Preliminary Evaluation of Minor Cereals as Non-Traditional Brewing Raw Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grain Quality Parameters
2.2. Malting Procedures
2.3. Amylase Activity
2.4. Statistical Analysis
3. Results and Discussion
3.1. Grain Quality
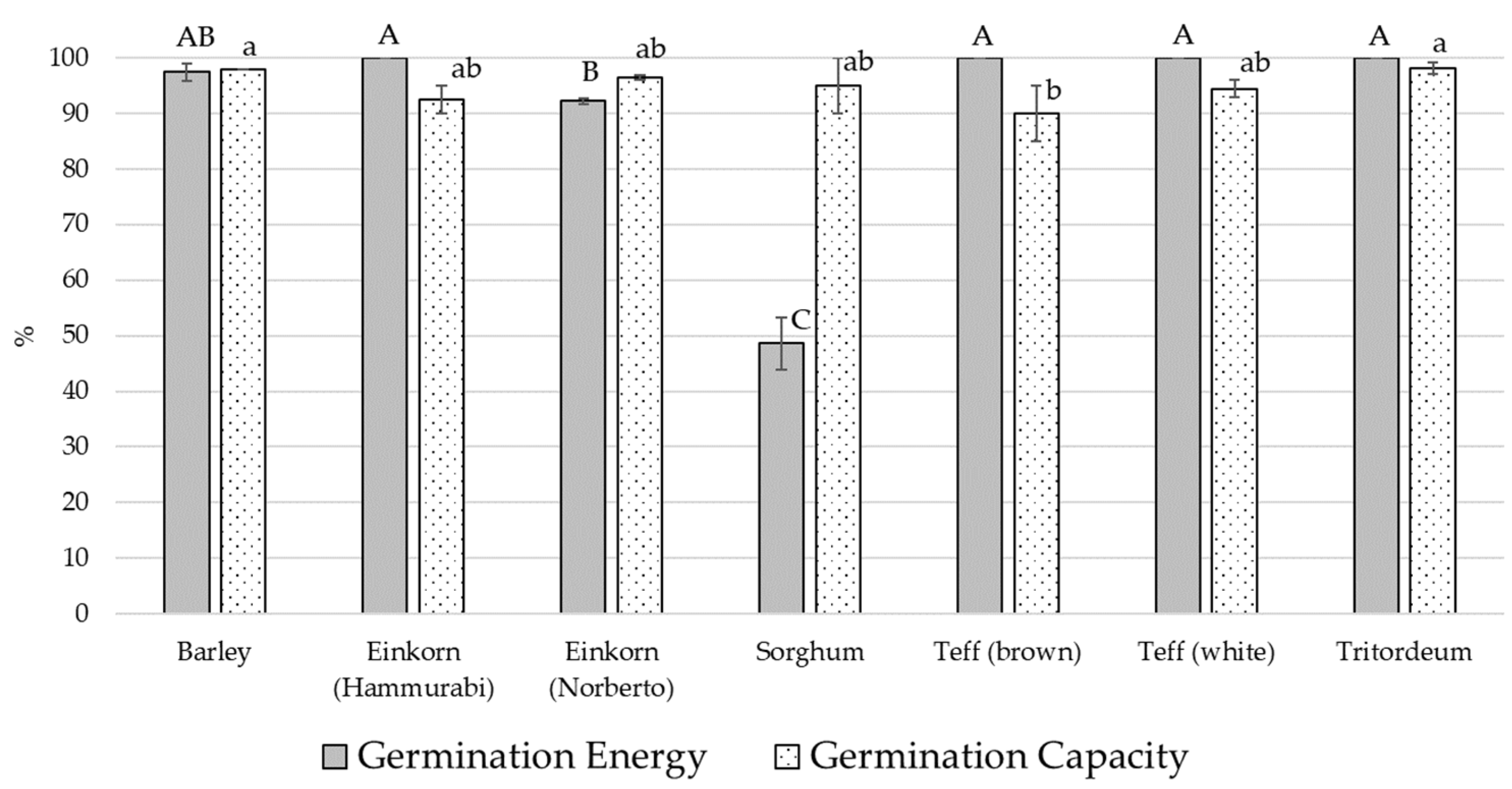
