Abstract
In the present work, the migration of three chemicals, benzophenone, 1,4-diphenylbutadiene and Uvitex® OB from low-density polyethylene samples into the food simulant, 50% ethanol (v/v), was studied. The key parameters of the diffusion process, the partition and diffusion coefficients, were calculated by using a mathematical model based on Fick’s Second Law. As expected, the diffusion coefficients increased with temperature and the values obtained ranged between 3.87 × 10−11 and 1.00 × 10−8 cm2/s. Furthermore, the migration in different fruit juices was also evaluated and the results indicated that benzophenone migrated to a greater extent in comparison with the other two migrants in all beverages analyzed. To quantify the migrants, a high-performance liquid chromatographic method with a diode array detector (HPLC-DAD) was used. The separation was performed on an Ace 3 C18-HL column (30 × 3 mm, 3 μm particle size) and using a gradient elution system consisting of Milli-Q water and acetonitrile. The total analysis time did not exceed 8 min.
1. Introduction
Polymeric materials including, polyethylene, polypropylene, polyvinyl chloride (PVC), polystyrene, polyethylene terephthalate (PET), and so on, have been widely used in the food packaging industry in many applications. The major concern related to their use is the migration of low molecular substances, such as additives, residual monomers and oligomers from the material to the food. Different factors, such as the time and temperature of contact, the surface area of the material in contact with the food, the nature of the food and the migrant, etc., control the migration process [1]. The migration from a plastic material into the food is a predictable physical process that, in most cases, follows Fick’s Laws [2].
It is generally accepted, that substances with low molecular weight (<1000 Da) can be absorbed in the gastrointestinal tract, and therefore may cause potentially hazardous effects on consumers’ health [3]. The Regulation EU No 10/2011 [4] issues the Union list of monomers, other starting substances, macromolecules obtained from microbial fermentation, additives and polymer production aids authorized in the manufacture of plastic materials and articles, as well as the restrictions which they are subject to.
Due to the complexity of the food matrices and the analytical difficulties to determine the migrating substances in foodstuffs, the legislation allows the use of food simulants to conduct the migration assays. For cloudy drinks like juices and nectars, the simulants that must be used are 3% acetic acid (w/v) (B) and 50% ethanol (v/v) (D1) [4].
In the present study, three model migrants with different physico-chemical properties and uses, 1,4-diphenylbutadiene (DPBD), benzophenone and Uvitex® OB, were selected. DPBD belongs to fluorescent whitening agents; Uvitex OB is an optical brightener, both compounds act protecting the colour of plastic materials from adverse environmental conditions and aid to prevent yellowing and discoloration that polymers can suffer from under unfavorable conditions. Benzophenone, is a photoinitiator for UV-cured inks, commonly used for printing the external face of packaging [5].
The aim of the present work was to study the migration kinetics of these three selected migrants from LDPE (low-density polyethylene) samples into the food simulant, 50% ethanol (v/v); thus, the key parameters of the diffusion process, partition and diffusion coefficients, were calculated by using a mathematical model based on Fick’s Second Law. Additionally, the migration in fruit juices was also evaluated.
2. Materials and Methods
2.1. Chemicals and Standard Solutions
Standards of benzophenone (BZP) (CAS No. 119-61-9; M.W. 182.221; log P (octanol/water) 3.18) (purity 99%) and 1,4-diphenylbutadiene (DPBD) (CAS No. 886-65-7; M.W. 206.287; log P (octanol/water) 5.290) (purity 98%) were supplied from Sigma Aldrich (Steinheim, Germany) and Uvitex® OB (CAS No. 7128-64-5; M.W. 430.5694; log P (octanol/water) 7.22) (purity 99%) was obtained from Fluka. Ethanol absolute, acetonitrile and tetrahydrofuran were provided by Merck (Darmstadt, Germany). Water used for all solutions was obtained from a Milli-Q water purification system (Millipore) (Bedford, MA, USA).
A primary stock solution of the three model migrants, of known concentration (1000 µg/mL), was prepared in tetrahydrofuran. Working solutions within the range of 0.05–5 µg/mL were prepared by dilution with acetonitrile. Solutions were stored at 4 °C in amber flasks to protect from light.
2.2. Plastic Films
Low-density polyethylene (LDPE) films additivated with the three model migrants (BZP, DPBD and Uvitex® OB) were used to conduct the migration assays. The films were prepared by an extrusion process.
