential for the development of nutraceutical strategies [62], but not only in these contexts are they important. For example, elderberry (Sambucus nigra L.) stands out for its rich composition of anthocyanins and phenolic compounds that exert antioxidant and antimicrobial properties. Recent research highlights the role of peonidin from sweet potato (an O-methylated anthocyanidin derived from cyanidin), which showed potential to act as a prebiotic, inducing the proliferation of Bifidobacterium bifidum, Bifidobacterium adolescentis, Bifidobacterium infantis, and Lactobacillus acidophilus, which illustrates the potential of dietary compounds to benefit the overall intestinal microbiota health [11]. Sweet potato extract has also shown an impact on the intestinal microbiota composition, counteracting the negative symptoms of a high-fat/cholesterol diet and controlling the redox state through gut beneficial bacteria modulation (through Akkermansia, Lactobacillus, and Bifidobacteria increases) and a reduction in fecal LPS levels [12]. Anthocyanins from blueberry and cranberry extracts have also been explored as promoters of the growth of specialized bacteria in short-chain fatty acid (SCFA) production, such as Lachnoclostridium, Roseburia, Peptoclostridium, and Clostridium innocuum groups in C57BL/6 J mice following a high-fat diet (HFD). Decreased levels of Rikenella, a Gram-negative bacteria family associated with LPS production, were also reported [63]. The effects of black raspberry extract on the composition and metabolism of the intestinal microbiota have also been explored using a computer-controlled in vitro human colonic model using fresh fecal samples from healthy volunteers. Interestingly, the relative abundance of some pathogenic bacteria (i.e., Enterococcus, Citrobacter, and Pseudomonas) was significantly reduced by the black raspberry extract, although some bacteria related to health traits were not modulated (i.e., Akkermansia) [64]. In this line, Wu et al. [65] reported that high doses of fermented raspberry juice in vitro modified the production of SCFAs, promoting the growth of butyric acid-producing bacteria and modulating the gut microbiota community overall. In vivo, all the fermented juice doses improved the bacterial SCFA production, thus ameliorating the gene expression of ZO-1, Claudin-1, Claudin-4, Occludin, E-cadherin, and Muc-2 in healthy mice, which was positively correlated with the intestinal barrier function, yet the high dose of the active compound significantly inhibited Akkermansia [65].
It is also known that, in a HFD-induced mice model, purple-leaf tea (Camellia sinensis L.), a popular beverage and food supplement in China and Vietnam, displayed beneficial effects like the restoration of fasting blood glucose and a reduction in serum triglycerides attributed to the metabolism of flavonoids or anthocyanins by gut microbiota. The tea reduced the firmicutes/Bacteriodetes ratio [66]. A beneficial impact of blueberry polyphenolic fractions on glucose homeostasis can be also induced by a fecal microbiota transplant (FMT). Morissette et al. [67] reported that C57BL/6 germ-free mice who received an FMT from donor mice fed with a high-fat, high-sucrose diet and blueberry polyphenolic fractions gained less weight than mice that received an FMT from the high-fat, high-sucrose diet group. The authors suggest that proanthocyanidin intake induces Muribaculaceae growth, which protects against diet-induced obesity. In fact, this bacterial family is associated with an improved intestinal barrier function in mice [67]. Muribaculaceae members were also modulated by freeze-dried jabuticaba peel in an HFD mice model and reduced the abundance of Firmicutes and Actinobacteriota phyla while increasing the Muribaculaceae and Lachnospiraceae families and the Faecalicatena genus. Jabuticaba peel is mainly composed of dietary fiber, and anthocyanins and their effects were also observed in optimizing the glucose metabolism by avoiding insulin resistance via the lipopolysaccharide/Toll-like receptor-4 inflammatory pathway [68]. In the same way, pomegranate peels rich in anthocyanins significantly changed the composition of the gut microbiota, including Akkermansia, Lachnoclostridium, Parabacteroides, Ruminiclostridium_9, Ruminiclostridium, and Bacteroides, the proportions of which were increased by the supplementation and improved HFD-induced insulin resistance [69].
In 2020, it was also reported that the use of a black maize extract rich in cyanidin-3-glucoside and pelargonidin-3-O-glucoside in an animal Gallus gallus model increased Bifidobacterium and Clostridium populations, decreasing E. coli. However, functionally, the extract maintained the villi height, Paneth cell number, and goblet cell diameter (in the villi and crypt) [70].
Gut microbiota modulation by anthocyanins has also been studied at a clinical level. Two-hundred milligrams of different plant and/or fruit extracts of anthocyanin and prebiotics such as short-chain fructooligosaccharides and inulin were tested in male and female volunteers with uncomplicated obesity. After 8 weeks of daily supplementation, a decrease in Firmicutes and Actinobacteria and an increase in Bacteroidetes were observed, with no other significant changes reported, but with the normalization of or reduction in glycosylated hemoglobin (HbA1c) levels and improvements in bowel habits [71].
The study of the effects of anthocyanins on gut microbiota modulation has also been conducted in inflammation and colorectal cancer contexts. Polyphenols can inhibit the phosphorylation of p65, extracellular regulated protein kinase, and -Jun N-terminal kinase, thereby suppressing the activation of the nuclear factor-kappa B and mitogen-activated protein kinase signaling pathways [72]. This implies that antioxidant compounds could act in different ways by regulating oxidative stress and inflammation and by modulating the gut microbiota. In this case, some studies using maize-specific components have been also analyzed. Wu et al. [73] assessed isogenic maize lines enriched with flavonoids on a dextran sodium sulfate (DSS)-induced colitis model in mice. Maize diets enriched with anthocyanins and/or phlobaphenes effectively alleviated the induced colitis, reducing intestinal permeability, maintaining crypt structure and mucin-2 protein levels, and reducing inflammation. Anthocyanin-enriched diets were more effective in mice fed diets with anthocyanins and phlobaphenes, which had bacterial communities that were more like the healthy control than the DSS control group. Furthermore, anthocyanins could participate in tryptophan metabolism, increasing the production of the neuroprotective metabolite kynurenic acid [11]. Marques et al. [74] reported that blackberry anthocyanins (Rubus fruticosus) are associated with decreased TCK-1 expression in the hippocampus and the increased expression of fractalkine (a chemokine particularly important in the crosstalk between neurons and microglia). In an HFD mice model, TCK-1 was negatively correlated with Pseudoflavonifractor (associated with losing weight in obese patients) and Sporobacter and associated with a reduction in neuroinflammation and an influence on the metabolism of tryptophan. These findings suggest a mechanism by which anthocyanins may have anti-neuroinflammatory effects, possibly through the modulation of the intestinal microbiota [11,74]. The growth of beneficial intestinal microbiota such as Eubacterium rectale, Faecalibacterium prausnitzii, and Lactobacillus was increased by freeze-dried black raspberry anthocyanin supplementation in an azoxymethane (AOM)/dextran sodium sulfate (DSS) mouse model of inflammatory colorectal cancer, while the growth of intestinal pathogenic microflora, which included Campylobacter, Helicobacter pylori, Bacteroides, and Prevotella, was inhibited [75]. Black raspberry powder consistently lowered tumor multiplicity compared with AOM/DSS-treated mice by decreasing the level of p-STAT3 (signal transducer and activator of transcription-3, STAT3) in the intestinal epithelial cells. Furthermore, the genes involved in the β-catenin signaling pathway, including p-JNK, Bcl2, CDK4, CyclinD1, and c-Myc, which promote tumor proliferation and inhibit apoptosis, were significantly downregulated by black raspberry anthocyanins. The expression of Bax, the apoptosis-inducing factor, was upregulated by black raspberry anthocyanins [75]. In fact, probiotic bacteria are able to modulate the pro-apoptosis genes [76], and a synergistic effect of anthocyanins and probiotics, called synbiotics, could be another strategy to counter colorectal cancer.