3.2. Malting
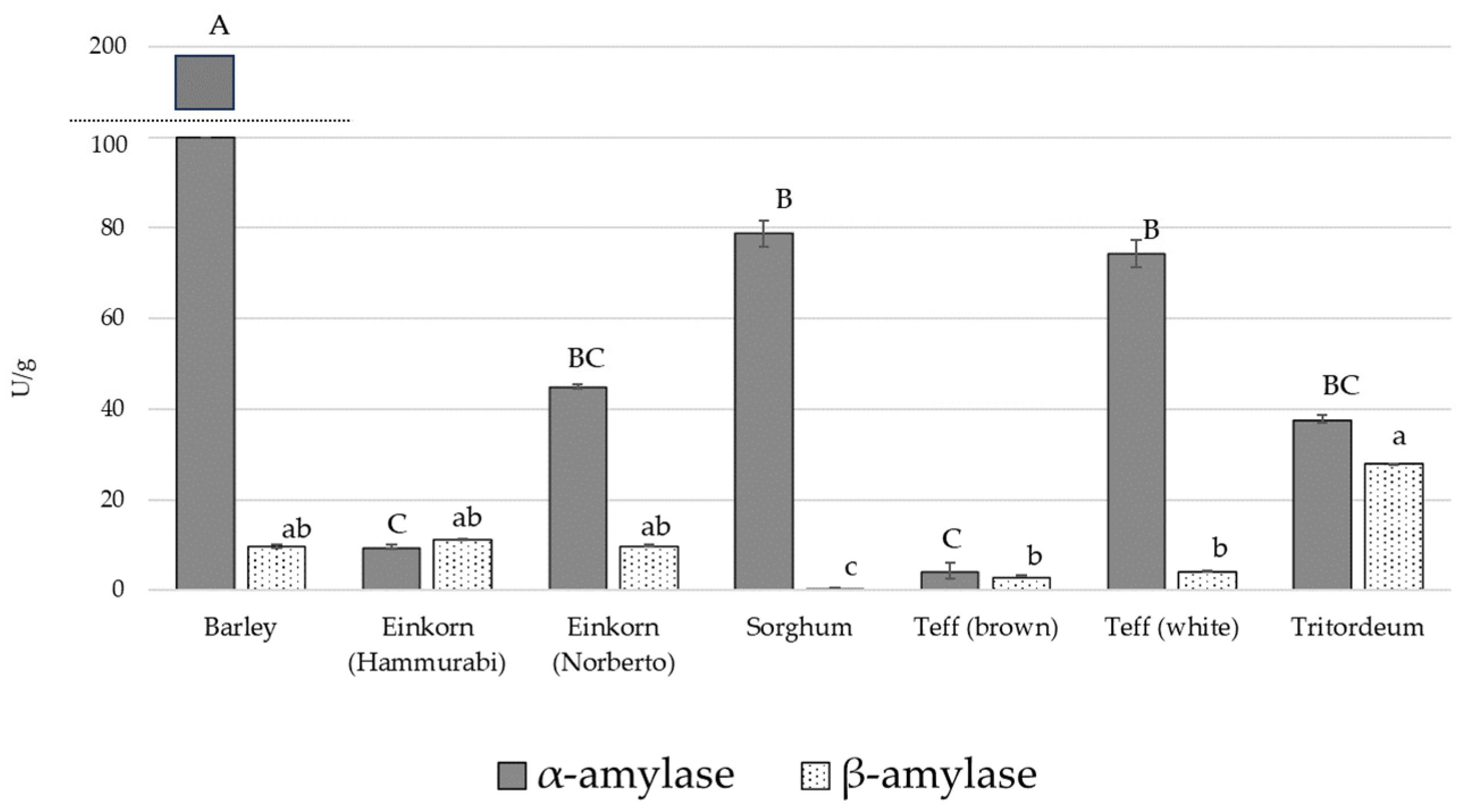
3.3. Amylase Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brewers Association. Craft Beer Industry Market Segments. Available online: https://www.brewersassociation.org/statistics-and-data/craft-beer-industry-market-segments (accessed on 30 October 2023).