2.3. Food Samples
The eight commercial juices used in the study were purchased in local supermarkets. Their fruit composition and characteristics are summarized in Table 1.

Table 1.
Composition and characteristics of the commercial fruit juices.
| Sample | Fruit Composition | Suspended Solids | pH |
|---|---|---|---|
| Juice 1 | Grape, apple, strawberry, raspberry, | 2.83 | |
| Currant, purple carrot, cranberry and ginseng extract | |||
| Juice 2 | Orange, mango and guarana extract | 3.67 | |
| Juice 3 | Tomato | x | 4.21 |
| Juice 4 | Orange, carrot puree, carrot, lemon and orange pulp | x | 3.39 |
| Juice 5 | Orange, carrot and lemon | 2.94 | |
| Juice 6 | Peach and soy seed | 3.92 | |
| Juice 7 | Orange | x | 3.44 |
| Juice 8 | Orange | 3.46 |
x, juice with suspended solids.
2.4. Migration Tests
2.4.1. Migration Kinetics into 50% Ethanol (v/v)
Films were cut into 2 cm × 5 cm (10 cm2) pieces and weighed. Then, they were put in tubes with screw caps containing 50 mL of the food simulant, 50% ethanol (v/v). The time-temperature conditions are presented in Table 2. At selected time intervals, aliquots of the food stimulant were removed, filtered through a 0.45 μm PTFE membrane filter (Advanted, Toyo Roshi Kaisha, Ltd., Utsunomiya-shi, Japan) and analyzed by high-performance liquid chromatography.

Table 2.
Time-temperature conditions used in the migration assays.
| Temperature (°C) | Time (h) |
|---|---|
| 10 | 2; 4; 12; 24; 48; 96; 168; 219; 675 |
| 20 | 2; 4; 8; 12; 24; 48; 96; 168; 219; 675 |
| 40 | 1; 2; 4; 8; 12; 24; 48; 96; 168; 219 |
| 60 | 0.5; 1; 2; 4; 8; 12; 24; 48; 96; 168 |
2.4.2. Migration in Fruit Juices
To perform the migration assays in fruit juices, 10 cm2 of the additivated films were put in contact with 50 mL of the beverages in tubes with screw caps. The time-temperature conditions were 10 days at 40 °C. Moreover, for samples 7 and 8, the tests were also carried out at 20 °C. After that, the film was removed and cleaned with paper tissue. In order to simplify the analysis instead of determining the quantity of migrants in the foodstuff, the migrant that remained in the polymer was determined and it was assumed that all the missing migrant was in the food.
The migrants were extracted as follows: the plastic films were placed in flasks with 50 mL pure ethanol and stored in an oven at 70 °C for 6 h. Aliquots were removed from the flasks, filtered and analyzed by HPLC. In order to check the initial concentration of the model migrants, the films were extracted as indicated. The determined concentrations were, 124.30, 43.75 and 568.56 mg/kg for BZP, DPBD and Uvitex® OB, respectively. A second extraction under the same conditions was performed to assure a complete extraction.
2.5. Chromatography
Analyses were performed in a chromatographic system consisting of an HP1100 quaternary pump, a degassing device, an autosampler, a column thermostatting system, a diode array UV detector and Agilent Chem-Station for LC and LC/MS systems software. Chromatographic separation was carried out on an Ace 3 C18-HL column (30 × 3 mm, 3 μm particle size) thermostated at 30 °C. Milli-Q water (A) and acetonitrile (B) were used as a mobile phase. The gradient elution program was as follows: 0–2 min, 50% B isocratic; 2–5 min, linear gradient 50%–100% B; 5–15 min, 100% B isocratic and then a post-time of 5 min. The flow rate was 0.7 mL/min and the injection volume was 20 μL. BZP was detected at 256 nm, DPBD at 330 nm and Uvitex® OB at 372 nm.
2.6. Identification and Quantification
The identification of the migrants was made by comparison of their retention times and spectra with those of pure standards. Quantitation was performed on the basis of linear calibration plots of peak area against concentration. Calibration lines were constructed within the 0.05–5 μg/mL range.