But why is the gut microbiota modulated by antioxidant compounds? In vitro experiments allow us to understand that gut microbiota enzymes participate in the degradation of antioxidant compounds. The degradation of glycosylated anthocyanins containing malvidin-3-glucoside, delphinidin-3-glucoside, peonidin-3-glucoside, petunidin-3-glucoside, and cyanidin-3-glucoside is underwent considerable metabolism by human fecal microbiota in 4 h and were totally degraded by 24 h. However, this does not mean that the loss of bioactivity due to newly formed compounds appears after anthocyanin and gallic acid metabolism. For example, the chemical breakdown of delphinid leads to the formation of derived compounds such as gallic acid and traces of homogentisic, syringic, and p-coumaric acids. Malvidin-3-glucoside, a syringic acid derivative, significantly increases the growth of beneficial bacteria such as Bididobacterium spp. and Lactobacillus spp. Intestinal bacteria with β-glucosidase activity include the glycoside hydrolase (GH) families GH1, GH3, GH5, GH9, GH30, and GH116 [77]. Besides Bifidobacterium spp. and Lactobacillus spp., malvidin-3-glucoside showed a tendency to promote the growth of Clostridium coccoides−Eubacterium rectale, a group of bacteria known to produce large amounts of butyrate [13]. Decarboxylase activities have been reported for Lactobacillus strains, particularly for Lacitplantibacillus plantarum; decarboxylases enzymes convert gallic acid to pyrogallol [78]. Other species, like Lactobacillus rhamnosus, are able to bioconvert crude polyphenols, flavonol + dihydrochalcone, anthocyanin, proanthocyanidin, and phenolic acid + catechin-rich fractions to 4-hydroxyphenylacetic acid, 3-(4-hydroxyphenyl) propionic acid, hydrocinnamic acid, catechol, and pyrogallol [79].
5. Mexican Traditional Beverages
Mexico uses corn as an important crop to produce a wide variety of foods, including beverages. Besides their nutritional value, they are also significant in the culinary and cultural heritage of Mexico. The fermentation process used for most traditional Mexican beverages made with maize is common, and here are some of the most researched examples:
Tejuino
Tejuino is made from maize using artisanal or commercial methods of germination–fermentation or nixtamalization–fermentation and is a traditional fermented beverage found in Mexico [80]. In terms of nutrition, this beverage contains compounds that have a high biological value, strains that produce prebiotics, and potential probiotics. Some of the strains that have been isolated in the Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco (CIATEJ, Guadalajara, México) are Weissella cibaria CIATEJ BI-48.1, Leuconostoc citreum CIATEJ BI-49.1, Bacillus safensis CIATEJ BI-75.1, Pseudomonas brenneri CIATEJ BI-26.2, Pseudomonas psychrophila CIATEJ BI-27.1, Chryseobacterium bovis CIATEJ BI-55.1, Acetobacter tropicalis CIATEJ BI-84.1, Brochothrix thermosphacta CIATEJ BI-2.1, Corynebacterium calluane CIATEJ BI-4.1, Kurthiagibsonii CIATEJ BI-4.5, and Pantoea vagans [81,82]. The latter strain has a high potential as a new biocatalyst for the industrial production of galactooligosaccharides (prebiotics) [82]. The strains Weissella Siberia and Leuconostoc showed potential for being classified as probiotics because they have tolerance to gastrointestinal conditions, antagonistic activity towards foodborne pathogens, short-chain fatty acid production, and adhesion to HT-29 cells [81].
The in vivo effects of tejuino consumption have not been confirmed. The colonic fermentation of the indigestible fraction of tejuino by human fecal microbiota is the only case that has been reported so far. Acetic and butyric acid were mainly produced by the colonial fermentation of tejuino after 6 h. Additionally, fermentation resulted in the production of soluble phenolic compounds and approximately one hundred volatile compounds [80].
Pozol
Pozol is fermented spontaneously by wrapping the nixtamalized maize dough in banana leaves until the pH reaches around 4.8. Then, water is used to suspend it after fermentation [83,84]. Although aflatoxins are inactivated after being nixtamalized, their activation can still occur due to the acidic pH during fermentation [85].
Pozol is regarded as an essential source of energy that offers the same amount of protein and fiber as a similar portion of tortilla [83]. Most research has focused on identifying the microorganisms involved in the fermentation process of this spontaneously fermented beverage [83,84,86,87]. This drink has a complex microbiota consisting mainly of lactic acid bacteria (LAB), Enterococcus, Lactococcus, Leuconostoc, Streptococcus, Weissella, and particularly, Streptococcus infantarius species. S. infantarius 25124 strain was reported to be the main amylolytic LAB, with a strong probiotic potential [10,88,89,90]. At the moment, there are no reports of the clinical effect of pozol; however, some healing properties such as reducing fever and helping in the treatment of diarrhea have been attributed, as well as the prevention or treatment of diseases associated with metabolic alterations [91,92]
Atole agrio
Atole agrio is a typical Mexican non-alcoholic, acidic, fermented maize beverage. There are different methods to prepare atole: Väkeväinen et al. [93] mention that one method uses young corn that is not nixtamalized, while Pérez-Armendáriz and Cardoso-Ugarte [87] describe two methods available for making atole agrio from nixtamalized maize. One method involves diluting fermented maize dough with water and bringing the mixture to a boil. The other method uses fresh maize dough and allows the resultant beverage to rest in a warm place until fermentation is accomplished. Fermentation time ranges from a few hours to several days, depending on the desired flavor. Atole agrio is unique in that it needs to be boiled before consumption and is typically drunk hot. The main lactic acid bacteria present in acidic “atole” are Weissella, Pediococcus, Lactococcus, and Lactobacillus. However, Enterobacteriaceae were detected throughout fermentation. The selection of starter cultures is a strategy to enhance the safety of atole and enable its commercialization at an industrial level [93].
Other Mexican maize-based beverages such as sendechó, tesgüino, tascalate, and tejate are also commonly consumed but have not yet been thoroughly analyzed [94]. Most of these beverages are homemade and consumed locally. Table 1 lists the varieties of traditional maize products that have already been commercialized.

Table 1.
Formulation of maize-based commercial beverages on the market.
6. Anthocyanins from Pigmented Maize as Potential Ingredients for the Development of New Functional Beverages
As mentioned previously, pigmented maize contains important flavonoids, one of the most important being cyanidin-3 glucoside [95]. Anthocyanins are natural dyes found in maize with powerful antioxidant, anti-inflammatory, and anti-carcinogenic properties [96]. They also help lower blood pressure and high cholesterol, improve blood circulation, and promote tissue regeneration [97]. In recent years, the interest in these compounds has increased due to their potential use as sources to replace synthetic colorants (e.g., indigo carmine and bright blue). In fact, one of the concerns of the use of these compounds has been the chemical degradation that can cause the loss of color and/or bioactivity, which may be disadvantageous when formulating specific products. This is why the stabilization and incorporation of anthocyanins into beverages can be achieved through methods including co-pigmentation, oxygen exclusion during processing and storage, coating methods, or even encapsulation techniques [98]. Table 2 summarizes the stabilization techniques for maize compounds.

Table 2.
Stabilization of corn anthocyanins in beverages.
In Mexico, maize in mainly consumed as tortillas, which requires a traditional nixtamalization process and the cooking of maize, resulting in losses of total anthocyanins, and this is influenced by location in the maize. Cortés et al. [104] and various authors have suggested ways to enhance the stability and bioavailability of anthocyanidins/anthocyanins, including acylation, metal complexation, co-pigmentation, self-association, and encapsulation [105]. In this regard, acylated anthocyanins showed better resistance to high temperatures, pH, oxygen, and light compared to unacylated anthocyanins [106]. The acylated anthocyanin could be obtained by both chemical and enzymatic acylation of the glycosyl moieties of anthocyanins, reducing attacks from hydrophilic groups and increasing steric hindrance [107]. Both methods, chemical and enzymatic acylation, have limits; for example, enzymatic acylation has mild reaction conditions, high catalytic efficiency, and selectivity but high cost. In contrast, chemical acylation requires the use of metals such as Fe and a stabilizing agent such as chondroitin sulfate.
