The Kavalactone Content and Profile of Fiji Kava Sold on the Local Market
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solvents and Materials
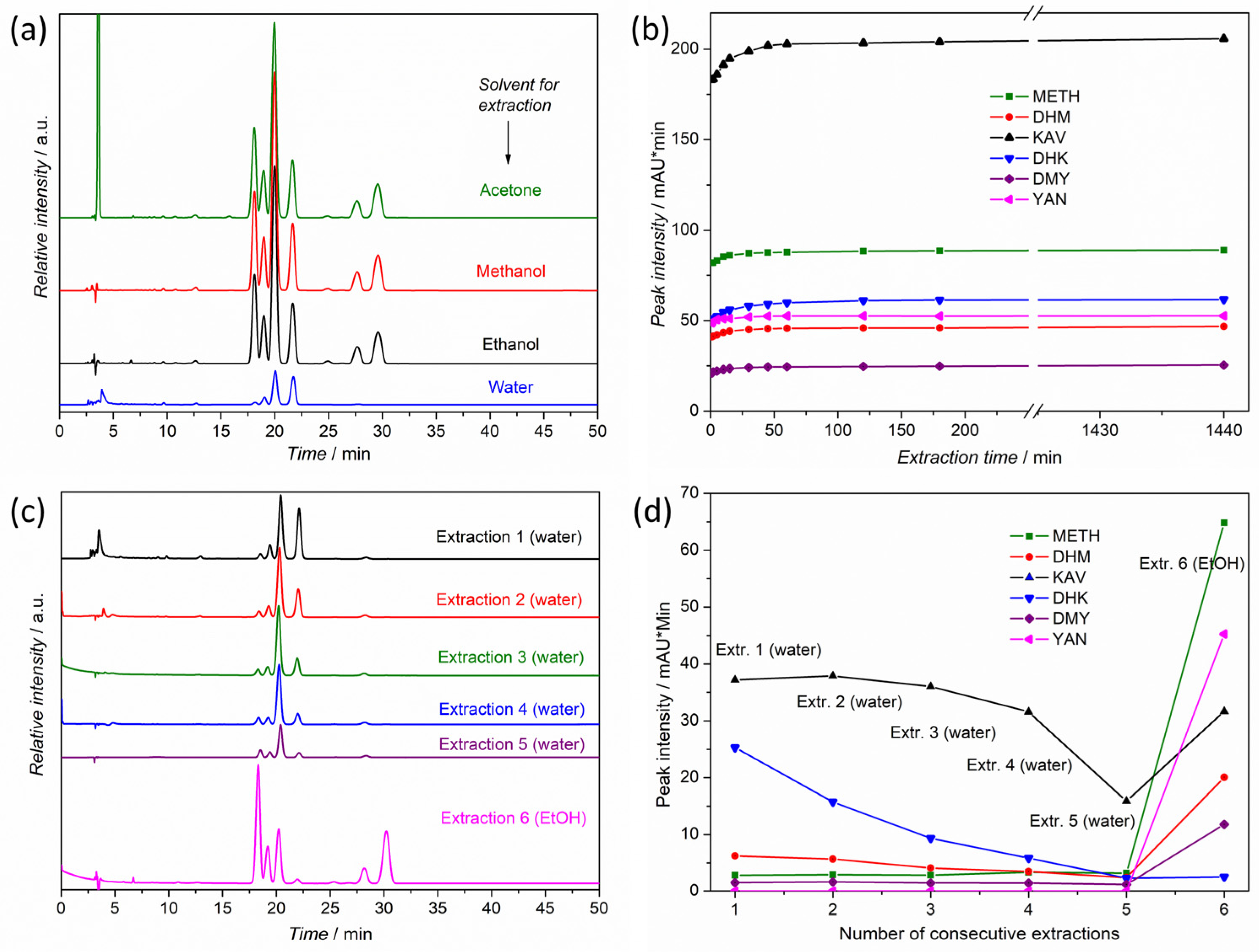
2.2. Extraction of Kava Powders and Plant Tissues
2.3. UV and HPLC Measurements
3. Results and Discussion
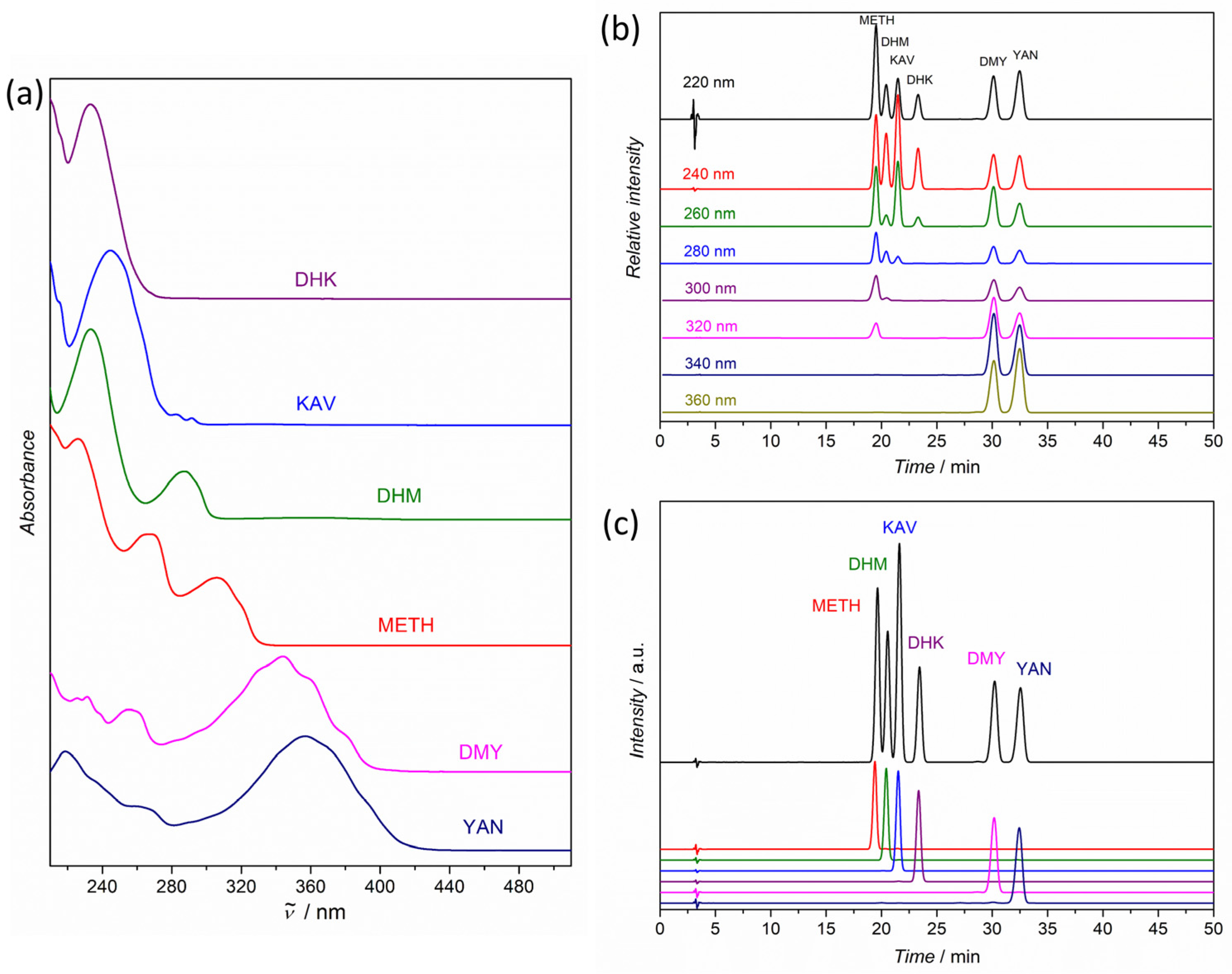
3.1. Method Selection and Validation
3.2. Kavalactone Content and Profile of a Selected Raw Kava Plant
3.3. Kavalactone Content and Profile of Kava Roots and Rhizomes from the Market
3.4. Kavalactone Content and Profile of Commercial Kava Powders
3.5. Chemotype of Kava Sold in the Fijian Market
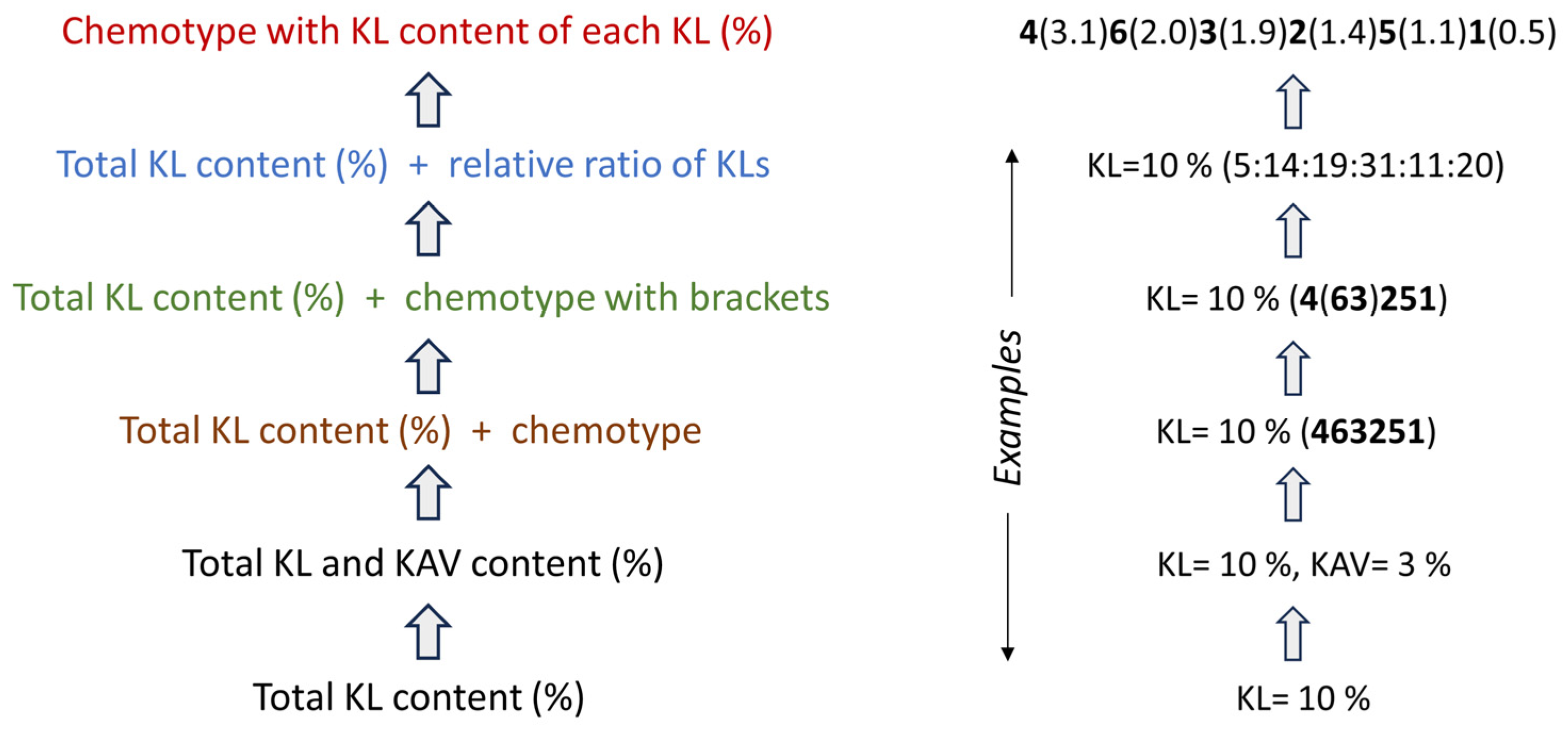
3.6. Suggestions for Improved Chemical Quality Code
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bian, T.; Corral, P.; Wang, Y.; Botello, J.; Kingston, R.; Daniels, T.; Salloum, R.G.; Johnston, E.; Huo, Z.; Lu, J.; et al. Kava as a Clinical Nutrient: Promises and Challenges. Nutrients 2020, 12, 3044. [Google Scholar] [CrossRef] [PubMed]
- Lebot, V.; Merlin, M.; Lindstrom, L. Kava: The Pacific Elixir: The Definite Guide to Its Ethnobotany, History, and Chemistry; Healing Arts Press: Rochester, VT, USA, 1997. [Google Scholar]
- Teschke, R.; Lebot, V. Proposal for a Kava Quality Standardization Code. Food Chem. Toxicol. 2011, 49, 2503–2516. [Google Scholar] [CrossRef] [PubMed]