- Gazza, L.; Nocente, F. The Contribution of Minor Cereals to Sustainable Diets and Agro-Food Biodiversity. Foods 2023, 12, 3500. [Google Scholar] [CrossRef] [PubMed]
- Cela, N.; Condelli, N.; Caruso, M.C.; Perretti, G.; Di Cairano, M.; Tolve, R.; Galgano, F. Gluten-Free Brewing: Issues and Perspectives. Fermentation 2020, 6, 53. [Google Scholar] [CrossRef]
- Gebremariam, M.M.; Zarnkow, M.; Becker, T. Teff (Eragrostis tef) as a raw material for malting, brewing and manufacturing of gluten-free foods and beverages: A review. J. Food Sci. Technol. 2014, 51, 2881–2895. [Google Scholar] [CrossRef] [PubMed]
- Cela, N.; Galgano, F.; Perretti, G.; Di Cairano, M.; Tolve, R.; Condelli, N. Assessment of brewing attitude of unmalted cereals and pseudocereals for gluten free beer production. Food Chem. 2022, 384, 132621. [Google Scholar] [CrossRef] [PubMed]
- Hager, A.-S.; Taylor, J.P.; Waters, D.M.; Arendt, E.K. Gluten free beer—A review. Trends Food Sci. Technol. 2014, 36, 44–54. [Google Scholar] [CrossRef]
- Ciccoritti, R.; Taddei, F.; Gazza, L.; Nocente, F. Influence of kernel thermal pre-treatments on 5-n-alkylresorcinols, polyphenols and antioxidant activity of durum and einkorn wheat. Eur. Food Res. Technol. 2021, 247, 353–362. [Google Scholar] [CrossRef]
- Martın, A.; Alvarez, J.B.; Martın, L.M.; Barro, F.; Ballesteros, J. The development of tritordeum: A novel cereal for food processing. J. Cereal Sci. 1999, 30, 85–95. [Google Scholar] [CrossRef]
- Galassi, E.; Taddei, F.; Ciccoritti, R.; Nocente, F.; Gazza, L. Biochemical and technological characterization of two C4 gluten-free cereals: Sorghum bicolor and Eragrostis tef. Cereal Chem. 2020, 97, 65–73. [Google Scholar] [CrossRef]
- Taylor, J.R.N.; Schober, T.J.; Bean, S.R. Novel food and non-food uses for sorghum and millets. J. Cereal Sci. 2006, 44, 252–271. [Google Scholar] [CrossRef]
- Owuama, C.I. Brewing beer with sorghum. J. Inst. Brew. 1999, 105, 23–34. [Google Scholar] [CrossRef]
- Gazza, L.; Hidalgo, A.; Brandolini, A. A high protein ancient wheat species: Einkorn. J. Cereal Sci. 2023, 114, 103790. [Google Scholar] [CrossRef]
- Vaquero, L.; Comino, I.; Vivas, S.; Rodríguez-Martín, L.; Giménez, M.J.; Pastor, J.; Sousa, C.; Barro, F. Tritordeum: A novel cereal for food processing with good acceptability and significant reduction in gluten immunogenic peptides in comparison with wheat. J. Sci. Food Agric. 2018, 98, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Bean, S.R.; Loerger, B.P.; Smith, B.M.; Blackwell, D.L. Sorghum protein structure and chemistry: Implications for nutrition and functionality. In Advances in Cereal Science: Implications to Food Processing and Health Promotion; Awika, J.M., Pironen, V., Bean, S., Eds.; ACS Publications: Washington, DC, USA, 2011; Volume 1089, pp. 131–147. [Google Scholar]
- Althwab, S.A.; Carr, T.; Weller, C.; Dweikat, I.; Schlegel, V. Advances in grain sorghum and its co-products as a human health promoting dietary system. Food Res. Int. 2015, 77, 349–359. [Google Scholar] [CrossRef]
- Rathnavati, C.V.; Komala, V.V. Sorghum grain quality. In Sorghum Biochemistry: An Industrial Perspective; Ratnavathi, C.V., Patil, J.V., Chavan, U.D., Eds.; Academic Press: Cambridge, MA, USA, 2016; Chapter 1; pp. 1–61. [Google Scholar]
