Fortification of White Wines with Antioxidants and Se: Impacts on Browning Development and Phenolic Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Samples
2.3. Accelerated Browning Test
2.4. Determination of Total Phenols (TP), Antioxidant Ability (AA), Free Sulfhydryl Groups (SH), High Performance Liquid Chromatography (HPLC)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Accelerated Browning Test
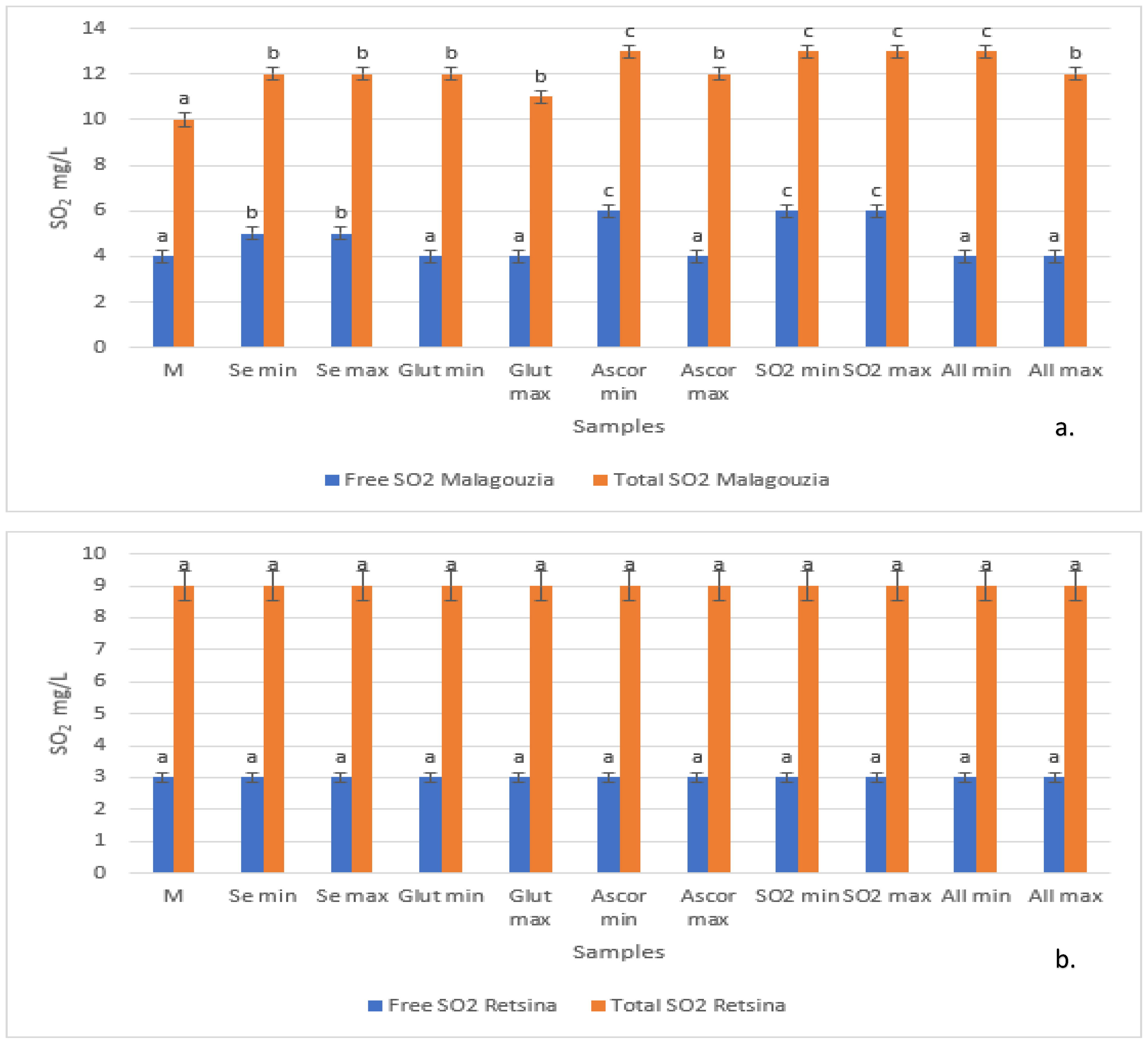
3.2. SO2 Content (Total and Free)
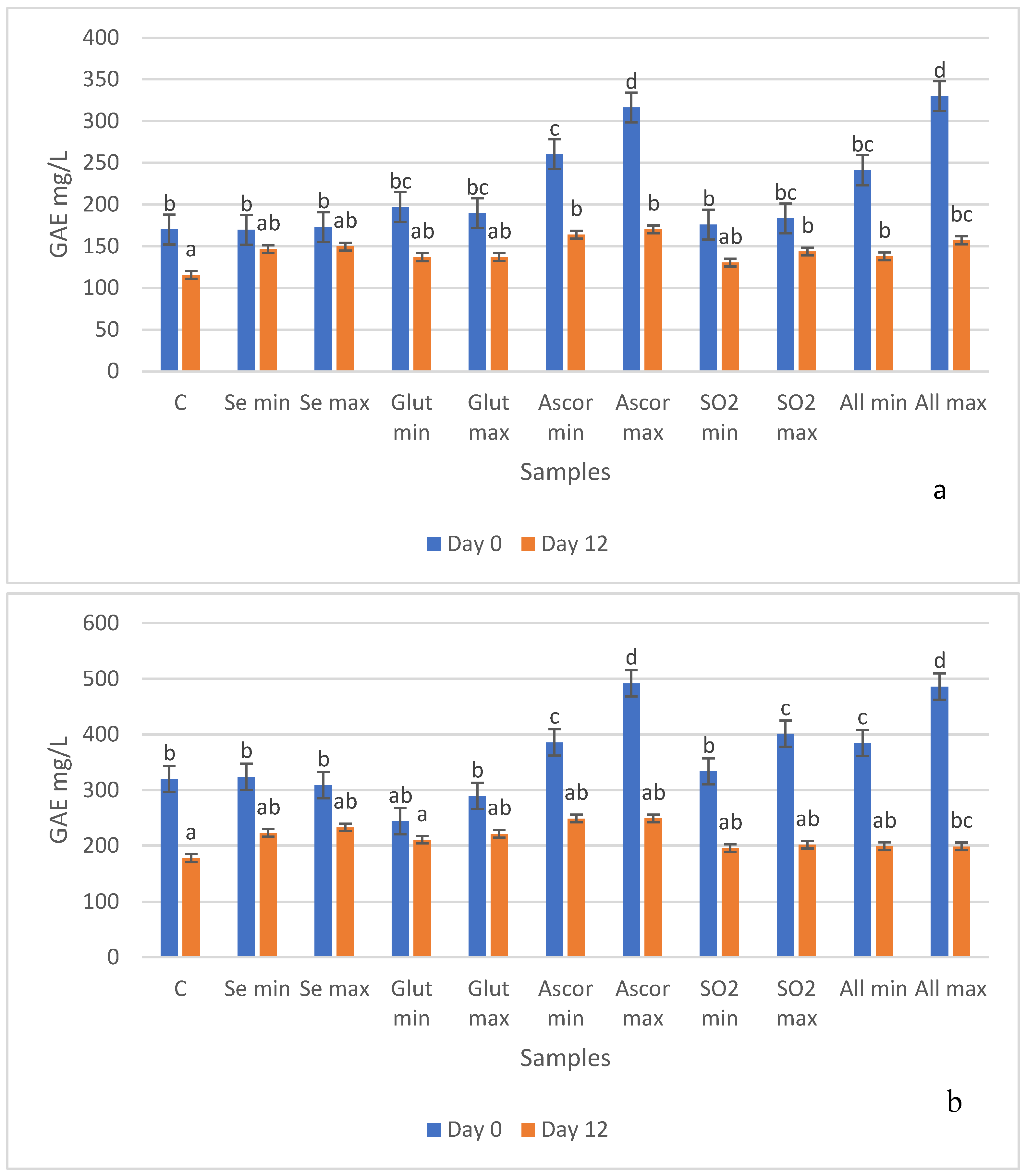
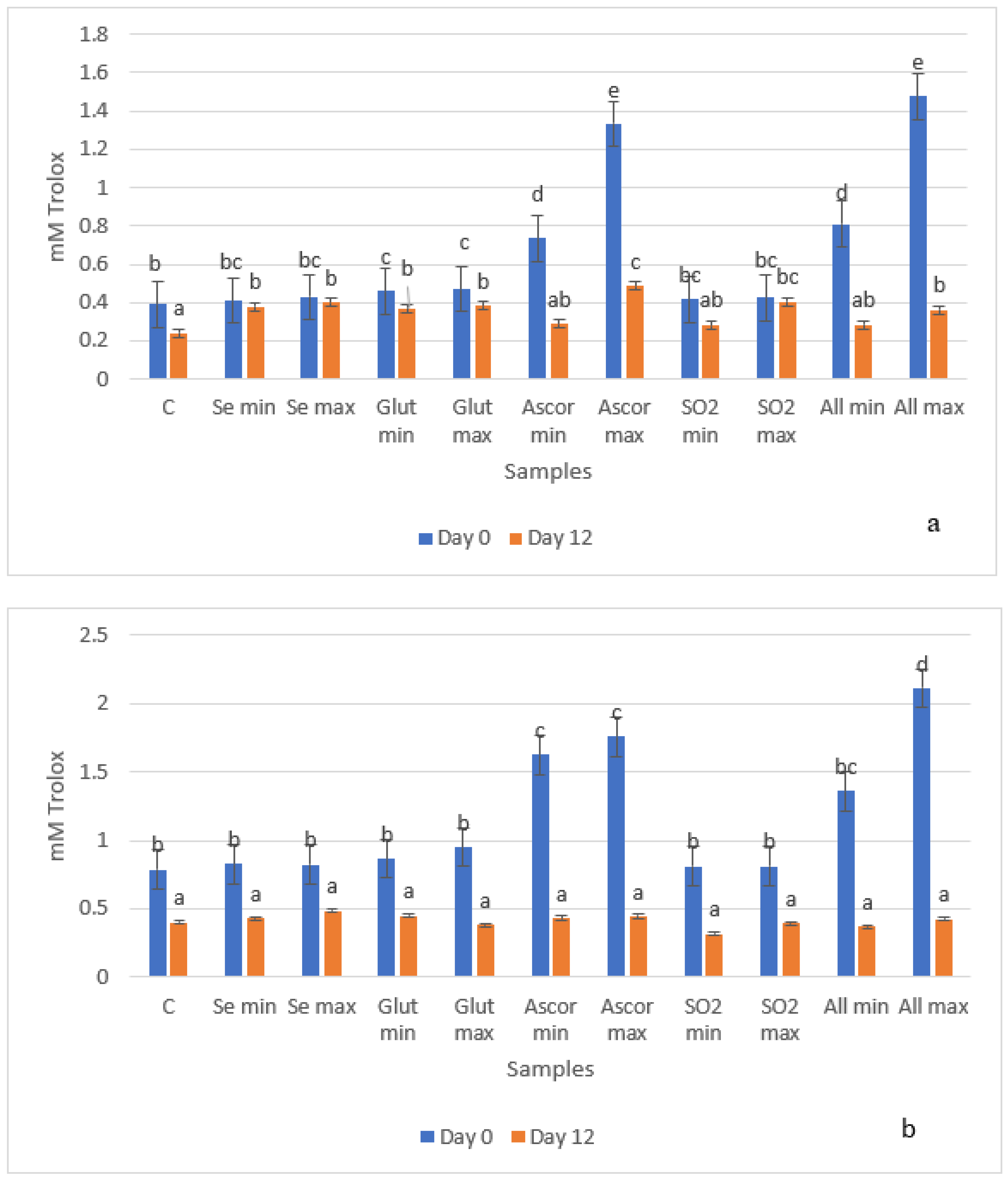
3.3. Antioxidant Activity (Folin and DPPH Methods)
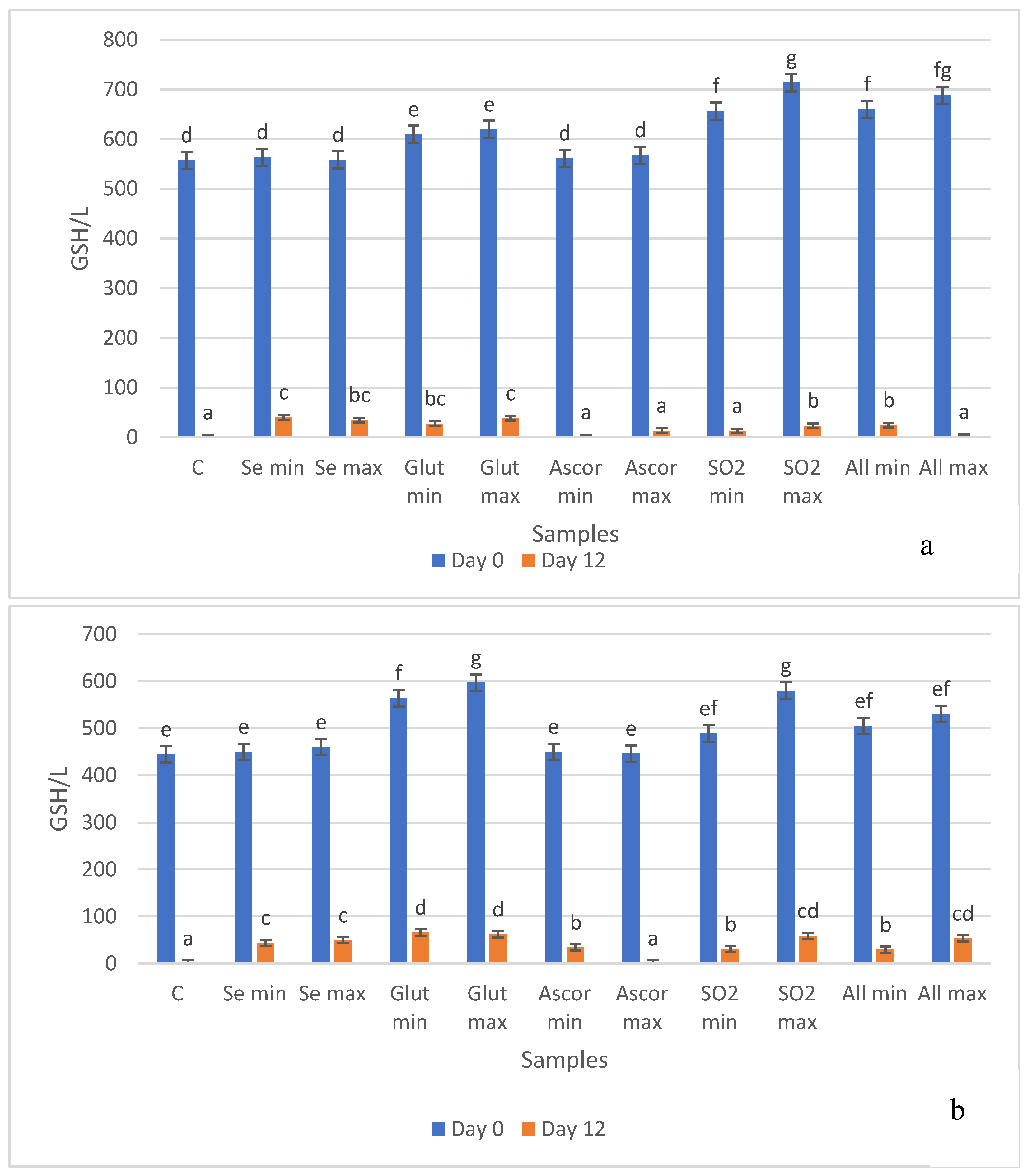
3.4. Free -SH Groups
3.5. Individual Phenolic Compounds Determined by HPLC (Flavanols and Hydroxycinnamic Acids)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Voltea, S.; Karabagias, K.I.; Roussis, I.G. Use of Fe (II) and H2O2 along with Heating for the Estimation of the Browning Susceptibility of White Wine. J. Appl. Sci. 2022, 12, 4422. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Siuta, M. The Impact of Oxygen at Various Stages of Vinification on the Chemical Composition and the Antioxidant and Sensory Properties of White and Red Wines. Int. J. Food Sci. 2020, 2020, 7902974. [Google Scholar] [CrossRef]
- Escudero, A.; Hernandez-Orte, P.; Cacho, J.E.; Ferreira, V. Clues about the role of 3-(methylthio)propionaldehyde as a character impact odorant of some oxidized wines. J. Agric. Food Chem. 2000, 48, 4268–4272. [Google Scholar] [CrossRef]
- Danilewicz, J.C. Review of reaction mechanisms of oxygen and proposed intermediate reduction products in wine: Central role of iron and copper. Am. J. Enol. Vitic. 2003, 54, 73–85. [Google Scholar] [CrossRef]