Another option for maintaining the stability of anthocyanins is intermolecular or intramolecular co-pigmentation. In the first case, the anthocyanin is noncovalently bound to an aromatic accessory pigment, mainly through π–π interactions. In contrast, in the second case, the co-pigmentation requires an interaction with the anthocyanin skeleton. In both cases, Huang and collaborators [108] mention that a color enhancement effect could result from the combination of free anthocyanins through co-pigmentation, which is unfavorable for maintaining a blue color. The stabilization and incorporation of anthocyanins into beverages can be achieved through various methods, including co-pigmentation, the exclusion of oxygen during processing and storage, coating methods, and encapsulation techniques [98]. On the other hand, encapsulation represents a strategy to develop various formulations to provide additional protection to the anthocyanins, improving the stability and bioavailability of anthocyanins. Encapsulating anthocyanin extract with the right wall material, such as gelatin, soy protein isolate, maltodextrin, and gum arabic, can facilitate the stability, bioavailability, release, and degradation of anthocyanins, leading to better colonic accessibility [109]. Biopolymer-based formulations are environmentally friendly, edible, cost-effective, and easily modified, and they are ideal to be incorporated into food applications. Stabilization, release, and bioavailability strategies for anthocyanins set the benchmark for supplementation or the design of new functional foods. As an example, bilberry anthocyanins have already been used as anti-cancer ingredients and were encapsulated in liposomal micelles called NutraNanoSpheres, which are freely water soluble [110]. Nanosytems can guarantee anthocyanin activities when supplementing them in other formulations; the crucial point of their use is the toxicological assessment of nanopackaging materials [111]. They could allow anthocyanins to be used as preventive supplements in beverages. Food nanosystems must tolerate the conditions of gastrointestinal digestion. In this context, Oancea et al. [112] have increased the availability of anthocyanins from sour cherry extract using whey protein isolates. Maltodextrin is widely used for the microencapsulation of bioactive compounds due to its high solubility, low viscosity at high solid concentrations, low relative cost, and neutral taste and aroma [99,113]. A spray-drying technique with the conventional wall material maltodextrin and a novel one, hydroxypropyl-β-cyclodextrin, was employed to encapsulate anthocyanin-rich blue maize. The microencapsulates had a good impact on stability and protected against adverse environmental conditions. This is a promising perspective from the standpoint of food and pharmaceutical applications that could be achieved by entrapping bioactive molecules in double microencapsulates by combining commonly used biopolymers with novel ones [114].
Traditional beverages (such as the ones described before) usually are made using conventional methods such as spontaneous fermentation by vegetable substrates. Actually, research into fermented plant-based beverages faces significant challenges related to the improvement of processing techniques, including the exploration of emerging non-thermal processing technologies that can ensure the safety of the product while accentuating nutritional and sensory qualities [115]. This would also be important to supply antioxidants, micro and macronutrients to populations that might be vulnerable, malnourished, aged, or having special health needs. In this regard, there are other novel techniques that contribute to preserving the functionality of the plant pigments and may later be used in the creation of functional products. Penha et al. [116] suggested the use of ecofriendly technologies on a laboratory scale, such as ultrasound, ultra-high-pressure homogenization (UHPH), enzymatic treatments, and fermentation for plant-based beverage production to improve the extraction of intracellular compounds such as proteins and bioactive compounds and also to improve the technological properties of plant matrices. Another emerging non-thermal technology is the pulsed electric field (PEF); the integration of PEF technology into the extraction process of anthocyanins, betalains, carotenoids, and chlorophylls stands out due to its mechanism of action on plant matrices. PEF technology increases the mass transfer of the pigments through the phenomenon of electroporation, avoiding their thermal degradation [117].
7. Discussion and Perspectives
This review seeks to highlight the use of pigmented maize in traditional beverages, explore the bioactive compounds present, and identify new opportunities in functional foods. Pigmented maize, especially purple maize, has gained consumer interest as a natural food additive due to its high flavonoid content. Anthocyanins, responsible for the vibrant maize colors, offer countless health benefits, including antioxidant, anti-inflammatory, and anti-cancer properties and gut microbiota modulation, as discussed above (Figure 2).
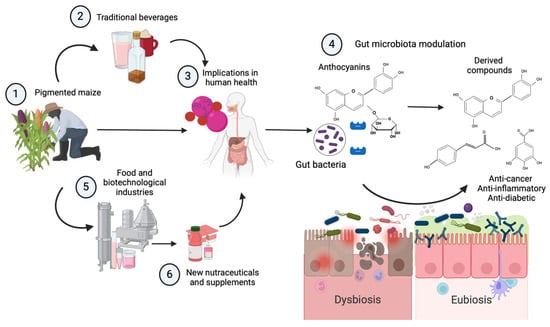
Figure 2.
Pigmented maize is harvested in Mesoamerica and other regions (1) and has been used for the preparation of traditional beverages (2) from the southeastern and western zones in Mexico. Especially used on festive occasions, these beverages are fermented with Enterobacteriaceae and various lactic acid bacteria (i.e., tejuino and pozol). One of the important bioactive agents of these beverages from pigmented maize are anthocyanins (3), one group of vegetal dyes with implications in human health. One of the most important is the intestinal effect on the intestinal barrier (4) and specifically on the microbiota protecting the intestinal lumen. Here, several bacteria use anthocyanins (3), fermenting them through gut enzymes (cyanidin-3 glucoside to phenolic acids and polyphenolic metabolites) and increasing their immunomodulatory, anti-inflammatory, anti-cancer, and anti-diabetic effects, and even in the aglycone form, they have also shown strong antioxidant effects. This is why the food and biotechnological industries (5) have developed methods that can be used in the production, fortification, and re-formulation of new nutraceuticals (6) and supplements with specific effects on nutrition and health. Created on BioRender.com (2024) (accessed on 28 June 2024).
The discussion about the implications of the use of this cereal is indeed interesting and has biological and socio-anthropological aspects. Here, the principal question would be to determine what is still missing to increase the quality of a principal crop that is nowadays still used for feeding animals and humans, especially in Latin American and African populations. In recent years, maize applications have expanded to include bioethanol production, leading to technological advances in hybridization and GMOs. Traditionally used in the livestock and bioethanol industries, there is renewed interest in its culinary uses, especially in Mexico, where it is consumed principally as tortillas. However, the nixtamalization process used for tortillas reduces the phenolic and anthocyanin contents, weakening their antioxidant properties [9]. Pre-Hispanic preparations like pozol and tejuino offer alternatives to preserve these benefits [10]. But in fact, other world civilizations have also explored maize-based beverages, such as “mahewu” in South Africa (a cooked maize porridge, fermented at room temperature for one or two days) [118], the highly antioxidant Peruvian “chicha” with purple maize [119], and even Korean “Oksusu-cha” maize tea [120], the bacterial and physicochemical properties of which have been already analyzed.
Would these ancient beverages be modified to offer additional benefits to the populations that consume them? It is well known that they are commonly accepted and consumed by the population in several states in these countries [121]. However, it is an interesting question, and to our knowledge, nowadays there are only a few proposals of teams working on industrial modifications or even the production of beverages containing pigmented maize extracts for health purposes; in 2018, Wolfgang-Engel and Robert-Brocker proposed an extract of the spindles and outer bracts of blue maize for the oral treatment and/or prophylaxis of human inflammatory bowel disease [122]. However, some other groups are already testing extrusion and microwave methods, for example, to produce functional foods that include blue maize as a major source of anthocyanins and incorporates the antioxidant activity into third-generation snack production [123].
Another essential aspect of these applications is the natural anthocyanin pigment from maize. This provides a natural, non-allergenic, yet economical alternative when contrasting with artificial dyes [124]. The interesting point here is that the analysis for optimal colorant production requires not only an understanding of pigment biochemistry but also the integration of all the genetic aspects of maize production, which implies the genetic analysis of the activating and repressing genes [125]. The complex composition of anthocyanins in pigmented maize presents significant potential for developing natural food colorants, therapeutical matrices for several health conditions, and functional foods replacing synthetic additives. Hence, several groups have started to develop strategies such as pharmacological supplementation and industrial fortification of the foods their nutrition depends on. However, the biofortification of food crops with macro- or micronutrients has proven to be effective in the general improvement of crops. We know, for example, that the development of maize with increased micromineral content by projects such as HarvestPlus, an international agriculture research-based method of plant breeding, works to address micronutrient deficiency [126]. Several studies that have analyzed the effects of the genetic changes in the cultivars have proven these to be an effective alternative, as these changes allow for up to 89% and 100% of estimated average mineral requirements to be fulfilled for vulnerable populations such as pregnant women and children, demonstrating that fresh, high-zinc maize accumulates a substantial amount of this micronutrient and highlighting the potential of this alternative for health approaches [127].