- Lebot, V.; Michalet, S.; Legendre, L. Kavalactones and Flavokavins Profiles Contribute to Quality Assessment of Kava (Piper methysticum G. Forst.), the Traditional Beverage of the Pacific. Beverages 2019, 5, 34. [Google Scholar] [CrossRef]
- Petersen, G.E.; Tang, Y.; Fields, C. Chemical and in vitro toxicity analysis of a supercritical fluid extract of Kava kava (Piper methysticum). J. Ethnopharm. 2019, 235, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Scalise, L.; Bergonzi, M.C.; Vincieri, F.F. Analysis of kavalactones from Piper methysticum (kava-kava). J. Chromatogr. B 2004, 812, 203–214. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Guo, L.; Yu, H.; Chan, P.-C. Toxicity of Kava Kava. J. Environ. Sci. Health Part C 2008, 26, 89–112. [Google Scholar] [CrossRef] [PubMed]
- Whitton, P.A.; Lau, A.; Salisbury, A.; Whitehouse, J.; Evans, C.S. Kava lactones and the kava-kava controversy. Phytochemistry 2003, 64, 673–679. [Google Scholar] [CrossRef] [PubMed]
- White, C.M. The Pharmacology, Pharmacokinetics, Efficacy, and Adverse Events Associated with Kava. J. Clin. Pharmacol. 2018, 58, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Chetty, A. (Ministry of Agriculture, Suva, Fiji). Personal Communication. 2023. [Google Scholar]
- Pacific Data Hub. Kava Data. Available online: https://pacificdata.org/data/dataset/kava-df-kava/resource/f8c41e00-c5a8-44c0-80b1-7a4d7a8dc103 (accessed on 17 December 2023).