- Galassi, E.; Gazza, L.; Nocente, F.; Kouagang Tchakoutio, P.; Natale, C.; Taddei, F. Valorization of Two African Typical Crops, Sorghum and Cassava, by the Production of Different Dry Pasta Formulations. Plants 2023, 12, 2867. [Google Scholar] [CrossRef]
- Kutyauripo, J.; Parawira, W.; Tinofa, S.; Kudita, I.; Ndengu, C. Investigation of shelf-life extension of sorghum beer (Chibuku) by removing the second conversion of malt. Int. J. Food Microbiol. 2009, 129, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Rathnavati, C.V.; Chavan, U.D. Malting and Brewing of Sorghum. In Sorghum Biochemistry: An Industrial Perspective; Ratnavathi, C.V., Patil, J.V., Chavan, U.D., Eds.; Academic Press: Cambridge, MA, USA, 2016; Chapter 2; pp. 63–106. [Google Scholar]
- Dabija, A.; Ciocan, M.E.; Chetrariu, A.; Codină, G.G. Maize and Sorghum as Raw Materials for Brewing, a Re-view. Appl. Sci. 2021, 11, 3139. [Google Scholar] [CrossRef]
- Ciocan, M.E.; Salamon, R.V.; Ambrus, Á.; Codină, G.G.; Chetrariu, A.; Dabija, A. Brewing with Unmalted and Malted Sorghum: Influence on Beer Quality. Fermentation 2023, 9, 490. [Google Scholar] [CrossRef]
- Patanè, C.; Cosentino, S.L.; Cavallaro, V.; Saita, A. Screening for cold tolerance during germination within sweet and fiber sorghums [Sorghum bicolor (L.) Moench] for Energy Biomass. Agronomy 2021, 11, 620. [Google Scholar] [CrossRef]
- International Seed Testing Association (ISTA). ISTA Handbook on Seedling Evaluation; ISTA: Bassersdorf, Switzerland, 2006. [Google Scholar]
- International Association for Cereal Science and Technology. ICC Standard Methods (Method No. 110/1); ICC: Vienna, Austria, 1976. [Google Scholar]
- S352.2; Moisture Measurement Unground Grain and Seeds. American Society of Agricultural Engineers: St. Joseph, MI, USA, 1999.
- ISO 520:2010; Cereals and Pulses-Determination of the Mass of 1000 Grains. International Organization for Standardization (ISO): Geneva, Switzerland, 2010; p. 10.
- ISO 7971-1:2009; Determination of Bulk Density, Called Mass per Hectolitre-Part 1: Reference Method. International Organization for Standardization (ISO): Geneva, Switzerland, 2009; p. 8.
- International Association for Cereal Science and Technology. ICC Standard Methods (Method No. 105/2); ICC: Vienna, Austria, 2003. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis 996.11, 18th ed.; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- American Association of Cereal Chemists. Approved Methods of Analysis, 10th ed.; AACC International: St. Paul, MN, USA, 2000. [Google Scholar]
- Djameh, C.; Kwesi Saalia, F.; Sinayobye, E.; Budu, A.; Essilfie, G.; Mensah-Brown, H.; Sefa-Dedeh, S. Optimization of the sorghum malting process for pito production in Ghana. J. Inst. Brew. 2015, 121, 106–112. [Google Scholar] [CrossRef]
- Di Ghionno, L.; Sileoni, V.; Marconi, O.; De Francesco, G.; Perretti, G. Comparative study on quality attributes of gluten-free beer from malted and unmalted teff [Eragrostis tef (zucc.) trotter]. LWT Food Sci. Technol. 2017, 84, 746–752. [Google Scholar] [CrossRef]
- Turner, H.M.; Elmore, L.; Walling, J.; Lachowiec, J.; Mangel, D.; Fischer, A.; Sherman, J. Effect of Steeping Regime on Barley Malt Quality and Its Impacts on Breeding Program Selection. J. Am. Soc. Brew. Chem. 2019, 77, 267–281. [Google Scholar] [CrossRef]