An important axis in the comprehensive approach to food safety and the biological importance of genetic engineering is still the analysis of modified crops. However, one crucial perspective of this article is the possible genetic engineering aspect of maize crops. The possibility of introducing genes with special effects from other species (plants or bacteria) into maize, for example, the use of zein promoters that specifically express bacterial crtB and crtI genes in maize endosperm, has already been evaluated, resulting in a thirty-four fold increase in total carotene contents in the crop, which boosts the possibilities of the plant advantage [128]. Even so, the use of other plants such as blueberry and cranberry, which are high antioxidant producers, has not been analyzed to ameliorate the genetic traits of pigmented maize. Specifically, for Vaccinium corymbosum, V. macrocarpon, and V. oxycoccus, their specific genes ANS (anthocyanidin synthase) and UFGT2 have already shown homology between these species and have also been tested as leaders in anthocyanin biosynthesis modulation for anthocyanin production [129]. It would be an interesting starting point to propose alternatives to understand how to boost the anthocyanin contents in pigmented maize. Together with the full understanding of C1, C2, Pl1, Pl2, ZmCOP1, and ZmHY5, as the genes in the anthocyanin production pathway in maize [130], and other important genes such as Sh2, researchers may be able to understand if changes in the resistant starch control may be applied to the plant, maybe even by microbiological modification, in order to produce higher-power symbiotics with specific biological effects [131]. Other implications in the development of transcriptomics and metabolomics would be necessary to understand information about pigmented maize, nutrient compound synthesis, and even infection resistance of the plant and the availability of specific desired components.
But in fact, other perspectives on the improvement of the biological traits of the plant, for example, the increase in anthocyanins or bioactive compounds, implies the fermentation and inclusion of beneficial bacteria to modify different patterns of the bioavailability of the plant compounds. The fermentation of other cereals like red sorghum grains (in both spontaneous and artificial ways) has already been linked to an increase in the anthocyanin concentration; for example, Pantoea dispersa LMG 2603 led to a higher increase in anthocyanin bioactivities [132]. In order to apply this knowledge, the food industry benefits from it, knowing that the fermentation process in black wheat has also been explored in order to produce beer by the actions of Saccharomyces cerevisiae CMS12 isolated from fruit waste [133]. This product, with up to 6.43 mg/L anthocyanins and higher levels of minerals that are already on the market, shows how basic science can also improve other important fields such as the food industry. In fact, an analysis of the synergy between microorganisms and bioactive compounds reveals a well-assessed strategy that enhances their beneficial capacity, as evidenced in other cases. These include bacterial-derived metabolites from broccoli, such as glucosinolates, which, when converted into isothiocyanates, enhance their anti-cancer effect on the colon [134], or the involvement of intestinal bacteria in the hydrolysis of tryptophan to serotonin (5-hydroxytryptamine), quinurenine (Kyn), and indole derivatives important in serotonin regulation [135]. Indeed, it is known that several polyphenols, such as those found in blueberries, often escape absorption in the small intestine because of their substantial molecular size. Instead, they accumulate in the large intestine, where they undergo transformation by gut bacteria [67]. Yet some authors have already suggested that anthocyanin metabolites produced by colonic fermentation by the intestinal microbiota might be responsible for the observed in vivo health effects [13].
As analyzed in Section 4, the complete microbiota effects of anthocyanins have already been analyzed for other food matrices, although it remains a short-term goal to assess how the different maize components impact identified bacteria consortia with proven functionalities or provide them with other ones that are interesting for health. Another critical question is also the dose of these maize anthocyanins to obtain a positive health effect. Although no reports on the toxicity of this compound upon ingestion in humans have been presented, it is important to say that most of the assays are still preclinical. That is why that Reagan-Shaw formula [136] is one of the more appropriate calculus methods for converting drug doses from animal studies to human studies using the body surface area normalization method. Furthermore, all the microbiological fermentative phenomena of anthocyanins and pigmented maize components analyzed so far not only highlight the importance of diet in maintaining a healthy microbial balance; they also motivate the creation and further analysis of functional foods, supplements, and nutritional modules to respond to specific nutritional demands looking to improve gastrointestinal and systemic health. Innovative approaches for research and clinical applications will have to be built on a solid foundation of basic and applied sciences such as nano- and microencapsulation, emerging extraction techniques, and maybe even edible films and other physicochemical innovative techniques. These findings also open new opportunities for understanding how different diet patterns, lifestyles, and specific dietary components may not only influence gut health but also systemic and microbiota health, highlighting the potential role of anthocyanins as prebiotics for the host commensal microbiota and also for novel allochthone colonization species, which could later constitute symbiotic pharmaceutical preparations. Finally, we are now landing into the analysis of this complex through the novel interactome, which will help us to understand intestinal and colonic niche colonization, tissue remodeling, bacterial living patterns, and even immunomodulation.
In summary, pigmented maize is not only a staple food crop but also a valuable source of bioactive compounds that enhance health and nutrition. Future research and development can connect the ancient knowledge of beverage preparation to the translational science-based innovative nutritional alternatives and functional foods. These may utilize the unique properties of anthocyanins and other bioactive compounds in pigmented maize to propose novel health prophylactic and/or therapeutic effects.
Author Contributions
D.R.-P., K.S.S.-S. and E.T.-M.: writing—Gut microbiota modulation. E.C.-S.: Writing—Mexican traditional beverages and maize compounds as additives in beverages. V.M.-T. and M.J.N.-I.: writing—Technological aspects section, review, and editing. R.P.-P.-B.: writing—Principal antioxidant compounds section. C.O.-S.: writing—Antioxidant compounds in maize and health effects section. D.R.-P. and E.T.-M.: Writing—original draft, conceptualization and discussion section, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
K.S.S.-S. received a scholarship from the Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCyT, Mexico City, Mexico). E.T.M., was supported by the CONAHCyT repatriation program (Mexico City, Mexico).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- OECD, Food, Nations AOotU. OECD-FAO Agricultural Outlook 2023–2032; OECD: Paris, France, 2023. [Google Scholar]