- Simeoni, P.; Lebot, V. Identification of factors determining kavalactone content and chemotype in Kava (Piper methysticum Forst. f.). Biochem. Syst. Ecol. 2002, 30, 413–424. [Google Scholar] [CrossRef]
- Fiji Kava Quality Manual. Available online: https://phamaplus.com.au/resources/technical-reports/fiji-kava-quality-manual/ (accessed on 10 October 2023).
- Zhang, N.; Wu, L.; Liu, X.; Shen, X. Plant-Derived Kavalactones and Their Bioactivities. Med. Res. 2018, 2, 170019. [Google Scholar] [CrossRef]
- Lebot, V.; Lévesque, J. The origin and distribution of kava (Piper methysticum Forst. f. Piperaceae): A phytochemical approach. Allertonia 1989, 5, 223–281. [Google Scholar]
- Lebot, V.; Merlin, M.D.; Lindstrom, L. Kava: The Pacific Drug; Yale University Press: New Haven, CT, USA, 1992. [Google Scholar]
- Lebot, V. An overview of kava production in the Pacific Islands: What we do know and what we don’t know. J. South Pac. Agric. 1997, 4, 55–62. [Google Scholar]
- Tang, Y.; Fields, C. A UHPLC-UV Method Development and Validation for Determining Kavalactones and Flavokavains in Piper methysticum (Kava). Molecules 2019, 24, 1245. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; He, K.; Zheng, B.; Zheng, Q. Reversed-phase high-performance liquid chromatographic method for quantitative analysis of the six major kavalactones in Piper methysticum. J. Chromatogr. A 1998, 825, 1–8. [Google Scholar] [CrossRef]