- Tan, J.; Vincken, J.P.; Zadelhoff, A.; Hilgers, R.; Lin, Z.; Bruijn, W.J.C. Presence of free gallic acid and gallate moieties reduces auto-oxidative browning of epicatechin (EC) and epicatechin gallate (ECg). J. Food Chem. 2023, 425, 136446. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, A.; Wang, H. Mechanisms of oxidative browning of wine. J. Agric. Food Chem. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Danilewicz, J.C. Interaction of sulfur dioxide, polyphenols, and oxygen in a wine-model system: Central role of iron and copper. Am. J. Enol. Vitic. 2007, 58, 53–60. [Google Scholar] [CrossRef]
- Es-Safi, N.E.; Cheynier, V.; Moutounet, M. Role of aldehydic derivatives in the condensation of phenolic compounds with emphasis on the sensorial properties of fruit-derived foods. J. Agric. Food Chem. 2002, 50, 5571–5585. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, R.; Aliano, M.J.; Cantos, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Kutz, M. Handbook of Environmental Degradation of Materials, 2nd ed.; William Andrew-Elsevier: Oxford, UK, 2012. [Google Scholar] [CrossRef]
- Godden, P.W.; Francis, I.L.; Field, J.B.F.; Gishen, M.; Coulter, A.D.; Valente, P.J.; Hoj, P.B.; Robinson, E.M.C. An evaluation of the technical performance of wine bottle closures. In Proceedings of the 11th Wine Industry Technical Conference, Adelaide, Australia, 7–11 October 2001; Blair, R., Williams, P., Hoj, P., Eds.; AWITC: Glen Osmond, Australia, 2001; pp. 44–52. [Google Scholar] [CrossRef]
- Lemos, W.J.F.; Binati, R.L.; Bersani, N.; Torriani, S. Investigating the glutathione accumulation by non-conventional wine yeasts in optimized growth conditions and multi-starter fermentations. J. Food Sci. Technol. 2021, 142, 110990. [Google Scholar] [CrossRef]
- Antoce, O.A. Oenology: Chemistry and Sensory Analysis; Universitaria Publishing House: Craiova, Romania, 2007. [Google Scholar] [CrossRef]
- Echave, J.; Barral, M.; Fraga, M.; Prieto, M.A.; Simal, J. Bottle Aging and Storage of Wines: A Review. Molecules 2021, 26, 713. [Google Scholar] [CrossRef]
- Witkowska, A.M.; Zujko, M.E.; Borawska, M.H.; Socha, K. Antioxidant Properties and Selenium Content of Wines. Pol. J. Environ. Stud. 2006, 15, 208–211. [Google Scholar]
