Fucoidan from Marine Macroalgae: Biological Actions and Applications in Regenerative Medicine, Drug Delivery Systems and Food Industry
Abstract
:1. Introduction
2. Marine Macroalgal Sources of Fucoidan
3. Cultivation of Marine Macroalga
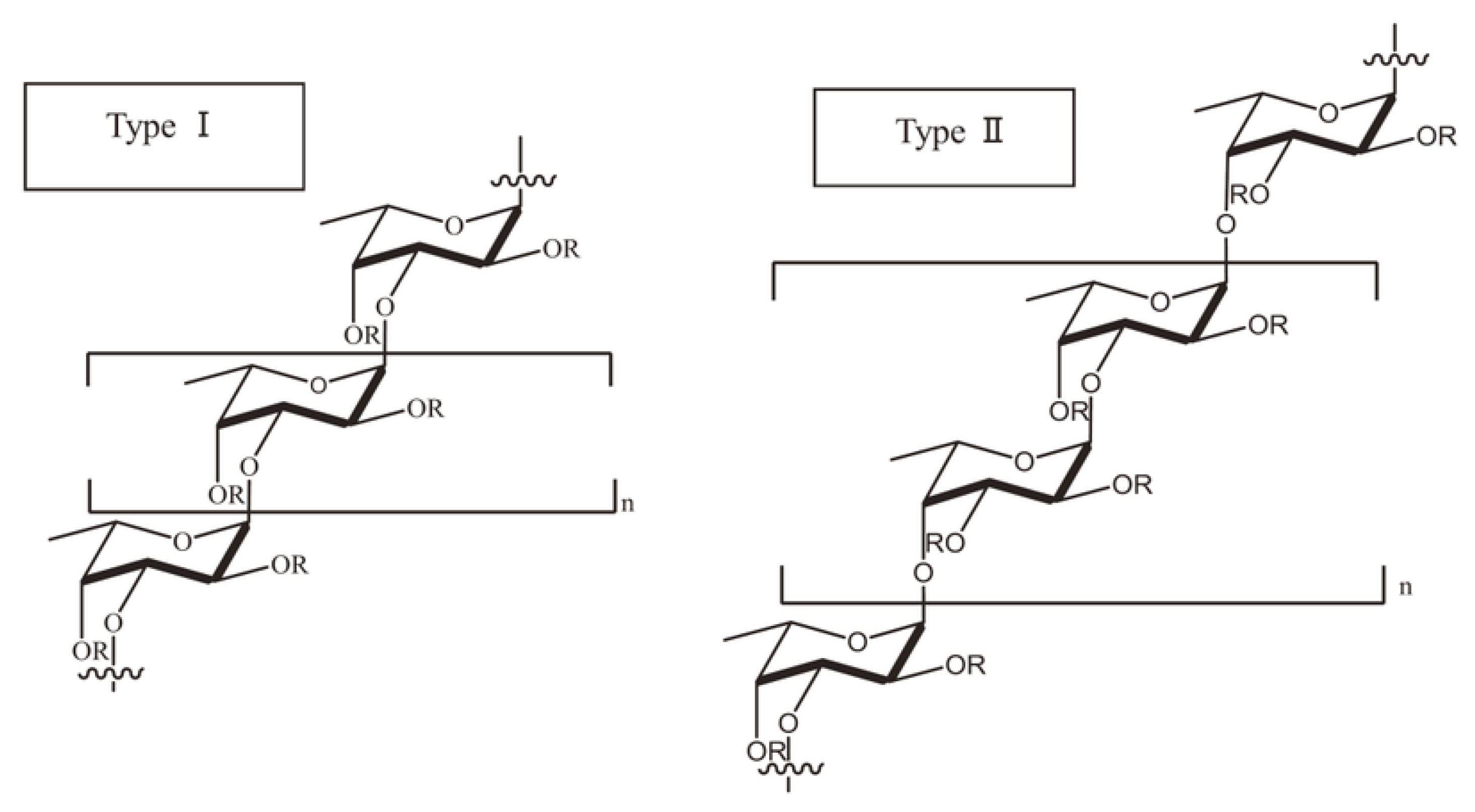
4. Composition, Structure and Physicochemical Properties of Fucoidan
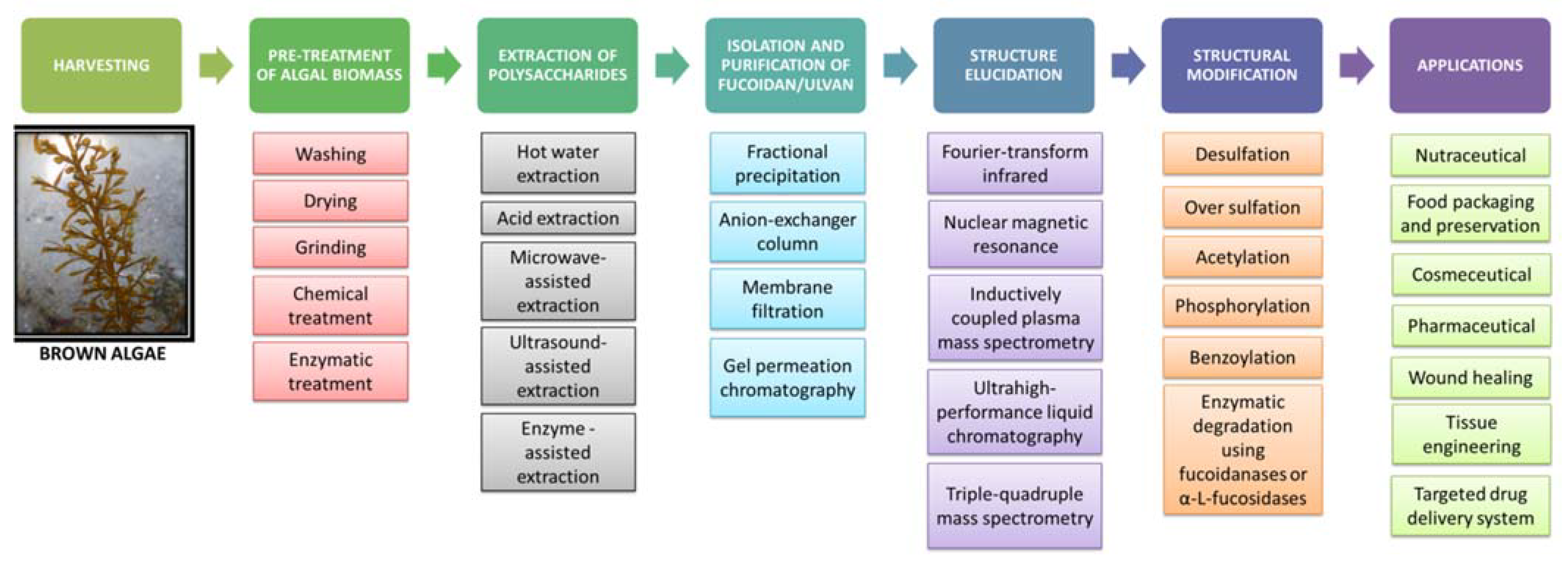
5. Extraction, Purification and Structural Modification of Fucoidan
5.1. Harvesting and Pretreatment of Algal Biomass
5.2. Extraction of Fucoidan
5.3. Isolation and Purification of Fucoidan
5.4. Structure Elucidation of Fucoidan
5.5. Structural Modification of Fucoidan
6. Industrial Production Scenario
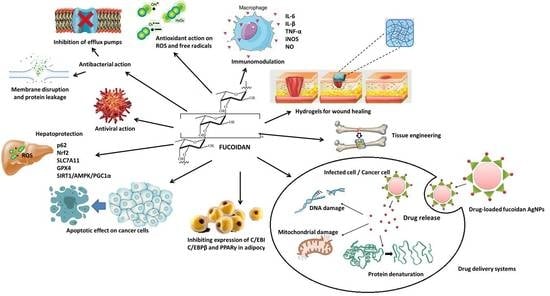
7. Biological Action and Health Benefits of Fucoidan
7.1. Antioxidant Action
7.2. Anticancer Action/Apoptotic Effect
7.3. Immunomodulating Action
7.4. Lipolytic and Anti-Adipogenic/Anti-Obesogenic Activity
7.5. Hepatoprotective Action
7.6. Neuroprotective Action
7.7. Anticoagulant Action
7.8. Antibacterial Action
7.9. Antiviral Action
7.10. Cosmeceutical Applications
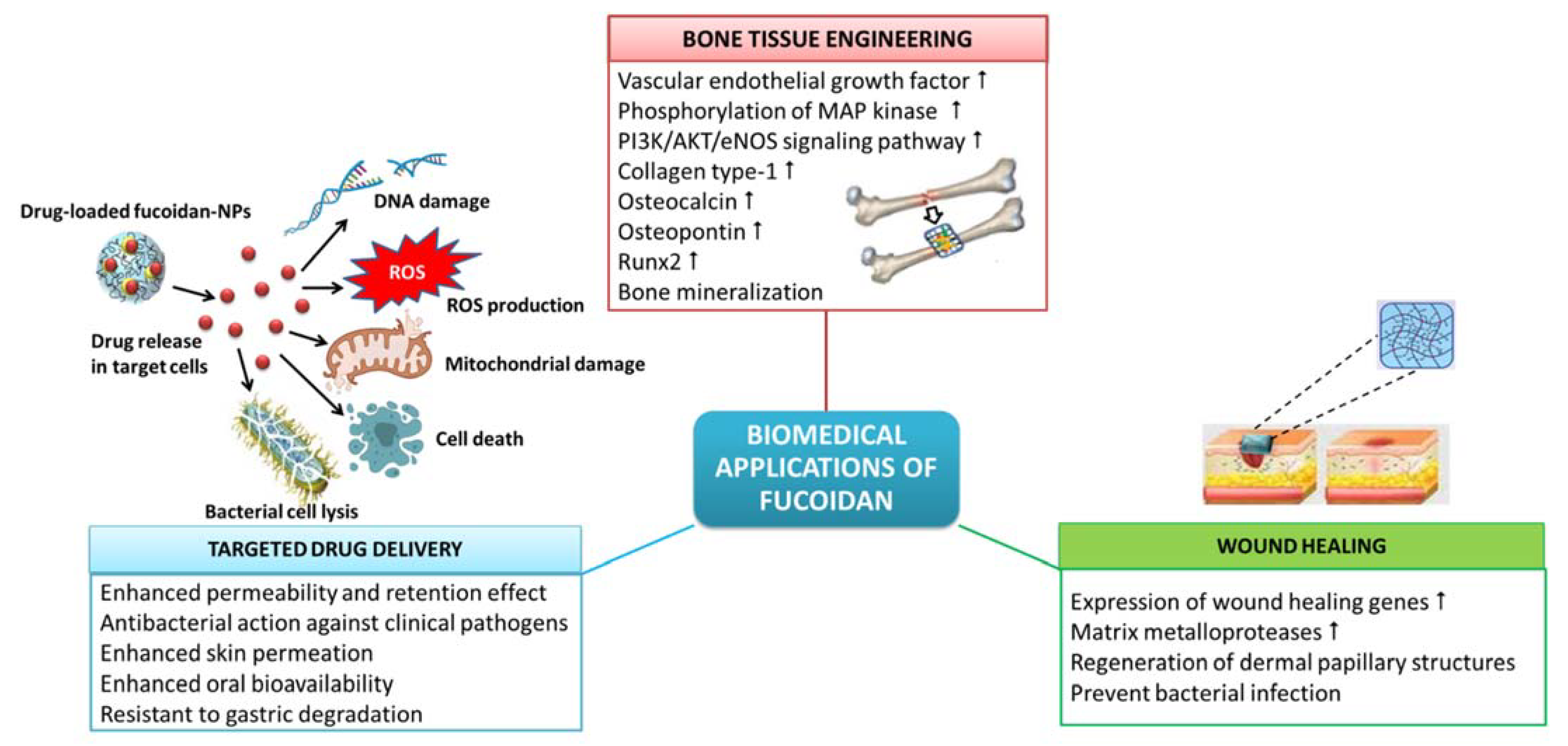
8. Biomedical Applications of Fucoidan
8.1. Wound Healing
8.2. Tissue Engineering and Regenerative Medicine
8.3. Targeted Drug Delivery Systems
9. Food and Feed Applications of Fucoidan
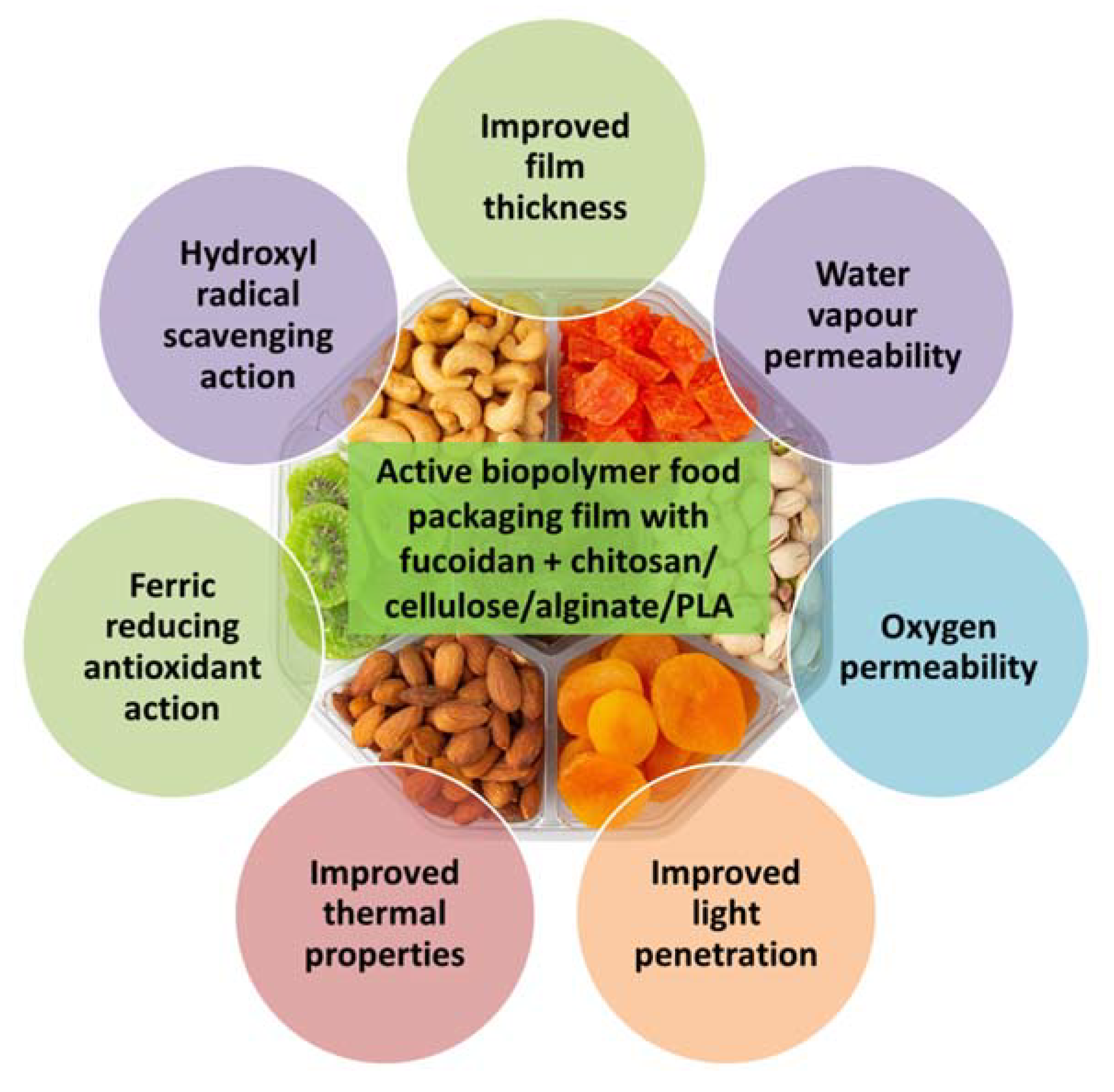
10. Food Packaging and Preservation
11. Challenges and Outlook
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdulrahman, A.S.; Omar, H.H.; Bahabri, F.S. Health Benefits of Edible Seaweeds and Their Nano-Applications. J. Am. Sci. 2020, 16, 40–72. [Google Scholar] [CrossRef]
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydr. Polym. 2017, 175, 395–408. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Jeon, Y. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Zayed, A.; El-Aasr, M.; Ibrahim, A.S.; Ulber, R. Fucoidan Characterization: Determination of Purity and Physicochemical and Chemical Properties. Mar. Drugs 2020, 18, 571. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Patel, A.K.; Vadrale, A.P.; Singhania, R.R.; Michaud, P.; Pandey, A.; Chen, S.-J.; Chen, C.-W. Algal polysaccharides: Current status and future prospects. Phytochem. Rev. 2022, 5, 1–30. [Google Scholar] [CrossRef]