- Vallebueno-Estrada, M.; Rodríguez-Arévalo, I.; Rougon-Cardoso, A.; Martínez González, J.; García Cook, A.; Montiel, R.; Vielle-Calzada, J.-P. The earliest maize from San Marcos Tehuacán is a partial domesticate with genomic evidence of inbreeding. Proc. Natl. Acad. Sci. USA 2016, 113, 14151–14156. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Valdivia, I.; Perkins, A.C.; Schneider, H.M.; Vallebueno-Estrada, M.; Burridge, J.D.; González-Orozco, E.; Montufar, A.; Montiel, R.; Lynch, J.P.; Vielle-Calzada, J.-P. Gradual domestication of root traits in the earliest maize from Tehuacán. Proc. Natl. Acad. Sci. USA 2022, 119, e2110245119. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.J. Why we need GMO crops in agriculture. Mo. Med. 2014, 111, 492–507. [Google Scholar] [PubMed]
- Broa Rojas, E.; Vázquez Carrillo, M.G.; Estrella Chulím, N.G.; Hernández Salgado, J.H.; Ramírez Valverde, B.; Bahena Delgado, G. Características fisicoquímicas y calidad de la proteína de maíces nativos pigmentados de Morelos en dos años de cultivo. Rev. Mex. de Cienc. Agrícolas 2019, 10, 683–697. [Google Scholar] [CrossRef]
- Halbwirth, H.; Martens, S.; Wienand, U.; Forkmann, G.; Stich, K. Biochemical formation of anthocyanins in silk tissue of Zea mays. Plant Sci. 2003, 164, 489–495. [Google Scholar] [CrossRef]
- Cappellini, F.; Marinelli, A.; Toccaceli, M.; Tonelli, C.; Petroni, K. Anthocyanins: From Mechanisms of Regulation in Plants to Health Benefits in Foods. Front. Plant Sci. 2021, 12, 748049. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vega, H.; Vázquez-Carrillo, G.; Muñóz-Rosales, G.M.; Martínez-Loperena, R.; Heredia-Nava, D.; Martínez-Sifuentes, J.Á.; Anaya-Esparza, L.M.; Gómez-Rodríguez, V.M. Physical and Chemical Characteristics of Native Maize from the Jalisco Highlands and Their Influence on the Nixtamalization Process. Agriculture 2022, 12, 1293. [Google Scholar] [CrossRef]
- Lopez-Martinez, L.X.; Parkin, K.L.; Garcia, H.S. Phase II-Inducing, Polyphenols Content and Antioxidant Capacity of Corn (Zea mays L.) from Phenotypes of White, Blue, Red and Purple Colors Processed into Masa and Tortillas. Plant Foods Hum. Nutr. 2011, 66, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Ruiz, G.; Guyot, J.P.; Ruiz-Teran, F.; Morlon-Guyot, J.; Wacher, C. Microbial and physiological characterization of weakly amylolytic but fast-growing lactic acid bacteria: A functional role in supporting microbial diversity in pozol, a Mexican fermented maize beverage. Appl. Environ. Microbiol. 2003, 69, 4367–4374. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, P.; Zhu, Y.; Lou, Q.; He, S. Antioxidant and prebiotic activity of five peonidin-based anthocyanins extracted from purple sweet potato (Ipomoea batatas (L.) Lam.). Sci. Rep. 2018, 8, 5018. [Google Scholar] [CrossRef]
- Liu, D.; Ji, Y.; Wang, K.; Guo, Y.; Wang, H.; Zhang, H.; Li, L.; Li, H.; Cui, S.W.; Wang, H. Purple sweet potato anthocyanin extract regulates redox state related to gut microbiota homeostasis in obese mice. J. Food Sci. 2022, 87, 2133–2146. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Guzzon, F.; Arandia Rios, L.W.; Caviedes Cepeda, G.M.; Céspedes Polo, M.; Chavez Cabrera, A.; Muriel Figueroa, J.; Medina Hoyos, A.E.; Jara Calvo, T.W.; Molnar, T.L.; Narro León, L.A.; et al. Conservation and Use of Latin American Maize Diversity: Pillar of Nutrition Security and Cultural Heritage of Humanity. Agronomy 2021, 11, 172. [Google Scholar] [CrossRef]
- Cui, L.; Gao, R.; Dong, S.; Zhang, J.; Liu, P.; Zhang, H.; Meng, J.; Shi, D. Effects of ear shading on the anthocyanin contents and quality of kernels in various genotypes of maize. Aust. J. Crop Sci. 2012, 6, 704–710. [Google Scholar]
- Zilić, S.; Serpen, A.; Akıllıoğlu, G.; Gökmen, V.; Vančetović, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Croft, K.D. The chemistry and biological effects of flavonoids and phenolic acids. Ann. N. Y. Acad. Sci. 1998, 854, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kondo, T. Structure and Molecular Stacking of Anthocyanins—Flower Color Variation. Angew. Chem. Int. Ed. Engl. 1991, 30, 17–33. [Google Scholar] [CrossRef]
- Mazza, G.J.; Miniati, E. Anthocyanins in Fruits Vegetables & Grains; CRC-Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Abdel-Aal, E.-S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef]
- Jing, P.; Noriega, V.; Schwartz, S.J.; Giusti, M.M. Effects of growing conditions on purple corncob (Zea mays L.) anthocyanins. J. Agric. Food Chem. 2007, 55, 8625–8629. [Google Scholar] [CrossRef]
- de la Parra, C.; Saldivar, S.O.; Liu, R.H. Effect of processing on the phytochemical profiles and antioxidant activity of corn for production of masa, tortillas, and tortilla chips. J. Agric. Food Chem. 2007, 55, 4177–4183. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Aulis, F.; Hernandez-Vazquez, L.; Aguilar-Osorio, G.; Arrieta-Baez, D.; Navarro-Ocana, A. Extraction and Identification of Anthocyanins in Corn Cob and Corn Husk from Cacahuacintle Maize. J. Food Sci. 2019, 84, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Rosa, G.G.; Edna Alarcón, A.; Oscar García, B.; Jose, C.S.; Tania, A.Z. Chemical, Antioxidant, and Cytotoxic Properties of Native Blue Corn Extract. In Natural Products and Cancer Drug Discovery; Farid, A.B., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 67–77. [Google Scholar]
- Salinas Moreno, Y.; Sánchez, G.S.; Hernández, D.R.; Lobato, N.R. Characterization of Anthocyanin Extracts from Maize Kernels. J. Chromatogr. Sci. 2005, 43, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Ge-Zhang, S.; Song, M. Anthocyanins in metabolites of purple corn. Front. Plant Sci. 2023, 14, 1154535. [Google Scholar] [CrossRef] [PubMed]
- Carrera, E.J.; Cejudo-Bastante, M.J.; Hurtado, N.; Heredia, F.J.; González-Miret, M.L. Revalorization of Colombian purple corn Zea mays L. by-products using two-step column chromatography. Food Res. Int. 2023, 169, 112931. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhu, Y.; Wang, Y.; Wang, T.; Zhao, S.; Feng, K.; Li, L.; Wu, P. Molecular identification of phenylalanine ammonia lyase-encoding genes EfPALs and EfPAL2-interacting transcription factors in Euryale ferox. Front. Plant Sci. 2023, 14, 1114345. [Google Scholar] [CrossRef] [PubMed]
- Garzón, G.A. Las antocianinas como colorantes naturales y compuestos bioactivos: Revisión. Acta Biológica Colomb. 2008, 13, 27–36. [Google Scholar]
- Chatham, L.A.; Juvik, J.A. Linking anthocyanin diversity, hue, and genetics in purple corn. G3 Genes Genomes Genet. 2021, 11, jkaa062. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Lawson, D.; Xie, D.-Y. Metabolic engineering of anthocyanins in dark tobacco varieties. Physiol. Plant. 2017, 159, 2–12. [Google Scholar] [CrossRef]
- Shi, M.Z.; Xie, D.Y. Biosynthesis and metabolic engineering of anthocyanins in Arabidopsis thaliana. Recent. Pat. Biotechnol. 2014, 8, 47–60. [Google Scholar] [CrossRef]
- Nurkhasanah, A.; Fardad, T.; Carrera, C.; Setyaningsih, W.; Palma, M. Ultrasound-Assisted Anthocyanins Extraction from Pigmented Corn: Optimization Using Response Surface Methodology. Methods Protoc. 2023, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Vargas, F.; Paredes-Lopez, O. Natural Colorants for Food and Nutraceutical Uses; CRC Press: Boca Raton, Florida, USA, 2002. [Google Scholar] [CrossRef]
- McDougall, G.J.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red cabbage—Stability to simulated gastrointestinal digestion. Phytochemistry 2007, 68, 1285–1294. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, L.; Deng, Y.; Chi, J.; Zhang, Y.; Wei, Z.; Zhang, M. Phenolic content and antioxidant activity of eight representative sweet corn varieties grown in South China. Int. J. Food Prop. 2017, 20, 3043–3055. [Google Scholar] [CrossRef]