- Battin, E.E.; Brumaghim, J.L. Antioxidant Activity of Sulfur and Selenium: A Review of Reactive Oxygen Species Scavenging, Glutathione Peroxidase and Metal-Binding Antioxidant Mechanisms. Cell Biochem. Biophys. 2009, 55, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Perrone, D.; Monteiro, M.; Nunes, J.C. The Chemistry of Selenium. R. Soc. Chem. 2015, 9, 11–12. [Google Scholar] [CrossRef]
- Tinggi, U. Essentiality and toxicity of selenium and its status in Australia: A review. Toxicol. Lett. 2003, 137, 103–110. [Google Scholar] [CrossRef]
- Adadi, P.; Barakova, N.V.; Muravyov, K.Y.; Krivoshapkina, E.F. Designing selenium functional foods and beverages: A review. Food Res. Int. 2019, 120, 708–725. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Bei, Y. Thermodynamics and Dynamic Kinetics of the Oxidation of Selenomethionine to Methionine Selenoxide: A DFT Study. Sci. Rev. 2000 LTD 2010, 35, 417–422. [Google Scholar] [CrossRef]
- Rahmanto, A.D.; Davies, M.J. Selenium-containing Amino Acids as Direct and Indirect Antioxidants. Life 2012, 64, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Perez-Corona, M.T.; Sanchez-Martinez, M.; Valderrama, M.J.; Rodriguez, M.E.; Camara, C.; Madrid, Y. Selenium biotransformation by Saccharomyces cerevisiae and Saccharomyces bayanus during white wine manufacture: Laboratory-scale experiments. J. Food Chem. 2011, 124, 1050–1055. [Google Scholar] [CrossRef]
- Ferreira-Lima, N.E.; Burin, V.M.; Caliari, V.; Bordignon-Luiz, M.T. Impact of Pressing Conditions on the Phenolic Composition, Radical Scavenging Activity and Glutathione Content of Brazilian Vitis vinifera White Wines and Evolution During Bottle Ageing. Food Bioprocess Technol. 2016, 9, 944–957. [Google Scholar] [CrossRef]
- Assuncao, M.; Martins, L.L.; Mourato, M.P.; Baleiras-Couto, M.M. Effect of selenium on growth and antioxidant enzyme activities of wine related yeasts. World J. Microbiol. Biotechnol. 2015, 31, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Barbulescu, I.; Feredes, M.; Carmen, B.; Rodica, A.; Teodoerscu, R. Improving wine-making products with selenium and total polyphenols. Rom. Biotechnol. Lett. 2012, 17, 7646–7655. [Google Scholar]