- Li, J.; He, Z.; Liang, Y.; Peng, T.; Hu, Z. Insights into Algal Polysaccharides: A Review of Their Structure, Depolymerases, and Metabolic Pathways. J. Agric. Food Chem. 2022, 70, 1749–1765. [Google Scholar] [CrossRef]
- Priyanka, K.R.; Rajaram, R.; Sivakumar, S.R. A critical review on pharmacological properties of marine macroalgae. Biomass Conv. Bioref. 2022. online first. [Google Scholar] [CrossRef]
- Zayed, A.; Avila-Peltroche, J.; El-Aasr, M.; Ulber, R. Sulfated Galactofucans: An Outstanding Class of Fucoidans with Promising Bioactivities. Mar. Drugs 2022, 20, 412. [Google Scholar] [CrossRef]
- Kawai, H.; Henry, E.C. Phaeophyta. In Handbook of the Protists; Archibald, J., Simpson, A., Slamovits, C., Eds.; Springer: Cham, Switzerland, 2017; pp. 267–304. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a Fucoidan from the Brown Seaweed Fucus serratus L. Carbohydr. Res. 2006, 341, 238–245. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Kelly, M.; Sanderson, C.J.; Nifantiev, N.E.; Usov, A.I. Further Studies on the Composition and Structure of a Fucoidan Preparation from the Brown Alga Saccharina latissima. Carbohydr. Res. 2010, 345, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Thuy, T.T.T.; Van, T.T.T.; Ly, B.M.; Nifantiev, N.E.; Usov, A.I. Preliminary Investigation of a Highly Sulfated Galactofucan Fraction Isolated from the Brown Alga Sargassum polycystum. Carbohydr. Res. 2013, 377, 48–57. [Google Scholar] [CrossRef]
- Chevolot, L.; Mulloy, B.; Ratiskol, J.; Foucault, A.; Colliec-Jouault, S. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydr. Res. 2001, 330, 529–535. [Google Scholar] [CrossRef]
- Elizondo-Gonzalez, R.; Cruz-Suarez, L.E.; Ricque-Marie, D.; Mendoza-Gamboa, E.; Rodriguez-Padilla, C.; Trejo-Avila, L.M. In vitro characterization of the antiviral activity of fucoidan from Cladosiphon okamuranus against Newcastle Disease Virus. Virol. J. 2012, 9, 307. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Structural Characterization of Laminaran and Galactofucan Extracted from the Brown Seaweed Saccharina longicruris. Phytochemistry 2010, 71, 1586–1595. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Jeon, Y.-J. Enzyme-assistant extraction (EAE) of bioactive components: A useful approach for recovery of industrially important metabolites from seaweeds: A review. Fitoterapia 2012, 83, 6–12. [Google Scholar] [CrossRef]