3. Results and Discussion
3.1. Chromatographic Method
A suitable chromatographic separation and peak-resolution of the three model migrants were achieved by using a reversed stationary phase and a binary solvent gradient composed of Milli-Q water and acetonitrile. Furthermore, the proposed method was rapid, under these conditions the analysis was completed within 8 min.
The linearity of the method was tested by using a series of BZP, DPBD and Uvitex® OB standards of known concentrations. Calibration curves were constructed using seven concentration levels of standard solutions, and they were fitted to a linear equation. Each point of the calibration curve is the average of two peak-area measurements. Parameters of linearity, the linear equation, and the determination coefficients are shown in Table 3. All compounds showed a good linearity, correlation coefficients were, in all cases, greater than 0.9988.

Table 3.
Parameters of linearity of the model migrants.
| Migrant | Retention Time | Equation | r2 |
|---|---|---|---|
| BZP | 1.383 min | y = 183.39x + 6.78 | 0.9994 |
| DPBD | 5.208 min | y = 485.65x + 11.57 | 0.9998 |
| Uvitex® OB | 7.180 min | y = 213.17x + 6.88 | 0.9988 |
The limits of detection, calculated according to ACS guidelines [6] (defined as signal three times the height of the noise level), were 0.025 mg/L for BZP, 0.01 mg/L for DPBD and 0.005 mg/L for Uvitex® OB. Similar LOQ were obtained by Gandhimathi et al. [7].
3.2. Migration Kinetics in 50% Ethanol (v/v)
Mathematical Model
The key parameters of the migration process, namely partition and diffusion coefficients were determined. A mathematical model based on Fick’s Second Law (Equation (1)) was used to assess the migration of the model contaminants into the food simulant, 50% ethanol (v/v):
where Cp is the concentration of the migrant in the polymer at time t (s) and position x and D is the diffusion coefficient in p (cm2/s).
An analytical solution of this differential equation that describes the diffusion kinetics was proposed by Crank (1975) [8]; after a slight modification, this can be expressed by the following equation (Simoneau, 2010) [9]:
where, mF,t is the mass of the migrant transferred from P (LDPE films) into F (food simulant) after time t, (mg); A is the area of P in contact with F (cm2); CP,0 is the initial concentration of the migrant in P (mg/kg); ρP is the density of P (g/cm3); t is the migration time (s); dP is the thickness of P (cm); VP is the volume of P (cm3); VF is the volume of F (cm3); qn is the positive roots of the equation tan qn = −α·qn; DP is the diffusion coefficient of the migrant in the polymer (cm2/s); KP/F is the partition coefficient of the migrant between P and F.
The partition coefficient (KP/S) indicates the relative solubility of the model migrant between the polymer and the food simulant when equilibrium is reached [10,11,12].
Partition coefficients between the film and food simulants were calculated according to the following equation:
where: KP/S is the partition coefficient between the polymer and the food simulant. CP is the concentration of substance in the polymer at equilibrium, in µg/g. CS is the concentration of substance in the food simulant at equilibrium, in µg/g.
The migration kinetics of the selected model migrants were carried out at four different temperatures and in the food simulant, 50% ethanol (v/v). This is the food simulant of choice for “juices and nectars and soft drinks containing fruit pulp, musts containing fruit pulp, liquid chocolate” [4].
Table 4 summarized the key parameters—partition and diffusion coefficients—of the migration process. The migrant Uvitex® OB presented the highest KP/S values (Table 4) in comparison with the other migrants studied, showing that this compound has a higher affinity to the polyethylene; on the contrary, BZP presented the lowest values.

Table 4.
Partition (KP/S) and Diffusion (D) coefficients for the food simulant studied.