- Sultan, S.M.; Dikshit, N.; Mohanty, C.S.; Rout, P.K.; Raina, S.K. Biochemical evaluation of dent corn (Zea mays L.) genotypes cultivated under rainfed conditions in the hills of north western Indian Himalayan state of Jammu and Kashmir. J. Appl. Nat. Sci. 2018, 10, 196–201. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Cadenas, E. Mitochondrial free radical production and cell signaling. Mol. Asp. Med. 2004, 25, 17–26. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Zolla, G.; Afaray-Carazas, A.; Vera-Vega, M.; Huanuqueño, H.; Begazo-Gutiérrez, H.; Chirinos, R.; Pedreschi, R.; Shetty, K. Integrated metabolite analysis and health-relevant in vitro functionality of white, red, and orange maize (Zea mays L.) from the Peruvian Andean race Cabanita at different maturity stages. Front. Nutr. 2023, 10, 1132228. [Google Scholar] [CrossRef]
- Urias-Lugo, D.A.; Heredia, J.B.; Muy-Rangel, M.D.; Valdez-Torres, J.B.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Anthocyanins and Phenolic Acids of Hybrid and Native Blue Maize (Zea mays L.) Extracts and Their Antiproliferative Activity in Mammary (MCF7), Liver (HepG2), Colon (Caco2 and HT29) and Prostate (PC3) Cancer Cells. Plant Foods Hum. Nutr. 2015, 70, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Duangpapeng, P.; Lertrat, K.; Lomthaisong, K.; Paul Scott, M.; Suriharn, B. Variability in Anthocyanins, Phenolic Compounds and Antioxidant Capacity in the Tassels of Collected Waxy Corn Germplasm. Agronomy 2019, 9, 158. [Google Scholar] [CrossRef]
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Ranilla, L.G.; Huamán-Alvino, C.; Flores-Báez, O.; Aquino-Méndez, E.M.; Chirinos, R.; Campos, D.; Sevilla, R.; Fuentealba, C.; Pedreschi, R.; Sarkar, D.; et al. Evaluation of phenolic antioxidant-linked in vitro bioactivity of Peruvian corn (Zea mays L.) diversity targeting for potential management of hyperglycemia and obesity. J. Food Sci. Technol. 2019, 56, 2909–2924. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vital, D.A.; Gonzalez de Mejia, E. Anthocyanins from purple corn activate free fatty acid-receptor 1 and glucokinase enhancing in vitro insulin secretion and hepatic glucose uptake. PLoS ONE 2018, 13, e0200449. [Google Scholar] [CrossRef] [PubMed]
- Zaa, C.A.; Marcelo, Á.J.; An, Z.; Medina-Franco, J.L.; Velasco-Velázquez, M.A. Anthocyanins: Molecular Aspects on Their Neuroprotective Activity. Biomolecules 2023, 13, 1598. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, J.; Wang, W.; Lyu, L.; Wu, W.; Li, W. The Extraction and High Antiproliferative Effect of Anthocyanin from Gardenblue Blueberry. Molecules 2023, 28, 2850. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Leopold, L.; Rugină, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and Antioxidant Properties of Anthocyanin Rich Extracts from Blueberry and Blackcurrant Juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The omega-6/omega-3 fatty acid ratio: Health implications. OCL 2010, 17, 267–275. [Google Scholar] [CrossRef]
- Melo, R.B.; de Barros Silva, P.G.; Oriá, R.B.; Melo, J.U.d.S.; da Silva Martins, C.; Cunha, A.M.; Vasconcelos, P.R.L. Anti-inflammatory effect of a fatty acid mixture with high ω-9:ω-6 ratio and low ω-6:ω-3 ratio on rats submitted to dental extraction. Arch. Oral. Biol. 2017, 74, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Andjelkovic, V.; Vukadinović, J.; Srebric, M.; Mladenović-Drinić, S. Pigmented maize—A potential source of β-carotene and α-tocopherol. J. Eng. Process. Manag. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Hossain, A.; Jayadeep, A. Determination of tocopherol and tocotrienol contents in maize by in vitro digestion and chemical methods. J. Cereal Sci. 2018, 83, 90–95. [Google Scholar] [CrossRef]
- Zhai, J.; Zhu, Y.; Wu, Y.; Li, N.; Cao, Y.; Guo, Y.; Xu, L. Antioxidant Effect of Tyr-Ala Extracted from Zein on INS-1 Cells and Type 2 Diabetes High-Fat-Diet-Induced Mice. Antioxidants 2022, 11, 1111. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R. Zein and zein -based nano-materials for food and nutrition applications: A review. Trends Food Sci. Technol. 2018, 79, 184–197. [Google Scholar] [CrossRef]
- Salguero, M.V.; Al-Obaide, M.A.I.; Singh, R.; Siepmann, T.; Vasylyeva, T.L. Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp. Ther. Med. 2019, 18, 3461–3469. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, S.; Shin, S.J.; Park, Y.H.; Nam, Y.; Kim, C.W.; Lee, K.W.; Kim, S.M.; Jung, I.D.; Yang, H.D.; et al. Gram-negative bacteria and their lipopolysaccharides in Alzheimer’s disease: Pathologic roles and therapeutic implications. Transl. Neurodegener. 2021, 10, 49. [Google Scholar] [CrossRef]
- Haș, I.M.; Teleky, B.-E.; Szabo, K.; Simon, E.; Ranga, F.; Diaconeasa, Z.M.; Purza, A.L.; Vodnar, D.-C.; Tit, D.M.; Nițescu, M. Bioactive Potential of Elderberry (Sambucus nigra L.): Antioxidant, Antimicrobial Activity, Bioaccessibility and Prebiotic Potential. Molecules 2023, 28, 3099. [Google Scholar] [CrossRef]
- Liu, J.; Hao, W.; He, Z.; Kwek, E.; Zhu, H.; Ma, N.; Ma, K.Y.; Chen, Z.-Y. Blueberry and cranberry anthocyanin extracts reduce bodyweight and modulate gut microbiota in C57BL/6 J mice fed with a high-fat diet. Eur. J. Nutr. 2021, 60, 2735–2746. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, M.; Sun, X.; Liu, X.; Choueiry, F.; Xu, R.; Shi, H.; Zhu, J. Black raspberry extract shifted gut microbe diversity and their metabolic landscape in a human colonic model. J. Chromatogr. B 2022, 1188, 123027. [Google Scholar] [CrossRef]
- Wu, T.; Chu, X.; Cheng, Y.; Tang, S.; Zogona, D.; Pan, S.; Xu, X. Modulation of Gut Microbiota by Lactobacillus casei Fermented Raspberry Juice In Vitro and In Vivo. Foods 2021, 10, 3055. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lu, H.F.; Chen, J.C.; Huang, H.C.; Chen, Y.H.; Su, Y.S.; Tung, C.Y.; Huang, C. Purple-leaf tea (Camellia sinensis L.) ameliorates high-fat diet induced obesity and metabolic disorder through the modulation of the gut microbiota in mice. BMC Complement. Med. Ther. 2020, 20, 376. [Google Scholar] [CrossRef]
- Morissette, A.; Kropp, C.; Songpadith, J.-P.; Moreira, R.J.; Costa, J.; Mariné-Casadó, R.; Pilon, G.; Varin, T.V.; Dudonné, S.; Boutekrabt, L.; et al. Blueberry proanthocyanidins and anthocyanins improve metabolic health through a gut microbiota-dependent mechanism in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E965–E980. [Google Scholar] [CrossRef]
- Loubet Filho, P.S.; Baseggio, A.M.; Vuolo, M.M.; Reguengo, L.M.; Telles Biasoto, A.C.; Correa, L.C.; Junior, S.B.; Alves Cagnon, V.H.; Betim Cazarin, C.B.; Maróstica Júnior, M.R. Gut microbiota modulation by jabuticaba peel and its effect on glucose metabolism via inflammatory signaling. Curr. Res. Food Sci. 2022, 5, 382–391. [Google Scholar] [CrossRef]
- Song, H.; Shen, X.; Deng, R.; Chu, Q.; Zheng, X. Pomegranate peel anthocyanins prevent diet-induced obesity and insulin resistance in association with modulation of the gut microbiota in mice. Eur. J. Nutr. 2022, 61, 1837–1847. [Google Scholar] [CrossRef]
- Agrizzi Verediano, T.; Agarwal, N.; Stampini Duarte Martino, H.; Kolba, N.; Grancieri, M.; Dias Paes, M.C.; Tako, E. Effect of Black Corn Anthocyanin-Rich Extract (Zea mays L.) on Cecal Microbial Populations In Vivo (Gallus gallus). Nutrients 2022, 14, 4679. [Google Scholar] [CrossRef] [PubMed]
- Hester, S.N.; Mastaloudis, A.; Gray, R.; Antony, J.M.; Evans, M.; Wood, S.M. Efficacy of an Anthocyanin and Prebiotic Blend on Intestinal Environment in Obese Male and Female Subjects. J. Nutr. Metab. 2018, 2018, 7497260. [Google Scholar] [CrossRef]
- Gao, M.; Peng, X.; Tang, J.; Deng, J.; Wang, F.; Zhang, Y.; Zhao, P.; Kan, H.; Liu, Y. Anti-Inflammatory Effects of Camellia fascicularis Polyphenols via Attenuation of NF-κB and MAPK Pathways in LPS-Induced THP-1 Macrophages. J. Inflamm. Res. 2022, 15, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Cox, A.D.; Chang, H.; Kennett, M.; Rosa, C.; Chopra, S.; Li, S.; Reddivari, L. Maize near-isogenic lines with enhanced flavonoids alleviated dextran sodium sulfate-induced murine colitis via modulation of the gut microbiota. Food Funct. 2023, 14, 9606–9616. [Google Scholar] [CrossRef]