- Krylova, N.V.; Ermakova, S.P.; Lavrov, V.F.; Leneva, I.A.; Kompanets, G.G.; Iunikhina, O.V.; Nosik, M.N.; Ebralidze, L.K.; Falynskova, I.N.; Silchenko, A.S.; et al. The Comparative Analysis of Antiviral Activity of Native and Modified Fucoidans from Brown Algae Fucus evanescens In Vitro and In Vivo. Mar. Drugs 2020, 18, 224. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Rasin, A.B.; Kusaykin, M.I.; Kalinovsky, A.I.; Miansong, Z.; Changheng, L.; Malyarenko, O.; Zueva, A.O.; Zvyagintseva, T.N.; Ermakova, S.P. Structure, enzymatic transformation, anticancer activity of fucoidan and sulphated fucooligosaccharides from Sargassum horneri. Carbohydr. Polym. 2017, 175, 654–660. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Shevchenko, N.M.; Malyarenko, O.S.; Anastyuk, S.D.; Kasprik, A.E.; Zvyagintsev, N.V.; Ermakova, S.P. Fucoidans from brown algae Laminaria longipes and Saccharina cichorioides: Structural characteristics, anticancer and radiosensitizing activity in vitro. Carbohydr. Polym. 2019, 221, 157–165. [Google Scholar] [CrossRef]
- Kopplin, G.; Rokstad, A.M.; Mélida, H.; Bulone, V.; Skjåk-Bræk, G.; Aachmann, F.L. Structural Characterization of Fucoidan from Laminaria hyperborea: Assessment of Coagulation and Inflammatory Properties and Their Structure-Function Relationship. ACS Appl. Bio Mater. 2018, 1, 1880–1892. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. A highly regular fraction of a fucoidan from the brown seaweed Fucus distichus L. Carbohydr. Res. 2004, 339, 511–517. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Camarena-gómez, M.T. European Union legislation on macroalgae products. Aquac. Int. 2021, 29, 487–509. [Google Scholar] [CrossRef]
- Sasaki, Y.; Yoshikuni, Y. Metabolic engineering for valorization of macroalgae biomass. Metab. Eng. 2022, 71, 42–61. [Google Scholar] [CrossRef]
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4. 0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef]
- Sebök, S.; Hanelt, D. Examining the capacity for cultivating marine macroalgae using process liquids from biogas digestate as nutrient source and cultivation medium. Biomass Bioener. 2020, 142, 105762. [Google Scholar] [CrossRef]
- Godvin, S.V.; Dinesh, K.M.; Pugazhendi, A.; Bajhaiya, A.K.; Gugulothu, P.; Rajesh, B.J. Biofuel production from Macroalgae: Present scenario and future scope. Bioengineered 2021, 12, 9216–9238. [Google Scholar] [CrossRef]