| Migrant | Temp. (°C) | KP/S | D (cm2/s) |
|---|---|---|---|
| BZP | 10 | 2.84 | 1.78 × 10−9 |
| 20 | 2.84 | 5.74 × 10−9 | |
| 40 | |||
| 60 | |||
| DPBD | 10 | 64.01 | 6.45 × 10−1° |
| 20 | 5.08 | 1.48 × 10−9 | |
| 40 | 7.68 | 7.81 × 10−9 | |
| 60 | 34.72 | 1.00 × 10−8 | |
| Uvitex® OB | 10 | >1000 | 3.87 × 10−11 |
| 20 | 606.62 | 6.79 × 10−11 | |
| 40 | 168.27 | 4.74 × 10−1° | |
| 60 | 414.24 | 2.08 × 10−9 |
When evaluating the diffusion coefficients, the highest values correspond to BZP, which could be explained because of its lower molecular weight; this effect has been reported in a study with different photoinitiators [13]. Naturally, for all migrants, D augmented with the temperature. The results obtained for DPBD at 20 °C and 40 °C were three orders of magnitude higher than the values at 25 °C (3.7 × 10−12 cm2/s) and 40 °C (7.5 × 10−12 cm2/s) obtained by Sanches-Silva et al. (2008) [14] for an LDPE-orange juice system. It could be attributed to the insoluble solids presented in the orange juice. In the case of BZP, Sanches-Silva et al. (2009) [12] obtained a similar D value at 25 °C (3.1 × 10−9 cm2/s) in 30% ethanol (v/v). The migration kinetics of selected migrants into 50% ethanol (v/v) at 10 and 20 °C are illustrated in Figure 1.
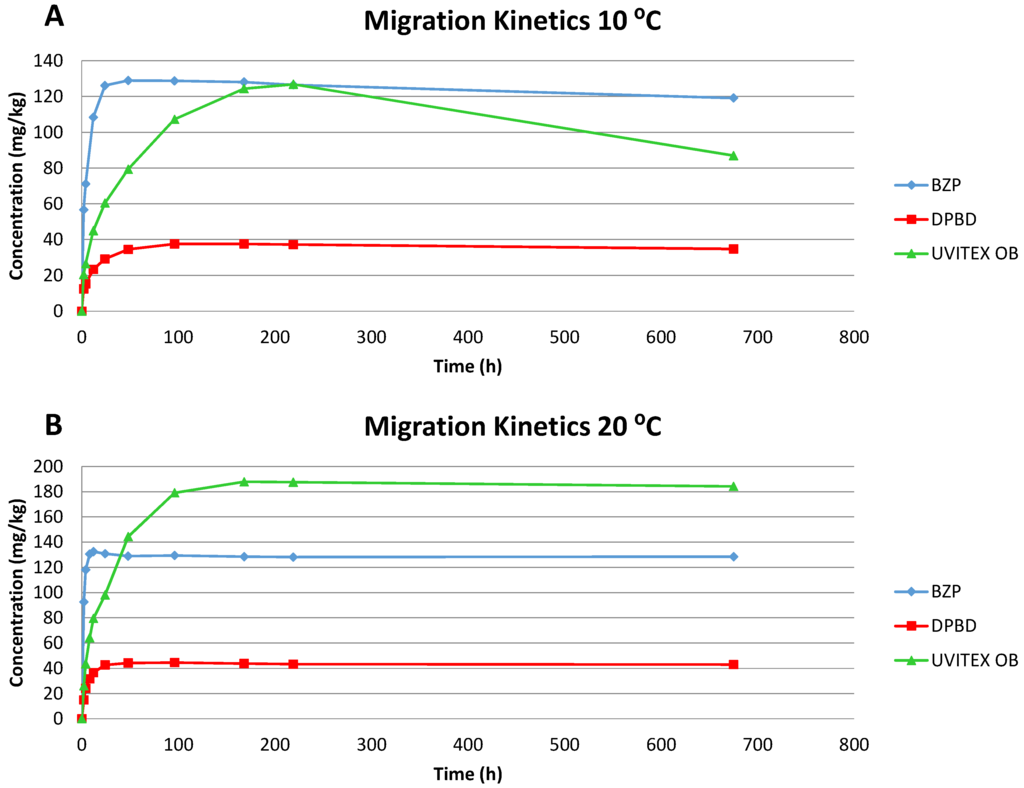
Figure 1.
Migration kinetics of BZP, DPBD and Uvitex® OB obtained from LDPE films into 50% ethanol (v/v) at 10 °C (A) and 20 °C (B).
To study the effect of the temperature on the diffusion of the model migrants, DPBD and Uvitex® OB into 50% ethanol the Arrhenius Equation was used.
where D is the diffusion coefficient (cm2/s), D0 is the preexponential factor (cm2/s), EA is the activation energy (kJ/mol), R is the gas constant (8.314 × 10−3 kJ/molK), and T is the temperature (K). EA values were determined from the slope by representing the 1/T versus logarithm of D. The EA is the energy necessary for a given compound to move through the polymeric matrix [15,16]. The D0, EA and correlation coefficients (r2) obtained were 0.16 cm2/s; 45.1 kJ/mol; 0.931 and 26.4 cm2/s; 64.51 kJ/mol; 0.993 for DPBD and Uvitex® OB, respectively. The Arrhenius Equation allows the prediction of the diffusion coefficient for any temperature between 10 °C and 60 °C.