- Tillah, M.; Batubara, I.; Kartika-Sari, R. Antimicrobial and Antioxidant Activities of Resins and Essential Oil from Pine (Pinus merkusii, Pinuso ocarpa, Pinus insularis) and Agathis (Agathis loranthifolia). J. Biol. Biol. Educ. 2017, 9, 134–139. [Google Scholar] [CrossRef]
- Netsika, M. Wines of Greece, 2nd ed.; IANOS: Thessaloniki, Greece, 2019. [Google Scholar]
- Vlahou, E.; Christofi, S.; Roussis, I.G.; Kallithraka, S. Browning Development and Antioxidant Compounds in White Wines after Selenium, Iron, and Peroxide Addition. J. Appl. Sci. 2022, 12, 3834. [Google Scholar] [CrossRef]
- OIV—International Organisation of Vine and Wine. SO2 and Wine: A review. In OIV Publications, 1st ed.; OIV—International Organisation of Vine and Wine: Dijon, France, 2021; Available online: https://www.oiv.int/public/medias/7840/oiv-collective-expertise-document-so2-and-wine-a-review.pdf (accessed on 12 February 2023).
- Ma, L.; Waterhouse, A.L. Flavanols react preferentially with quinones through an electron transfer reaction, stimulating rather than preventing wine browning. Anal. Chim. Acta 2018, 1039, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Kramling, T.E. Browning of white wines and accelerated test for browning capacity. Am. J. Enol. Vitic. 1976, 27, 157–160. [Google Scholar] [CrossRef]
- Lamuela-Raventos, R.M. Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications, 1st ed.; 6th Chapter; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Romanet, R.; Coelho, C.; Liu, Y.; Bahut, F.; Ballester, J.; Nikolantonaki, M.; Gougeon, R.D. The Antioxidant Potential of White Wines Relies on the Chemistry of Sulfur-Containing Compounds: An Optimized DPPH Assay. Molecules 2019, 24, 1353. [Google Scholar] [CrossRef] [PubMed]
- Kontogeorgos, N.; Roussis, I.G. Research Note: Total Free Sulphydryls of Several White and Red Wine. J. Enol. Vitic. 2014, 35, 125–127. [Google Scholar] [CrossRef][Green Version]