- Araújo, R.; Calderón, F.V.; López, J.S.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Tasende, M.G.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Califano, G.; Kwantes, M.; Abreu, M.H.; Costa, R.; Wichard, T. Cultivating the Macroalgal Holobiont: Effects of Integrated Multi-Trophic Aquaculture on the Microbiome of Ulva rigida (Chlorophyta). Front. Mar. Sci. 2020, 7, 52. [Google Scholar] [CrossRef]
- Luthuli, S.; Siya, W.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, Q.; Wu, J.; Yang, X.; Yang, S.; Zhu, W.; Liu, Y. Fucoidan Extracted From Sporophyll of Undaria pinnatifida Grown in Weihai, China—Chemical Composition and Comparison of Antioxidant Activity of Different Molecular Weight Fractions. Front. Nutr. 2021, 8, 636930. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Choi, J.; Park, H. Antioxidant activities of fucoidan degraded by gamma irradiation and acidic hydrolysis. Radiat. Phys. Chem. 2015, 109, 23–26. [Google Scholar] [CrossRef]
- Gotteland, M.; Riveros, K.; Gasaly, N.; Carcamo, C. The Pros and Cons of Using Algal Polysaccharides as Prebiotics. Front. Nutr. 2020, 7, 163. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important Determinants for Fucoidan Bioactivity: A Critical Review of Structure-Function Relations and Extraction Methods for Fucose-Containing Sulfated Polysaccharides from Brown Seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.E.R.; Cardoso, M.A.; Noseda, M.D.; Cerezo, A.S. Structural studies on fucoidans from the brown seaweed Sargassum stenophyllum. Carbohydr. Res. 2001, 333, 281–293. [Google Scholar] [CrossRef]
- Ponce, N.M.A.; Pujol, C.A.; Damonte, E.B.; Flores, M.L.; Stortz, C.A. Fucoidans from the brown seaweed Adenocystis utricularis: Extraction methods, antiviral activity and structural studies. Carbohydr. Res. 2003, 338, 153–165. [Google Scholar] [CrossRef]
- Cui, M.; Li, X.; Geng, L.; Wu, N.; Wang, J.; Deng, Z.; Li, Z.; Zhang, Q. Comparative study of the immunomodulatory effects of different fucoidans from Saccharina japonica mediated by scavenger receptors on RAW264.7 macrophages. Int. J. Biol. Macromol. 2022, 215, 253–261. [Google Scholar] [CrossRef]