- Xuan, T.D.; Fukuta, M.; Wei, A.C.; Elzaawely, A.A.; Khanh, T.D.; Tawata, S. Efficacy of extracting solvents to chemical components of kava (Piper methysticum) roots. J. Nat. Med. 2008, 62, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qu, W.; Bittenbender, H.C.; Li, Q.X. Kavalactone content and chemotype of kava beverages prepared from roots and rhizomes of Isa and Mahakea varieties and extraction efficiency of kavalactones using different solvents. J. Food Sci. Technol. 2015, 52, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Cote, C.S.; Kor, C.; Cohen, J.; Auclair, K. Composition and biological activity of traditional and commercial kava extracts. Biochem. Biophys. Res. Comm. 2004, 322, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Glaser, J.A.; Foerst, D.L.; McKee, G.D.; Quave, S.A.; Budde, W.L. Trace analyses for wastewaters. Environ. Sci. Technol. 1981, 15, 1426–1435. [Google Scholar] [CrossRef]

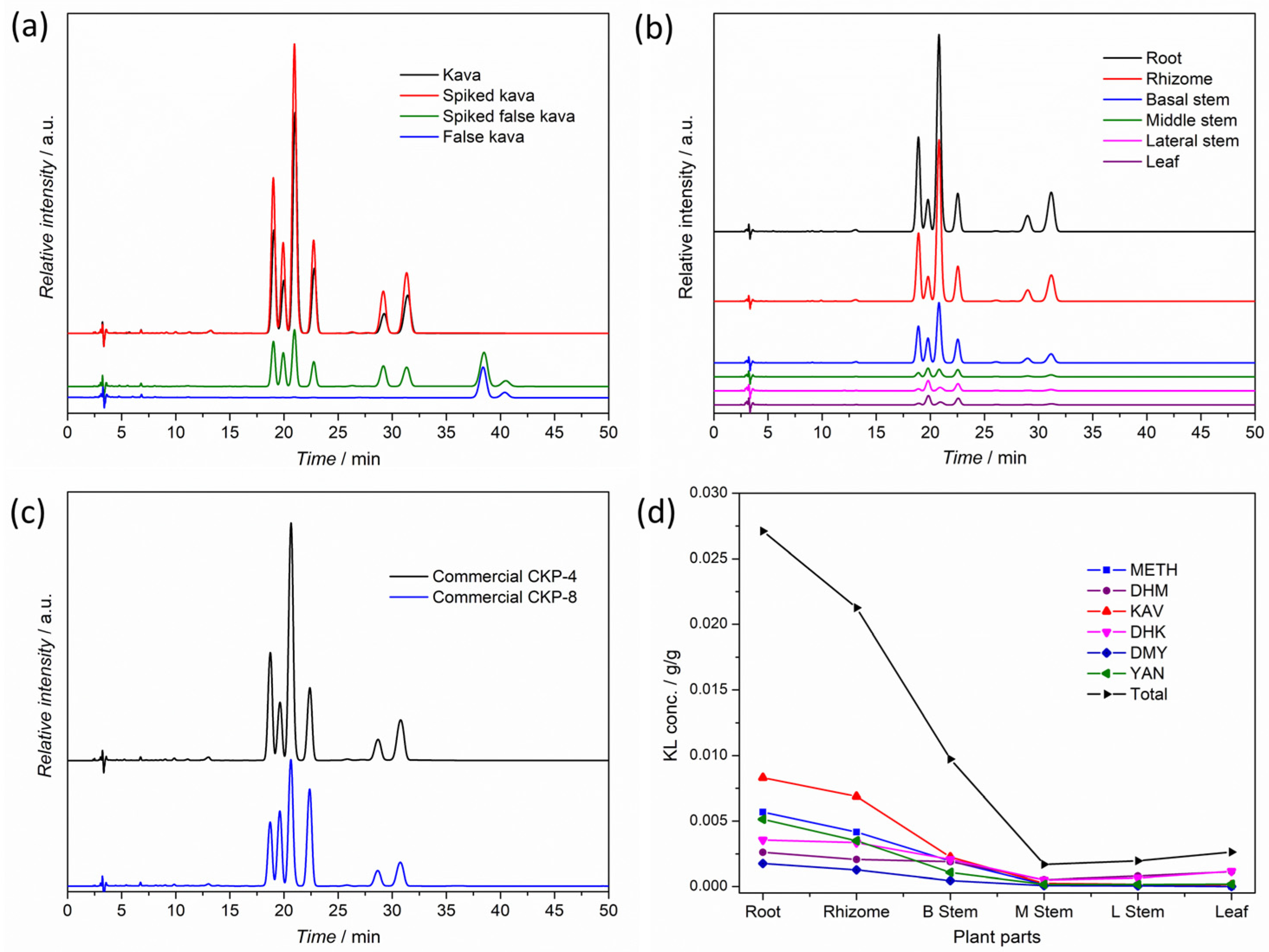
| Plant Part | Water Cont. (%) | Total KL Cont. (%) | Kavain Cont. (%) | Relative Mass Ratio of KLs a | Chemotype (Mass Based) b | Chemotype (Mol Based) b |
|---|---|---|---|---|---|---|
| Green plant | ||||||
| Root | 73.5 | 2.71 ± 0.019 | 0.83 ± 0.002 | 21:43:62:100:32:69 | 4(63)251 | 4(63)251 |
| Rhizome | 65.9 | 2.13 ± 0.031 | 0.69 ± 0.004 | 19:49:51:100:30:61 | 46(32)51 | 4(623)51 |
| Basal stem | 71.9 | 0.97 ± 0.042 | 0.23 ± 0.005 | 20:92:48:100:84:86 | (42)(65)31 | (42)(65)31 |