- Kallithraka, S.; Arvanitoyannis, I.; Kefalas, P.; El-Zajouli, A.; Soufleros, E.; Psarra, E. Instrumental and sensory analysis of Greek wines: Implementation of principal component analysis (PCA) for classification according to geographical origin. J. Food Chem. 2001, 73, 501–514. [Google Scholar] [CrossRef]
- Kanavouras, A.; Coutelieris, F.; Karanika, E.; Kotseridis, Y.; Kallithraka, S. Colour change of bottled white wines as a quality indicator. OENO One 2020, 2, 189–197. [Google Scholar] [CrossRef]
- Pavon, C.; Aldas, M.; Lopez-Martinez, J.; Hernandez-Fernandez, J.; Arrieta, M.P. Films Based on Thermoplastic Starch Blended with Pine Resin Derivatives for Food Packaging. Polymers 2021, 13, 1171. [Google Scholar] [CrossRef] [PubMed]
- Koutsouris, A.N. Quality Improvement Wine Retinite from the Savvatiano Variety Using Different Yeasts and Adding Oak Chips. Master’s Thesis, Agricultural University of Athens, Athens, Greece, 2018. [Google Scholar]
- Bradshaw, M.P.; Barril, C.; Clark, A.C.; Prenzler, P.D.; Scollary, G. Ascorbic Acid: A Review of its Chemistry and Reactivity in Relation to a Wine Environment. Crit. Rev. Food Sci. Nutr. 2011, 51, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Barril, C.; Rutledge, D.N.; Scollary, G.R.; Clark, A.C. Ascorbic acid and white wine production a review of beneficial versus detrimental impacts. Aust. J. Grape Wine Res. 2016, 2, 169–181. [Google Scholar] [CrossRef]
- Wilkes, E. Practical measurements of total SO2 in wine. Wine Vitic. J. 2018, 33, 32–34. [Google Scholar]
- Pati, S.; Crupi, P.; Savastano, M.L.; Benucci, I.; Esti, M. Evolution of phenolic and volatile compounds during bottle storage of a white wine without added sulfite. J. Sci. Food Agric. 2020, 100, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Skouroumounis, G.K.; Kwiatkowski, M.J.; Francis, I.L.; Oakey, H.; Capone, D.L.; Peng, Z.; Duncan, B.; Sefton, M.A.; Waters, E.J. Influence of ascorbic acid on the composition, colour and flavour properties of a Riesling and a wooded Chardonay wine over five years storage. Aust. J. Grape Wine Res. 2005, 11, 355–368. [Google Scholar] [CrossRef]