- Silva, M.M.C.L.; dos Santos Lisboa, L.; Paiva, W.S.; Batista, L.A.N.C.; Luchiari, A.C.; Rocha, H.A.O.; Camara, R.B.G. Comparison of in vitro and in vivo antioxidant activities of commercial fucoidans from Macrocystis pyrifera, Undaria pinnatifida, and Fucus vesiculosus. Int. J. Biol. Macromol. 2022, 216, 757–767. [Google Scholar] [CrossRef]
- Nishino, T.; Aizu, Y.; Nagumo, T. The relationship between the molecular weight and the anticoagulant activity of two types of fucan sulfates from the brown seaweed Ecklonia kurome. Agric. Biol. Chem. 1991, 55, 791–796. [Google Scholar] [CrossRef]
- Fitton, J.H.; Dell’Acqua, G.; Gardiner, V.-A.; Karpiniec, S.S.; Stringer, D.N.; Davis, E. Topical Benefits of Two Fucoidan-Rich Extracts from Marine Macroalgae. Cosmetics 2015, 2, 66–81. [Google Scholar] [CrossRef]
- Tako, M. Rheological Characteristics of Fucoidan Isolated from Commercially Cultured Cladosiphon okamuranus. Bot. Mar. 2003, 46, 465. [Google Scholar] [CrossRef]
- Sezer, A.D.; Cevher, E.; Hatipoglu, F.; Ogurtan, Z.; Bas, A.L.; Akbuga, J. Preparation of Fucoidan-Chitosan Hydrogel and Its Application as Burn. Biol. Pharm. Bull. 2008, 31, 2326–2333. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Kim, J.-K.; Cho, T.-S. Applications of ophthalmic biomaterials embedded with fucoidan. J. Ind. Eng. Chem. 2012, 18, 1197–1201. [Google Scholar] [CrossRef]
- Badrinathan, S.; Shiju, T.M.; Christa, A.S.S.; Arya, R.; Pragasam, V. Purification and Structural Characterization of Sulfated Polysaccharide from Sargassum myriocystum and its Efficacy in Scavenging Free Radicals. Indian J. Pharm. Sci. 2012, 74, 549–555. [Google Scholar]
- Shanthi, N.; Arumugam, P.; Murugan, M.; Sudhakar, M.P.; Arunkumar, K. Extraction of Fucoidan from Turbinaria decurrens and the Synthesis of Fucoidan-Coated AgNPs for Anticoagulant Application. ACS Omega 2021, 6, 30998–31008. [Google Scholar] [CrossRef]
- Luan, F.; Zou, J.; Rao, Z.; Ji, Y.; Lei, Z.; Peng, L.; Yang, Y.; He, X.; Zeng, N. Polysaccharides from Laminaria japonica: An insight into the current research on structural features and biological properties. Food Funct. 2021, 12, 4254–4283. [Google Scholar] [CrossRef]
- Aguilar-Briseño, J.A.; Cruz-Suarez, L.E.; Ricque-Marie, D.; Zapata-Benavides, P.; Mendoza-Gamboa, E.; Rodríguez-Padilla, C.; Maria Trejo-Avila, L. Sulphated Polysaccharides from Ulva clathrata and Cladosiphon okamuranus Seaweeds both Inhibit Viral Attachment/Entry and Cell-Cell Fusion, in NDV Infection. Mar. Drugs 2015, 13, 697–712. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef]