| Middle stem | 80.5 | 0.17 ± 0.013 | 0.03 ± 0.002 | 13:97:32:49:100:36 | (52)4(63)1 | (25)4(63)1 |
| Lateral stem | 85.8 | 0.20 ± 0.011 | 0.02 ± 0.002 | 6:81:19:21:100:13 | 52(43)61 | (52)4361 |
| Leaf | 78.9 | 0.26 ± 0.024 | 0.01 ± 0.001 | 2:100:34:12:99:2 | (25)34(61) | 2534(61) |
| Freeze-dried | ||||||
| Root | 10.31 ± 0.013 | 3.05 ± 0.016 | 25:43:65:100:33:71 | 4(63)251 | 4(63)2(51) | |
| Rhizome | 6.63 ± 0.047 | 2.17 ± 0.011 | 18:52:48:100:30:58 | 46(23)51 | 4(26)351 | |
| Basal stem | 3.81 ± 0.058 | 0.89 ± 0.002 | 21:90:51:100:83:84 | 4(265)31 | 42(65)31 | |
| Middle stem | 1.00 ± 0.031 | 0.15 ± 0.007 | 13:95:35:51:100:37 | (52)4(63)1 | (25)4(36)1 | |
| Lateral stem | 1.41 ± 0.047 | 0.12 ± 0.008 | 6:83:24:22:100:14 | 52(34)61 | (52)(34)61 | |
| Leaf | 1.50 ± 0.043 | 0.07 ± 0.001 | 2:99:43:12:100:3 | (52)34(61) | (25)34(61) | |
| Oven-dried | ||||||
| Root | 7.8 | 9.4 ± 0.028 | 2.7 ± 0.044 | 28:50:59:100:36:70 | 463251 | 4632(51) |
| Rhizome | 8.4 | 6.0 ± 0.078 | 1.9 ± 0.025 | 19:53:46:100:33:63 | 462351 | 4(62)351 |
| RB b | Water Cont. (%) | Total KL Cont. (%) c | Kavain Cont. (%) d | Relative Mass Ratio of KLs e | Chemotype (Mass Based) f | Chemotype (Mol Based) f |
|---|---|---|---|---|---|---|
| RB-1a | 14.2/15.4 | 7.9/2.8 | 2.7/0.9 | 21:46:48:100:17:63/18:64:38:100:32:66 | 46(32)15/4(62)351 | 46(23)15/426351 |
| RB-1b | 13.2/14.6 | 8.7/4.2 | 2.5/1.1 | 21:48:69:100:29:77/20:80:60:100:56:68 | 463251/426351 | 4(63)251/42(63)51 |