- Talbi, W.; Ghazouani, T.; Braconi, D.; Ben Abdallah, D.; Raboudi, F.; Santucci, A.; Fattouch, S. Effects of selenium on oxidative damage and antioxidant enzymes of eukaryotic cells: Wine Saccharomyces cerevisiae. J. Appl. Microbiol. 2019, 126, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Cucciniello, R.; Forino, M.; Picariello, L.; Coppola, F.; Moio, L.; Gambuti, A. How acetaldehyde reacts with low molecular weight phenolics in white and red wines. Eur. Food Res. Technol. 2021, 247, 2935–2944. [Google Scholar] [CrossRef]
- Kallithraka, S.; Kotseridis, Y.; Kyraleou, M.; Proxenia, N.; Tsakiris, A.; Karapetrou, G. Analytical phenolic composition and sensory assessment of selected rare Greek cultivars after extended bottle ageing. J. Sci. Food Agric. 2015, 95, 2353. [Google Scholar] [CrossRef]
- Scrimgeour, N.; Nordestgaard, S.; Lloyd, N.D.R.; Wilkes, E.N. Exploring the effect of elevated storage temperature on wine composition. Aust. J. Grape Wine Res. 2015, 21, 713–722. [Google Scholar] [CrossRef]
- Kallithraka, S.; Salacha, M.I.; Tzourou, I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. J. Food Chem. 2009, 20, 500–505. [Google Scholar] [CrossRef]
- Ekumah, J.N.; Ma, Y.; Akpabli-Tsigbe, N.D.; Kwaw, E.; Jie, H.; Quaisie, J.; Manqing, X.; Johnson, N.A.N. Effect of selenium supplementation on yeast growth, fermentation efficiency, phytochemical and antioxidant activities of mulberry wine. J. Food Sci. Technol. 2021, 146, 111425. [Google Scholar] [CrossRef]
- Johnson, N.A.N.; Ekumah, J.N.; Ma, Y.; Akpabli-Tsigbe, N.D.; Adade, S.Y.S.S.; Manching, X.; Quaisie, J.; Kwaw, E.; Wang, C. Optimization of fermentation parameters for the production of a novel selenium enriched mulberry (Morus nigra) wine. J. Food Sci. Technol. 2023, 178, 114608. [Google Scholar] [CrossRef]