- Getachew, A.T.; Holdt, S.L.; Meyer, A.S.; Jacobsen, C. Effect of Extraction Temperature on Pressurized Liquid Extraction of Bioactive Compounds from Fucus vesiculosus. Mar. Drugs 2022, 20, 263. [Google Scholar] [CrossRef]
- Rodriguez-Jasso, R.; Mussatto, S.; Pastrana, L.; Aguilar, C.; Teixeira, J. Chemical composition and antioxidant activity of sulphated polysaccharides extracted from Fucus vesiculosus using different hydrothermal processes. Chem. Pap. 2014, 68, 203–209. [Google Scholar] [CrossRef]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydr. Polym. 2011, 86, 1137–1144. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; You, S. Ultrasound-assisted extraction of sulfated polysaccharide from Nizamuddinia zanardinii: Process optimization, structural characterization, and biological properties. J. Food Process Eng. 2019, 42, e12979. [Google Scholar] [CrossRef]
- Devi, G.V.Y.; Nagendra, A.H.; Sudheer, S.P.; Chatterjee, K.; Venkatesan, J. Isolation and purification of fucoidan from Sargassum ilicifolium: Osteogenic differentiation potential in mesenchymal stem cells for bone tissue engineering. J. Taiwan Inst. Chem. Eng. 2022, 136, 104418. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Barba, F.J.; Lorenzo, J.M. Antioxidant Potential of Extracts Obtained from Macro- (Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and Micro-Algae (Chlorella vulgaris and Spirulina platensis) Assisted by Ultrasound. Medicines 2018, 5, 33. [Google Scholar] [CrossRef]
- My, P.L.T.; Sung, V.V.; Dat, T.D.; Nam, H.M.; Phong, M.T.; Hieu, N.H. Ultrasound-Assisted Extraction of Fucoidan from Vietnamese Brown Seaweed Sargassum mcclurei and Testing Bioactivities of the Extract. Chem. Sel. 2020, 5, 4371–4380. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Doherty, J.V.O.; Tiwari, B.K.; Sweeney, T. Enhancing the Extraction of Polysaccharides and Antioxidants from Macroalgae Using Sequential Hydrothermal-Assisted Extraction Followed by Ultrasound and Thermal Technologies. Mar. Drugs 2019, 17, 457. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Balboa, E.M.; Domínguez, H. Extraction and Purification of Fucoidan from Marine Sources. In Encyclopedia of Marine Biotechnology; Kim, S.