- Yding, E.D.; Pagenstecher, M.; Trummer, J.; Poreda, A.; Andersen, M.L.; Jespersen, B.M. Effect of malting regimes on the malt quality of tritordeum for beer brewing. Eur. Food Res. Technol. 2023, 249, 95–102. [Google Scholar] [CrossRef]
- Fogarasi, A.; Kun, S.; Kiss, Z.; Vecseri-Hegyes, B. Einkorn (Triticum monococcum L.) in Organic Beer Production. In Proceedings of the Malting of Organic Einkorn, Third International Young Scientists Symposium for the Brewing, Distilling and Malting Sectors, Nottingham, UK, 23–25 October 2012. [Google Scholar]
- Taddei, F.; Gazza, L.; Conti, S.; Muccilli, V.; Foti, S.; Pogna, N.E. Starch-bound 2S proteins and kernel texture in einkorn, Triticum monococcum ssp monococcum. Theor. Appl. Genet. 2009, 119, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- McCleary, B.V.; Sheehan, H.J. Measurement of cereal a-amylase: A new assay procedure. J. Cereal Sci. 1987, 6, 237–251. [Google Scholar] [CrossRef]
- McCleary, B.V.; Codd, R. Measurement of b-amylase in cereal flours and commercial enzyme preparations. J. Cereal Sci. 1989, 9, 17–33. [Google Scholar] [CrossRef]
- Belcar, J.; Matłok, N.; Gorzelany, J. Technological assessment of winter cultivar of common wheat (Triticum aestivum L.) and winter barley (Hordeum vulgare L.) for pale malt production. Acta Univ. Cibiniensis. Ser. E Food Technol. 2020, 1, 89–98. [Google Scholar] [CrossRef]
- Salamini, F.; Ozkan, H.; Brandolini, A.; Schäfer-Pregl, R.; Martin, W. Genetics and geography of wild cereal domestication in the Near East. Nat. Rev. Genet. 2002, 3, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Ilori, M.O.; Akingbala, J.O.; Oguntimein, G.B.; Ogundiwin, J.O. Effect of grain bed thickness, duration of steeping and germination on the malting properties of improved Nigerian sorghum varieties. LWT Food Sci. Technol. 1990, 23, 505–512. [Google Scholar]
- Bekele, A.; Bultosa, G.; Belete, K. The effect of germination time on malt quality of six sorghum (Sorghum bicolor) varieties grown at Melkassa, Ethiopia. J. Inst. Brew. 2012, 118, 76–81. [Google Scholar] [CrossRef]
- Armstrong, B.; Weiss, M.; Grieg, R.; Aldred, G. Using digital image analysis to accurately determine the thousand kernel weight of randomly distributed barley, malt and wheat samples. In Proceedings of the 51st Australian Cereal Chemistry Conference, Coogee, Australia, 9–13 September 2001; Wootton, M., Batey, I.L., Wrigley, C.W., Eds.; RACI-CCD: Melbourne, Australia; pp. 115–118.
- Cabral, A.L.; Jordan, M.C.; Larson, G.; Somers, D.J.; Humphreys, D.G.; McCartney, C.A. Relationship between QTL for grain shape, grain weight, test weight, milling yield, and plant height in the spring wheat cross RL4452/‘AC Domain’. PLoS ONE 2018, 13, e0190681. [Google Scholar] [CrossRef]
- Hilmarsson, H.S.; Rio, S.; Sánchez, J.I.Y. Genotype by Environment Interaction Analysis of Agronomic Spring Barley Traits in Iceland Using AMMI, Factorial Regression Model and Linear Mixed Model. Agronomy 2021, 11, 499. [Google Scholar] [CrossRef]