- Es-Safi, N.E.; Le Guerneve, C.; Fulcrand, H.; Cheynier, V.; Moutonet, M. Xanthylium salts formation involved in wine colour changes. Int. J. Food Sci. Technol. 2000, 35, 63–74. [Google Scholar] [CrossRef]
- Sartor, S.; Burin, V.M.; Ferreira-Lima, N.E.; Caliari, V.; Bordignon-Luiz, M.T. Polyphenolic Profiling, Browning, and Glutathione Content of Sparkling Wines Produced with Nontraditional Grape Varieties: Indicator of Quality During the Biological Aging. J. Food Sci. 2019, 84, 3546–3554. [Google Scholar] [CrossRef]
- De Beer, D.; Joubert, E.; Gelderblom, W.C.A.; Manley, M. Changes in the Phenolic Composition and Antioxidant Activity of Pinotage, Cabernet Sauvignon, Chardonnay and Chenin blanc Wines during Bottle Ageing. J. Enol. Vitic. 2005, 26, 6–15. [Google Scholar] [CrossRef][Green Version]
- Proestos, C.; Bakogiannis, A.; Komaitis, M. Determination of Phenolic Compounds in Wines. Int. J. Food Stud. 2012, 1, 33–41. [Google Scholar] [CrossRef]
| Sample | Glutathione (mg/L) | Selenomethionine (μg/L) | Ascorbic Acid (mg/L) | KMS (mg/L) |
|---|---|---|---|---|
| C | - | - | - | - |
| Glut. Min | 10 | - | - | - |
| Glut. Max | 20 | - | - | - |
| Se Min | - | 25 | - | - |
| Se Max | - | 50 | - | - |
| Ascor. Min | - | - | 100 | - |
| Ascor. Max | - | - | 200 | - |
| SO2 Min | - | - | - | 20 |
| SO2 Max | - | - | - | 40 |
| All Min | 10 | 25 | 100 | 20 |
| All Max | 20 | 50 | 200 | 40 |
| Sample | %ΔA420 Malagouzia | %ΔA420 Retsina | k (Day−1) * 10−3 Malagouzia | k (Day−1) * 10−3 Retsina |
|---|---|---|---|---|
| C | 182.90 b | 517.81 ab | 10.60 ab | 37.4 d |
| Semeth. Min | 204.63 bc | 577.17 bc | 11.00 ab | 38.1 d |
| Semeth. Max | 205.01 bc | 574.61 bc | 10.70 ab | 37.6 d |
| Glut Min | 185.70 b | 588.57 bc | 10.20 ab | 37.7 d |
| Glut Max | 203.67 bc | 552.66 b | 11.35 b | 36.9 cd |
| Ascor. Min | 233.11 d | 685.22 d | 14.85 c | 45.5 e |
| Ascor. Max | 264.10 e | 662.67 d | 18.85 d | 50.5 f |
| SO2 Min | 137.91 a | 549.91 b | 9.2 ab | 34.9 bc |
| SO2 Max | 141.69 a | 486.10 a | 8.55 a | 31.6 a |
| All Min | 213.80 cd | 602.40 c | 14.45 c | 44.1 c |
| All Max | 205.55 bc | 751.71 e | 18.8 d | 48.2 f |