- Marques, C.; Fernandes, I.; Meireles, M.; Faria, A.; Spencer, J.P.E.; Mateus, N.; Calhau, C. Gut microbiota modulation accounts for the neuroprotective properties of anthocyanins. Sci. Rep. 2018, 8, 11341. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, B.; Zhong, C.; Guo, J.; Zhang, L.; Mu, T.; Zhang, Q.; Bi, X. Chemoprevention of colorectal cancer by black raspberry anthocyanins involved the modulation of gut microbiota and SFRP2 demethylation. Carcinogenesis 2018, 39, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Torres-Maravilla, E.; Boucard, A.-S.; Al Azzaz, J.; Gontier, S.; Kulakauskas, S.; Langella, P.; Bermúdez-Humarán, L.G. Assessment of the safety of Levilactobacillus brevis CNCM I-5321, a probiotic candidate strain isolated from pulque with anti-proliferative activities. Benef. Microbes 2023, 14, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Modrackova, N.; Vlkova, E.; Tejnecky, V.; Schwab, C.; Neuzil-Bunesova, V. Bifidobacterium β-Glucosidase Activity and Fermentation of Dietary Plant Glucosides Is Species and Strain Specific. Microorganisms 2020, 8, 839. [Google Scholar] [CrossRef] [PubMed]
- Sáez, G.D.; Flomenbaum, L.; Zárate, G. Lactic Acid Bacteria from Argentinean Fermented Foods: Isolation and Characterization for their Potential Use as Starters for Fermentation of Vegetables. Food Technol. Biotechnol. 2018, 56, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Parmar, I.; Neir, S.V. Biotransformation of Cranberry Proanthocyanidins to Probiotic Metabolites by Lactobacillus rhamnosus Enhances Their Anticancer Activity in HepG2 Cells In Vitro. Oxidative Med. Cell. Longev. 2019, 2019, 4750795. [Google Scholar] [CrossRef]
- Rubio-Castillo, Á.E.; Zamora-Gasga, V.M.; Sánchez-Burgos, J.A.; Ruiz-Valdiviezo, V.M.; Montalvo-González, E.; Velázquez-Estrada, R.M.; González-Córdova, A.F.; Sáyago-Ayerdi, S.G. Gut metabolites produced during in vitro colonic fermentation of the indigestible fraction of a maize-based traditional Mexican fermented beverage, Tejuino. Food Chem. Mol. Sci. 2022, 5, 100150. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.S.; Ramos, C.L.; González-Avila, M.; Gschaedler, A.; Arrizon, J.; Schwan, R.F.; Dias, D.R. Probiotic properties of Weissella cibaria and Leuconostoc citreum isolated from tejuino—A typical Mexican beverage. LWT 2017, 86, 227–232. [Google Scholar] [CrossRef]
- Yañez-Ñeco, C.V.; Rodriguez-Colinas, B.; Amaya-Delgado, L.; Ballesteros, A.O.; Gschaedler, A.; Plou, F.J.; Arrizon, J. Galactooligosaccharide Production from Pantoea anthophila Strains Isolated from “Tejuino”, a Mexican Traditional Fermented Beverage. Catalysts 2017, 7, 242. [Google Scholar] [CrossRef]
- López-Sánchez, R.; Hernández-Oaxaca, D.; Escobar-Zepeda, A.; Ramos Cerrillo, B.; López-Munguía, A.; Segovia, L. Analysing the dynamics of the bacterial community in pozol, a Mexican fermented corn dough. Microbiology 2023, 169, 001355. [Google Scholar] [CrossRef]
- Rizo, J.; Guillén, D.; Díaz-Ruiz, G.; Wacher, C.; Encarnación, S.; Sánchez, S.; Rodríguez-Sanoja, R. Metaproteomic Insights Into the Microbial Community in Pozol. Front. Nutr. 2021, 8, 714814. [Google Scholar] [CrossRef]
- Méndez-Albores, J.A.; Arámbula-Villa, G.; Preciado-Ortíz, R.E.; Moreno-Martínez, E. Aflatoxins in pozol, a nixtamalized, maize-based food. Int. J. Food Microbiol. 2004, 94, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Escalante, A.; Wacher, C.; Farrés, A. Lactic acid bacterial diversity in the traditional Mexican fermented dough pozol as determined by 16S rDNA sequence analysis. Int. J. Food Microbiol. 2001, 64, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Armendáriz, B.; Cardoso-Ugarte, G.A. Traditional fermented beverages in Mexico: Biotechnological, nutritional, and functional approaches. Food Res. Int. 2020, 136, 109307. [Google Scholar] [CrossRef] [PubMed]
- Wacher, C.; Cañas, A.; Cook, P.E.; Barzana, E.; Owens, J.D. Sources of microorganisms in pozol, a traditional Mexican fermented maize dough. World J. Microbiol. Biotechnol. 1993, 9, 269–274. [Google Scholar] [CrossRef]
- Phister, T.G.; O’Sullivan, D.J.; McKay, L.L. Identification of Bacilysin, Chlorotetaine, and Iturin A Produced by Bacillus sp. Strain CS93 Isolated from Pozol, a Mexican Fermented Maize Dough. Appl. Environ. Microbiol. 2004, 70, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Ampe, F.; ben Omar, N.; Guyot, J.P. Culture-independent quantification of physiologically-active microbial groups in fermented foods using rRNA-targeted oligonucleotide probes: Application to pozol, a Mexican lactic acid fermented maize dough. J. Appl. Microbiol. 1999, 87, 131–140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bañuelos-Pineda, J.; Gómez-Rodiles Carmen, C.; Cuéllar- José, R.; Aguirre López Luis, O. The Maize Contribution in the Human Health. In Corn; Amanullah, Fahad, S., Eds.; IntechOpen: Rijeka, Croatia, 2018; Chapter 3. [Google Scholar]
- Hesseltine, C.W.; Wang, H.L. Indigenous Fermented Food of Non-Western Origin (Mycologia Memoir); J. Cramer: Lincoln, UK, 1986; Volume 11, 351p. [Google Scholar]
- Väkeväinen, K.; Hernández, J.; Simontaival, A.-I.; Severiano-Pérez, P.; Díaz-Ruiz, G.; von Wright, A.; Wacher-Rodarte, C.; Plumed-Ferrer, C. Effect of different starter cultures on the sensory properties and microbiological quality of Atole agrio, a fermented maize product. Food Control 2020, 109, 106907. [Google Scholar] [CrossRef]
- Robledo-Márquez, K.; Ramírez, V.; González-Córdova, A.F.; Ramírez-Rodríguez, Y.; García-Ortega, L.; Trujillo, J. Research opportunities: Traditional fermented beverages in Mexico. Cultural, microbiological, chemical, and functional aspects. Food Res. Int. 2021, 147, 110482. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Salinas, P.A.; Zavala-García, F.; Urías-Orona, V.; Muy-Rangel, D.; Heredia, J.B.; Niño-Medina, G. Chromatic, Nutritional and Nutraceutical Properties of Pigmented Native Maize (Zea mays L.) Genotypes from the Northeast of Mexico. Arab. J. Sci. Eng. 2020, 45, 95–112. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Almonaitytė, A.; Jensen, I.-J.; Kurek, M.; Lerfall, J. Alginate microbeads incorporated with anthocyanins from purple corn (Zea mays L.) using electrostatic extrusion: Microencapsulation optimization, characterization, and stability studies. Int. J. Biol. Macromol. 2023, 246, 125684. [Google Scholar] [CrossRef]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef] [PubMed]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Lao, F.; Giusti, M.M. The effect of pigment matrix, temperature and amount of carrier on the yield and final color properties of spray dried purple corn (Zea mays L.) cob anthocyanin powders. Food Chem. 2017, 227, 376–382. [Google Scholar] [CrossRef]
- Haggard, S.; Luna-Vital, D.; West, L.; Juvik, J.A.; Chatham, L.; Paulsmeyer, M.; Gonzalez de Mejia, E. Comparison of chemical, color stability, and phenolic composition from pericarp of nine colored corn unique varieties in a beverage model. Food Res. Int. 2018, 105, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Chatham, L.A.; Howard, J.E.; Juvik, J.A. A natural colorant system from corn: Flavone-anthocyanin copigmentation for altered hues and improved shelf life. Food Chem. 2020, 310, 125734. [Google Scholar] [CrossRef]
- Xiang, Y.; Chen, X.; Sun, H.; Zhan, Q.; Zhong, L.; Hu, Q.; Zhao, L. The critical roles of α-amylase and amyloglucosidase in improving the quality of black waxy corn beverages: Special attentions to the color and flavor. J. Cereal Sci. 2023, 110, 103625. [Google Scholar] [CrossRef]
- Ren, S.; Giusti, M.M. The effect of whey protein concentration and preheating temperature on the color and stability of purple corn, grape and black carrot anthocyanins in the presence of ascorbic acid. Food Res. Int. 2021, 144, 110350. [Google Scholar] [CrossRef]