| RB-2a | 11.8/13.5 | 9.9/5.2 | 3.1/1.7 | 33:42:54:100:27:61/26:49:51:100:31:57 | 463215/463251 | 4(63)215/4(63)2(51) |
| RB-2b | 11.3/13.2 | 10.2/5.8 | 3.0/1.6 | 22:45:68:100:32:68/22:76:63:100:44:65 | 4(36)251/42(63)51 | 4(36)251/42(36)51 |
| RB-3a | 12.0/13.4 | 11.5/6.7 | 3.6/2.0 | 20:43:55:100:30:70/17:55:54:100:43:71 | 463251/46(23)51 | 463251/462351 |
| RB-3b | 10.9/12.9 | 12.9/8.3 | 4.1/2.3 | 23:39:57:100:29:70/20:56:70:100:42:71 | 463251/4(63)251 | 4632(51)/4(36)251 |
| RB-4a | 11.2/13.8 | 9.8/6.8 | 2.9/1.8 | 31:41:70:100:29:66/23:74:56:100:49:61 | 4(36)2(15)/42(63)51 | 436215/42(63)51 |
| RB-4b | 11.4/13.6 | 11.8/4.9 | 3.5/1.4 | 31:44:68:100:29:65/29:56:66:100:39:62 | 4(36)2(15)/4(36)251 | 436215/4(326)(51) |
| RB-5a | 11.5/13.3 | 9.3/6.1 | 2.8/1.7 | 23:65:51:100:37:60/20:82:51:100:45:52 | 4(26)351/42(63)51 | 426351/42(36)51 |
| RB-5b | 11.3/12.5 | 17.7/3.8 | 5.7/1.1 | 33:100:76:91:65:63/22:100:42:70:80:56 | 243(56)1/254631 | 243(56)1/245631 |
| RB-6a | 11.6/13.5 | 7.5/2.3 | 2.0/0.6 | 22:49:67:100:46:89/20:69:68:100:69:92 | 463(25)1/46(523)1 | 463251/462(35)1 |
| RB-6b | 12.6/13.8 | 5.7/3.3 | 1.5/0.8 | 17:40:57:98:55:100/18:59:70:99:64:100 | (64)(35)21/(64)(35)21 | 463521/463(25)1 |
| Number | Measured | |||||
|---|---|---|---|---|---|---|
| CKP a | Water Cont. (%) | Total KL Cont. (%) | Kavain Cont. (%) | Relative Mass Ratio of KLs b | Chemotype (Mass Based) c | Chemotype (Mol Based) c |