| (+)-Catechin (% Reduction) * | ||
|---|---|---|
| Samples | Malagouzia | Retsina |
| C | 100% c | 100% c |
| Se min | 53.3% b | 54.8% a |
| Se max | 50% b | 61.9% a |
| Glut min | 53.3% b | 76.2% b |
| Glut max | 26.6% a | 78.6% b |
| Ascor min | 90.0% a | 238% d |
| Ascor max | 96.7% c | 238% d |
| SO2 min | 43.3% b | 238% d |
| SO2 max | 50.0% b | 238% d |
| All min | 46.7% b | 238% d |
| All max | 26.7% a | 238% d |
| Samples | Caftaric Acid | Coutaric Acid | Fertaric Acid |
|---|---|---|---|
| C | 100% c | 100% c | 100% c |
| Se min | 112.1% c | 78.6% bc | 17.6% a |
| Se max | 100% c | 42.9% b | 23.5% a |
| Glut min | 96.9% b | 50.0% b | 52.9% b |
| Glut max | 90.9% b | 57.1% b | 29.4% ab |
| Ascor min | 127.3% d | 78.6% bc | 82.3% c |
| Ascor max | 160.6% e | 71.4% bc | 88.2% c |
| SO2 min | 63.6% a | 114.3% c | 82.3% c |
| SO2 max | 63.6% a | 107.1% c | 64.7% bc |
| All min | 93.9% b | 14.2% a | 17.6% a |
| All max | 169.7% e | 57.1% b | 41.2% ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzistavridi, M.M.; Christofi, S.; Kallithraka, S. Fortification of White Wines with Antioxidants and Se: Impacts on Browning Development and Phenolic Content. Beverages 2024, 10, 31. https://doi.org/10.3390/beverages10020031
Chatzistavridi MM, Christofi S, Kallithraka S. Fortification of White Wines with Antioxidants and Se: Impacts on Browning Development and Phenolic Content. Beverages. 2024; 10(2):31. https://doi.org/10.3390/beverages10020031
Chicago/Turabian StyleChatzistavridi, Melina Maria, Stefania Christofi, and Stamatina Kallithraka. 2024. "Fortification of White Wines with Antioxidants and Se: Impacts on Browning Development and Phenolic Content" Beverages 10, no. 2: 31. https://doi.org/10.3390/beverages10020031
APA StyleChatzistavridi, M. M., Christofi, S., & Kallithraka, S. (2024). Fortification of White Wines with Antioxidants and Se: Impacts on Browning Development and Phenolic Content. Beverages, 10(2), 31. https://doi.org/10.3390/beverages10020031