-K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Anastyuk, S.D.; Shevchenko, N.M.; Surits, V.V.; Silchenko, A.S.; Isakov, V.V.; Zvyagintseva, T.N.; Thinh, P.D.; Ermakova, S.P. Polysaccharides from brown algae Sargassum duplicatum: The structure and anticancer activity in vitro. Carbohydr. Polym. 2017, 175, 547–556. [Google Scholar] [CrossRef]
- Galermo, A.G.; Nandita, E.; Barboza, M.; Amicucci, M.J.; Vo, T.-T.T.; Lebrilla, C.B. Liquid Chromatography–Tandem Mass Spectrometry Approach for Determining Glycosidic Linkages. Anal. Chem. 2018, 90, 13073–13080. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, F.; Zhang, Q.; Zhang, Z.; Shi, X.; Li, P. Synthesized different derivatives of low molecular fucoidan extracted from Laminaria japonica and their potential antioxidant activity in vitro. Int. J. Biol. Macromol. 2009, 44, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Zhang, J.; Li, P. Synthesized phosphorylated and aminated derivatives of fucoidan and their potential antioxidant activity in vitro. Int. J. Biol. Macromol. 2009, 44, 170–174. [Google Scholar] [CrossRef]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef]
- Pacheco, D.; Cotas, J.; Amm, G.; Pereira, L. Fucoidan—A valuable source from the ocean to pharmaceutical. Front. Drug. Chem. Clin. Res. 2020, 3, 1–4. [Google Scholar] [CrossRef]
- Cai, J.; Lovatelli, A.; Aguilar-Manjarrez, J.; Cornish, L.; Dabbadie, L.; Desrochers, A.; Diffey, S.; Garrido Gamarro, E.; Geehan, J.; Hurtado, A.; et al. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development, FAO Fisheries and Aquaculture Circular No. 1229; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Fortune Business Insights. Available online: https://www.fortunebusinessinsights.com/industry-reports/commercial-seaweed-market-100077 (accessed on 15 August 2022).
- Hafting, J.T.; Craigie, J.S.; Stengel, D.B.; Loureiro, R.R.; Buschmann, A.H.; Yarish, C.; Edwards, M.D.; Critchley, A.T. Prospects and challenges for industrial production of seaweed bioactives. J. Phycol. 2015, 51, 821–837. [Google Scholar] [CrossRef]
- Office of Food Additive Safety US Food and Drug Administration. GRAS Notification for Fucoidan Concentrate from Fucus vesiculosus; GRAS Notice No. 661; Office of Food Additive Safety, US Food and Drug Administration: College Park, MD, USA, 2016.