- Fox, G.P.; Osborne, B.; Bowman, J.; Kelly, A.; Cakir, M.; Poulsen, D.; Inkerman, A.; Henry, R. Measurement of genetic and environmental variation in barley (Hordeum vulgare) grain hardness. J. Cereal Sci. 2007, 46, 82–92. [Google Scholar] [CrossRef]
- Galano, T.; Fininsa, C.; Bultosa, G. Effects of net blotch (Pyrenophora teres) on malt barley yield and grain quality atholeta, Central Ethiopia. East Afr. J. Sci. 2008, 2, 150–158. [Google Scholar]
- Fox, G.P.; Panozzo, J.F.; Li, C.D.; Lance, R.C.M.; Inkerman, P.A.; Henry, R.J. Molecular basis of barley quality. Aust. J. Agric. Res. 2003, 54, 1081–1101. [Google Scholar] [CrossRef]
- Kakabouki, I.; Beslemes, D.F.; Tigka, E.L.; Folina, A.; Karydogianni, S.; Zisi, C.; Papastylianou, P. Performance of six genotypes of tritordeum compare to bread wheat under east mediterranean condition. Sustainability 2020, 12, 9700. [Google Scholar] [CrossRef]
- Pedrazzani, C.; Vanara, F.; Bhandari, D.R.; Bruni, R.; Spengler, B.; Blandino, M.; Righetti, L. 5-n-Alkylresorcinol Profiles in Different Cultivars of Einkorn, Emmer, Spelt, Common Wheat, and Tritordeum. J. Agric. Food Chem. 2021, 69, 14092–14102. [Google Scholar] [CrossRef] [PubMed]
- Tsegaye, G.A.; Abera, S. The Study of Some Engineering Properties of Teff [Eragrostis teff (ZUCC.) Trotter] Grain Varieties. Int. J. Food Eng. Technol. 2020, 4, 9–12. [Google Scholar] [CrossRef]
- Fox, G.P.; Bettenhausen, H.M. Variation in quality of grains used in malting and brewing. Front. Plant Sci. 2023, 14, 1172028. [Google Scholar] [CrossRef]
- Deme, G.D.; Asfaw, B.T.; Gari, M.T. Evaluation of malting potential of different barley varieties. J. Water Pollut. Purif. Res. 2020, 6, 24–35. [Google Scholar]
- Brennan, C.S.; Harris, N.; Smith, D.; Shewry, P.R. Structural differences in the mature endosperms of good and poormalting barley cultivars. J. Cereal Sci. 1996, 24, 171–177. [Google Scholar] [CrossRef]
- Rani, H.; Bhardwaj, R.D. Quality attributes for barley malt: “The backbone of beer”. J. Food Sci. 2021, 86, 3322–3340. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A. Application of enzymes in brewing. J. Nutr. Food Sci. Forecast 2018, 1, 1002. [Google Scholar]
- Cela, N.; Condelli, N.; Perretti, G.; Di Cairano, M.; Tolve, R.; Galgano, F. Gluten reduction in beer: Effect of sorghum: Quinoa ratio and protein rest time on brewing parameters and consumer acceptability. J. Cereal Sci. 2023, 109, 103607. [Google Scholar] [CrossRef]
- Jukić, M.; Šumanovac, F.; Nakov, G.; Šimić, G.; Komlenić, D.K.; Ivanova, N.; Lukinac, J. Application of the Falling Number Method in the Evaluation of the α-Amylase Activity of Malt Flour. Appl. Sci. 2023, 13, 3218. [Google Scholar] [CrossRef]
- Fujita, A.; Simsek, S.; Schwarz, P.B. Observations on the Malting of Ancient Wheats: Einkorn, Emmer and Spelt. Fermentation 2020, 6, 125. [Google Scholar] [CrossRef]
- Belcar, J.; Sekutowski, T.R.; Zardzewiały, M.; Gorzelany, J. Effect of malting process duration on malting losses and quality of wheat malts. Acta Univer. Cib. Ser. E Food Technol. 2021, 25, 221–232. [Google Scholar] [CrossRef]
- De Schepper, C.F.; Michiels, P.; Buvé, C.; Van Loey, A.M.; Courtin, C.M. Starch hydrolysis during mashing: A study of the activity and thermal inactivation kinetics of barley malt α-amylase and β-amylase. Carbohydr. Polym. 2021, 255, 117494. [Google Scholar] [CrossRef]