3.3. Migration in Fruit Juices
The amount of BZP, DPBD and Uvitex® OB that had migrated into the fruit juice after 10 days at 40 °C (and at 20 °C for samples 7 and 8) expressed as the percent with respect the initial concentration in the plastic films is presented in Figure 2. BZP migrated to a greater extent in comparison with the other two migrants in all beverages investigated, whereas Uvitex® OB migrated to a much lesser extent. The highest concentrations of BZP and DPBD were found in beverage 6—This juice has soy seed in its composition—on the contrary, the highest concentration of Uvitex® OB was found in beverage 1, which has the lowest pH (2.83).
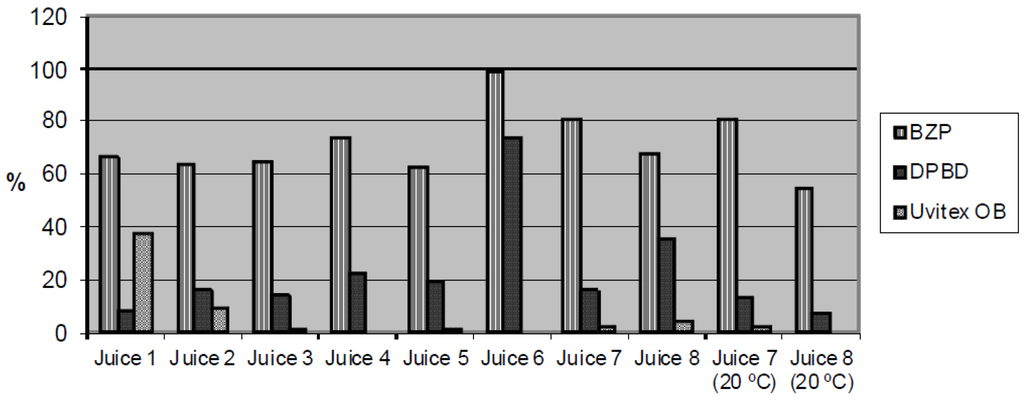
Figure 2.
Percentage of BZP, DPBD and Uvitex® OB migrated in fruit juices after 10 days at 40 °C and 10 days at 20 °C.
To evaluate the effect of the pulp on the migration process, two identical orange juices with and without pulp were analyzed. In general, higher concentrations of migrants were detected in the juice that contains pulp; this behavior was also observed in other studies [14,17]. As it has been reported in the study conducted by Sanches-Silva et al. [14], the fraction of fat associated to the insoluble solids seems to be a key factor that affect the migration.
4. Concluding Remarks
Briefly, in the present work, the migration kinetics of three model migrants, BZP, DPBD and Uvitex® OB from LDPE films into the food simulant, 50% ethanol (v/v), were determined. A mathematical model based on Fick’s Second Law was used to calculate the diffusion and partition coefficients, the D values obtained ranged between 3.87 × 10−11 and 1.00 × 10−8 cm2/s. The migration in fruit juices was also studied and results show that the presence of pulp in the beverage affects the migration process.
Acknowledgments
The study was financially supported by the “Consellería de Cultura Educación e Ordenación Universitaria” from the Xunta de Galicia (Galicia, Spain), Ref. No. GRC 2014/012.
R. Sendon is grateful to the “Parga Pondal” Program financed by “Consellería de Innovación e Industria, Xunta de Galicia” for her postdoctoral contract. The authors are also grateful to Gonzalo Hermelo Vidal, Cristina Casal and Patricia Blanco Carro for their excellent technical assistance.