| 1 | 10.7 | 5.4 ± 0.06 | 1.5 ± 0.02 | 23:65:53:100:46:68 | 4(62)351 | 426351 |
| 2 | 11.8 | 5.0 ± 0.07 | 1.4 ± 0.04 | 22:63:54:100:48:70 | 462351 | 4(26)351 |
| 3 | 6.7 | 4.4 ± 0.06 | 1.2 ± 0.03 | 24:64:54:100:44:66 | 4(62)351 | 426351 |
| 4 | 9.3 | 3.8 ± 0.03 | 1.1 ± 0.01 | 23:68:52:100:47:65 | 4(26)351 | 426351 |
| 5 | 15.8 | 4.5 ± 0.05 | 1.3 ± 0.01 | 22:71:51:100:51:64 | 426(35)1 | 426(35)1 |
| 6 | 6.0 | 4.8 ± 0.02 | 1.3 ± 0.02 | 22:74:52:100:52:68 | 426(53)1 | 426(35)1 |
| 7 | 13.2 | 4.1 ± 0.06 | 1.2 ± 0.03 | 25:57:56:100:37:63 | 4(623)51 | 4(263)51 |
| 8 | 9.8 | 3.0 ± 0.07 | 0.5 ± 0.03 | 19:100:34:59:68:43 | 254631 | 2(45)631 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasinszki, T.; Devi, D.D. The Kavalactone Content and Profile of Fiji Kava Sold on the Local Market. Beverages 2024, 10, 4. https://doi.org/10.3390/beverages10010004
Pasinszki T, Devi DD. The Kavalactone Content and Profile of Fiji Kava Sold on the Local Market. Beverages. 2024; 10(1):4. https://doi.org/10.3390/beverages10010004
Chicago/Turabian StylePasinszki, Tibor, and Deepti Darshani Devi. 2024. "The Kavalactone Content and Profile of Fiji Kava Sold on the Local Market" Beverages 10, no. 1: 4. https://doi.org/10.3390/beverages10010004
APA StylePasinszki, T., & Devi, D. D. (2024). The Kavalactone Content and Profile of Fiji Kava Sold on the Local Market. Beverages, 10(1), 4. https://doi.org/10.3390/beverages10010004