- Ledley, A.J.; Elias, R.J.; Hopfer, H.; Cockburn, D.W. A Modified Brewing Procedure Informed by the Enzymatic Profiles of Gluten-Free Malts Significantly Improves Fermentable Sugar Generation in Gluten-Free Brewing. Beverages 2021, 7, 53. [Google Scholar] [CrossRef]
- Espinosa-Ramírez, J.; Pérez-Carrillo, E.; Serna-Saldívar, S.O. Maltose and glucose utilization during fermentation of barley and sorghum lager beers as affected by β-amylase or amyloglucosidase addition. J. Cereal Sci. 2014, 6, 602–609. [Google Scholar] [CrossRef]

| Species | Steeping | Moisture after Steeping | Germination | Kilning |
|---|---|---|---|---|
| Barley | 10 h wet, 19 h dry, wet 6 h, dry 8 h, wet 4 h (total 47 h), 15 °C | 45% | 96 h, 15 °C | 2 h, 30 °C; 6 h, 40 °C;15 h, 50 °C; 5 h, 60 °C (total 28 h) |
| Einkorn cv. Hammurabi | 2 h wet, 19 h dry, wet 2 h (total 23 h), 15 °C | 42% | 96 h, 15 °C | 6 h, 40 °C; 15 h, 50 °C; 5 h, 60 °C (total 26 h) |
| Einkorn cv. Norberto | 4 h wet, 19 h dry, wet 2 h (total 25 h), 15 °C | 42% | 72 h, 15 °C | 6 h, 40 °C; 20 h, 50 °C; 5 h, 60 °C (total 31 h) |
| Sorghum | 4 h wet, 2 h dry, wet 4 h, dry 2 h, wet 4 h (total 16 h), 28 °C | 45% | 96 h, 28 °C | 1 h, 30 °C; 1 h, 35 °C; 15 h, 40 °C; 2 h, 50 °C; 2 h, 60 °C (total 21 h) |
| Teff | 3 h wet, 2 h dry, wet 2 h, (total 7 h), 24 °C | 48% | 96 h, 24 °C | 6 h, 30 °C; 10 h, 40 °C; 5 h, 60 °C (total 21 h) |
| Tritordeum | 6 h wet, 18 h dry, wet 6 h (total 30 h), 20 °C | 42% | 72 h, 20 °C | 2 h, 30 °C; 12 h, 40 °C; 5 h, 60 °C (total 19 h) |
| TKW | TW | TS | Protein | FN | |
|---|---|---|---|---|---|
| g | kg/hL | g/100 g | g/100 g | s | |
| Barley | 26.0 ± 0.1 ab | 73.5 ± 0.4 b | 64.4 ± 0.1 ab | 11.3 ± 0.1 b | 303 ± 7 b |
| Einkorn cv. Hammurabi | 33.4 ± 0.4 a | 79.4 ± 0.3 b | 54.9 ± 0.2 b | 19.0 ± 0.1 a | 414 ± 2 ab |
| Einkorn cv. Norberto | 23.8 ± 0.7 ab | 80 ± 1 b | 51.9 ± 0.2 b | 19.9 ± 0.2 a | 356 ± 14 b |
| Sorghum | 24.2 ± 0.4 ab | 72.6 ± 0.6 b | 79.5 ± 0.9 a | 10.5 ± 0.3 b | 473 ± 11 ab |
| Teff (brown) | 0.26 ± 0.02 c | 86.9 ± 0.3 ab | 77.3 ± 0.4 a | 11.6 ± 0.1 b | 431 ± 1 ab |
| Teff (white) | 0.27 ± 0.01 c | 88.9 ± 0.2 a | 79 ± 1 a | 11.0 ± 0.1 b | 623 ± 15 a |
| Tritordeum | 33.5 ± 0.9 a | 82.7 ± 0.7 ab | 61.9 ± 0.7 ab | 17.5 ± 0.1 ab | 383 ± 6 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocente, F.; Galassi, E.; Taddei, F.; Natale, C.; Gazza, L. Preliminary Evaluation of Minor Cereals as Non-Traditional Brewing Raw Materials. Beverages 2024, 10, 2. https://doi.org/10.3390/beverages10010002
Nocente F, Galassi E, Taddei F, Natale C, Gazza L. Preliminary Evaluation of Minor Cereals as Non-Traditional Brewing Raw Materials. Beverages. 2024; 10(1):2. https://doi.org/10.3390/beverages10010002
Chicago/Turabian StyleNocente, Francesca, Elena Galassi, Federica Taddei, Chiara Natale, and Laura Gazza. 2024. "Preliminary Evaluation of Minor Cereals as Non-Traditional Brewing Raw Materials" Beverages 10, no. 1: 2. https://doi.org/10.3390/beverages10010002
APA StyleNocente, F., Galassi, E., Taddei, F., Natale, C., & Gazza, L. (2024). Preliminary Evaluation of Minor Cereals as Non-Traditional Brewing Raw Materials. Beverages, 10(1), 2. https://doi.org/10.3390/beverages10010002