Author Contributions
All authors contributed equally to this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barnes, K.A.; Sinclair, R.C.; Watson, D.H. Chemical Migration and Food Contact Materials; Woodhead Publishing Limited: Abington Hall, Abington, Cambridge, UK; CRC Press LLC: Boca Raton, FL, USA, 2007; pp. 1–12. [Google Scholar]
- Brandsch, J.; Mercea, P.; Rüter, M.; Tosa, V.; Piringer, O. Migration modelling as a tool for quality assurance of food packaging. Food Addit. Contam. 2002, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Note for Guidance for Petitioners Presenting an Application for the Safety Assessment of a Substance to be Used in Food Contact Materials Prior to Its Authorization (Updated on 30/07/08). Available online: http://www.efsa.europa.eu/de/search/doc/21r.pdf (accessed on 30 July 2015).
- Union Guidelines on Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Available online: http://ec.europa.eu/food/food/chemicalsafety/foodcontact/docs/10-2011_plastic_guidance_en.pdf (accessed on 30 July 2015).
- Paseiro-Cerrato, R.; Rodríguez-Bernaldo de Quirós, A.; Sendón, R.; Bustos, J.; Santillana, M.I.; Cruz, J.M.; Paseiro-Losada, P. Chromatographic methods for the determination of polyfunctional amines and related compounds used as monomers and additives in food packaging materials: A state-of-the-art review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 676–694. [Google Scholar] [CrossRef]
- American Chemical Society (ACS). Subcommittee of environmental analytical chemistry. Anal. Chem. 1980, 52, 2242–2280. [Google Scholar]
- Gandhimathi, M.; Murugavel, K.; Ravi, T.K. Migration study of optical brighteners from polymerpacking materials to jam squeeze and fruit drink by spectrofluorimetry and RP-HPLC methods. J Food Sci. Technol. 2014, 51, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon: Oxford, UK, 1975; pp. 44–68. [Google Scholar]
- Simoneau, C. Applicability of Generally Recognised Diffusion Models for the Estimation of Specific Migration in Support of EU Directive 2002/72/EC. Available online: http://www.ibebvi.be/src/Frontend/Files/Labo/5/files/guideline%20modelling_70a.pdf (accessed on 30 July 2015).
- Tehrany, E.A.; Desobry, S. Partition coefficients in food/packaging systems: A review. Food Addit. Contam. 2004, 21, 1186–1202. [Google Scholar] [CrossRef] [PubMed]
- Sanches-Silva, A.; Cruz, J.M.; Sendón, R.; Franz, R.; Paseiro-Losada, P. Migration and diffusion of diphenylbutadiene from packages into foods. J. Agric. Food Chem. 2009, 57, 10225–10230. [Google Scholar] [CrossRef] [PubMed]
- Sanches-Silva, A.; Andre, C.; Castanheira, I.; Cruz, J.M.; Pastorelli, S.; Simoneau, C.; Paseiro-Losada, P. Study of the migration of photoinitiators used in printed food-packaging materials into food simulants. J. Agric. Food Chem. 2009, 57, 9516–9523. [Google Scholar] [CrossRef] [PubMed]
- Sanches-Silva, A.; Pastorelli, S.; Cruz, J.M.; Simoneau, C.; Castanheira, I.; Paseiro-Losada, P. Development of a method to study the migration of six photoinitiators into powdered milk. J. Agric. Food Chem. 2008, 56, 2722–2726. [Google Scholar] [CrossRef] [PubMed]
- Sanches-Silva, A.; Cruz Freire, J.M.; Franz, R.; Paseiro-Losada, P. Time-temperature study of the kinetics of migration of diphenylbutadiene from polyethylene films into aqueous foodstuffs. Food Res. Int. 2008, 41, 138–144. [Google Scholar] [CrossRef]
- Limm, W.; Hollifield, H.C. Modelling of additive diffusion in polyolefins. Food Addit. Contam. 1996, 13, 949–967. [Google Scholar] [CrossRef] [PubMed]
- Graciano-Verdugo, A.Z.; Soto-Valdez, H.; Peralta, E.; Cruz-Zárate, P.; Islas-Rubio, A.R.; Sánchez-Valdes, S.; Sánchez-Escalante, A.; González-Méndez, N.; González-Ríos, H. Migration of a-tocopherol from LDPE films to corn oil and its effect on the oxidative stability. Food Res. Int. 2010, 43, 1073–1078. [Google Scholar] [CrossRef]
- Franz, R.; Welle, F. Migration measurement and modelling from poly (ethylene terephthalate) (PET) into soft drinks and fruit juices in comparison with food simulants. Food Addit. Contam. 2008, 25, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).