- Cortés, G.A.; Salinas, M.Y.; Martín-Martinez, E.S.; Martínez-Bustos, F. Stability of anthocyanins of blue maize (Zea mays L.) after nixtamalization of seperated pericarp-germ tip cap and endosperm fractions. J. Cereal Sci. 2006, 43, 57–62. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, X.; Huang, G.; Xin, X.; Attaribo, T.; Zhang, Y.; Zhang, N.; Gui, Z. Enhancement of Stability and Antioxidant Activity of Mulberry Anthocyanins Through Succinic Acid Acylation. Food Technol. Biotechnol. 2022, 60, 321–329. [Google Scholar] [CrossRef]
- Cai, D.; Li, X.; Chen, J.; Jiang, X.; Ma, X.; Sun, J.; Tian, L.; Vidyarthi, S.K.; Xu, J.; Pan, Z.; et al. A comprehensive review on innovative and advanced stabilization approaches of anthocyanin by modifying structure and controlling environmental factors. Food Chem. 2022, 366, 130611. [Google Scholar] [CrossRef]
- Jokioja, J.; Yang, B.; Linderborg, K.M. Acylated anthocyanins: A review on their bioavailability and effects on postprandial carbohydrate metabolism and inflammation. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5570–5615. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhou, S.; Zhao, G.; Ye, F. Destabilisation and stabilisation of anthocyanins in purple-fleshed sweet potatoes: A review. Trends Food Sci. Technol. 2021, 116, 1141–1154. [Google Scholar] [CrossRef]
- Wu, Y.; Han, Y.; Tao, Y.; Li, D.; Xie, G.; Show, P.L.; Lee, S.Y. In vitro gastrointestinal digestion and fecal fermentation reveal the effect of different encapsulation materials on the release, degradation and modulation of gut microbiota of blueberry anthocyanin extract. Food Res. Int. 2020, 132, 109098. [Google Scholar] [CrossRef] [PubMed]
- Thibado, S.P.; Thornthwaite, J.T.; Ballard, T.K.; Goodman, B.T. Anticancer effects of Bilberry anthocyanins compared with NutraNanoSphere encapsulated Bilberry anthocyanins. Mol. Clin. Oncol. 2018, 8, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J.; León, I.E.; Ponce, A.G.; Alvarez, V.A. Active and pH-Sensitive Nanopackaging Based on Polymeric Anthocyanin/Natural or Organo-Modified Montmorillonite Blends: Characterization and Assessment of Cytotoxicity. Polymers 2022, 14, 4881. [Google Scholar] [CrossRef] [PubMed]
- Oancea, A.-M.; Hasan, M.; Vasile, A.M.; Barbu, V.; Enachi, E.; Bahrim, G.; Râpeanu, G.; Silvi, S.; Stănciuc, N. Functional evaluation of microencapsulated anthocyanins from sour cherries skins extract in whey proteins isolate. LWT 2018, 95, 129–134. [Google Scholar] [CrossRef]
- Xiao, Z.; Xia, J.; Zhao, Q.; Niu, Y.; Zhao, D. Maltodextrin as wall material for microcapsules: A review. Carbohydr. Polym. 2022, 298, 120113. [Google Scholar] [CrossRef] [PubMed]
- Ćujić Nikolić, N.; Žilić, S.; Simić, M.; Nikolić, V.; Živković, J.; Marković, S.; Šavikin, K. Microencapsulates of Blue Maize Polyphenolics as a Promising Ingredient in the Food and Pharmaceutical Industry: Characterization, Antioxidant Properties, and In Vitro-Simulated Digestion. Foods 2023, 12, 1870. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Fuentes, B.; de Jesús-José, E.; Cabrera-Hidalgo, A.d.J.; Sandoval-Castilla, O.; Espinosa-Solares, T.; González-Reza, R.M.; Zambrano-Zaragoza, M.L.; Liceaga, A.M.; Aguilar-Toalá, J.E. Plant-Based Fermented Beverages: Nutritional Composition, Sensory Properties, and Health Benefits. Foods 2024, 13, 844. [Google Scholar] [CrossRef]
- Penha, C.B.; Santos, V.D.P.; Speranza, P.; Kurozawa, L.E. Plant-based beverages: Ecofriendly technologies in the production process. Innov. Food Sci. Emerg. Technol. 2021, 72, 102760. [Google Scholar] [CrossRef]
- Bocker, R.; Silva, E.K. Pulsed electric field assisted extraction of natural food pigments and colorings from plant matrices. Food Chem. X 2022, 15, 100398. [Google Scholar] [CrossRef] [PubMed]
- Daji, G.A.; Green, E.; Abrahams, A.; Oyedeji, A.B.; Masenya, K.; Kondiah, K.; Adebo, O.A. Physicochemical Properties and Bacterial Community Profiling of Optimal Mahewu (A Fermented Food Product) Prepared Using White and Yellow Maize with Different Inocula. Foods 2022, 11, 3171. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Yana, D.; Aguilar-Morón, B.; Pezo-Torres, N.; Shetty, K.; Ranilla, L.G. Ancestral Peruvian ethnic fermented beverage “Chicha” based on purple corn (Zea mays L.): Unraveling the health-relevant functional benefits. J. Ethn. Foods 2020, 7, 35. [Google Scholar] [CrossRef]
- Shi, S.; Li, S.; Li, W.; Xu, H. Corn Silk Tea for Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. 2019, 2019, 2915498. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Linares, C.; Álvarez-Ríos, G.D.; Figueredo-Urbina, C.J.; Islas, L.A.; Lappe-Oliveras, P.; Nabhan, G.P.; Torres-García, I.; Vallejo, M.; Casas, A. Traditional Fermented Beverages of Mexico: A Biocultural Unseen Foodscape. Foods 2021, 10, 2390. [Google Scholar] [CrossRef] [PubMed]
- Wolfang-Engel, D.; Robert-Brocker, E. Orally Administered Preparations Containing Anthocyanins Made from Blue Maize. Spindles. Patent EP3417870A1, 26 December 2018. [Google Scholar]
- Neder-Suárez, D.; Lardizabal-Gutiérrez, D.; Zazueta-Morales, J.d.J.; Meléndez-Pizarro, C.O.; Delgado-Nieblas, C.I.; Ramírez Wong, B.; Gutiérrez-Méndez, N.; Hernández-Ochoa, L.R.; Quintero-Ramos, A. Anthocyanins and Functional Compounds Change in a Third-Generation Snacks Prepared Using Extruded Blue Maize, Black Bean, and Chard: An Optimization. Antioxidants 2021, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Chatham, L.A.; Paulsmeyer, M.; Juvik, J.A. Prospects for economical natural colorants: Insights from maize. Theor. Appl. Genet. 2019, 132, 2927–2946. [Google Scholar] [CrossRef] [PubMed]
- Paulsmeyer, M.N.; Juvik, J.A. R3-MYB repressor Mybr97 is a candidate gene associated with the Anthocyanin3 locus and enhanced anthocyanin accumulation in maize. Theor. Appl. Genet. 2023, 136, 55. [Google Scholar] [CrossRef]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Secur. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- Rosales, A.; Molina-Macedo, A.; Leyva, M.; San Vicente, F.; Palacios-Rojas, N. Fresh/High-Zinc Maize: A Promising Solution for Alleviating Zinc Deficiency through Significant Micronutrient Accumulation. Foods 2023, 12, 2757. [Google Scholar] [CrossRef]
- Aluru, M.; Xu, Y.; Guo, R.; Wang, Z.; Li, S.; White, W.; Wang, K.; Rodermel, S. Generation of transgenic maize with enhanced provitamin A content. J. Exp. Bot. 2008, 59, 3551–3562. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Putterill, J.; Dare, A.P.; Plunkett, B.J.; Cooney, J.; Peng, Y.; Souleyre, E.J.F.; Albert, N.W.; Espley, R.V.; Günther, C.S. Two genes, ANS and UFGT2, from Vaccinium spp. are key steps for modulating anthocyanin production. Front. Plant Sci. 2023, 14, 1082246. [Google Scholar] [CrossRef] [PubMed]
- Balcerowicz, M. Phytochrome-interacting factors at the interface of light and temperature signalling. Physiol. Plant 2020, 169, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Abna, F.; Avin, F.; Haron, N. Expression Level of Sh2 and Bt2 Genes in Some Advanced Corn Lines under Tropical Environment. J. Anim. Plant Sci. 2020, 30, 493–501. [Google Scholar] [CrossRef]
- Awe, S.; Aransiola, D.M.; Irondi, E.A. Microbial succession and anthocyanin concentration during sorghum fermentation. Meas. Food 2023, 12, 100109. [Google Scholar] [CrossRef]
- Singh, A.; Singh, S.; Kansal, S.K.; Garg, M.; Krishania, M. Production and characterization of anthocyanin-rich beer from black wheat by an efficient isolate Saccharomyces cerevisiae CMS12. Sci. Rep. 2023, 13, 5863. [Google Scholar] [CrossRef] [PubMed]
- Kellingray, L.; Le Gall, G.; Doleman, J.F.; Narbad, A.; Mithen, R.F. Effects of in vitro metabolism of a broccoli leachate, glucosinolates and S-methylcysteine sulphoxide on the human faecal microbiome. Eur. J. Nutr. 2021, 60, 2141–2154. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Mu, C.-l.; Farzi, A.; Zhu, W.-y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).