- Zayed, A.; Ulber, R. Fucoidans: Downstream Processes and Recent Applications. Mar. Drugs 2020, 18, 170. [Google Scholar] [CrossRef]
- Oliveira, R.M.; Barros, R.; Gomes, C.; Fernanda, J.; Monte, S.; Lucas, R.; Viana, S.; Rachel, K.; Melo, T.; Queiroz, M.F.; et al. Commercial Fucoidans from Fucus vesiculosus Can Be Grouped into Antiadipogenic and Adipogenic Agents. Mar. Drugs 2018, 16, 193. [Google Scholar] [CrossRef] [Green Version]
- Chauvierre, C.; Aid-Launais, R.; Aerts, J.; Chaubet, F.; Maire, M.; Chollet, L.; Rolland, L.; Bonafé, R.; Rossi, S.; Bussi, S.; et al. Pharmaceutical Development and Safety Evaluation of a GMP-Grade Fucoidan for Molecular Diagnosis of Cardiovascular Diseases. Mar. Drugs 2019, 17, 699. [Google Scholar] [CrossRef]
- Senni, K.; Gueniche, F.; Foucault-Bertaud, A.; Igondjo-Tchen, S.; Fioretti, F.; Colliec-Jouault, S.; Durand, P.; Guezennec, J.; Godeau, G.; Letourneur, D. Fucoidan a sulfated polysaccharide from brown algae is a potent modulator of connective tissue proteolysis. Arch. Biochem. Biophys. 2006, 445, 56–64. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Cao, M.-J.; Liu, G.-M.; Chen, Q.; Sun, L.; Chen, H. Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. Carbohydr. Polym. 2017, 172, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Wan Aida, W.M.; Maskat, M.Y.; Mamot, S.; Ropien, J.; Mazita Mohd, D. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocoll. 2014, 42, 280–288. [Google Scholar] [CrossRef]
- Narayani, S.S.; Saravanan, S.; Ravindran, J.; Ramasamy, M.S.; Chitra, J. In vitro anticancer activity of fucoidan extracted from Sargassum cinereum against Caco-2 cells. Int. J. Biol. Macromol. 2019, 138, 618–628. [Google Scholar] [CrossRef]
- Yuguchi, Y.; Tran, V.T.T.; Bui, L.M.; Takebe, S.; Suzuki, S.; Nakajima, N.; Kitamura, S.; Thanh, T.T.T. Primary structure, conformation in aqueous solution, and intestinal immunomodulating activity of fucoidan from two brown seaweed species Sargassum crassifolium and Padina australis. Carbohydr. Polym. 2016, 147, 69–78. [Google Scholar] [CrossRef]
- Hwang, P.-A.; Chien, S.-Y.; Chan, Y.-L.; Lu, M.-K.; Wu, C.-H.; Kong, Z.-L.; Wu, C.-J. Inhibition of Lipopolysaccharide (LPS)-Induced Inflammatory Responses by Sargassum hemiphyllum Sulfated Polysaccharide Extract in RAW264.7 Macrophage Cells. J. Agric. Food Chem. 2011, 59, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.-L.; Li, Y.; Ni, L.-Q.; Li, Y.-X.; Cui, Y.-S.; Jiang, S.-L.; Xie, E.-Y.; Du, J.; Deng, F.; Dong, C.-X. Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr. Polym. 2020, 229, 115487. [Google Scholar] [CrossRef]
- Dinesh, S.; Menon, T.; Hanna, L.E.; Suresh, V.; Sathuvan, M.; Manikannan, M. In vitro anti-HIV-1 activity of fucoidan from Sargassum swartzii. Int. J. Biol. Macromol. 2016, 82, 83–88. [Google Scholar] [CrossRef]
- Hayashi, K.; Lee, J.-B.; Nakano, T.; Hayashi, T. Anti-influenza A virus characteristics of a fucoidan from sporophyll of Undaria pinnatifida in mice with normal and compromised immunity. Microbes Infect. 2013, 15, 302–309. [Google Scholar] [CrossRef]
- Tan, M.T.H.; Gorji, M.E.; Toh, J.Y.L.; Park, A.Y.; Li, Y.; Gong, Z.; Li, D. Fucoidan from Fucus vesiculosus can inhibit human norovirus replication by enhancing the host innate immune response. J. Funct. Foods 2022, 95, 105149. [Google Scholar] [CrossRef]
- Kim, K.-J.; Yoon, K.-Y.; Lee, B.-Y. Low molecular weight fucoidan from the sporophyll of Undaria pinnatifida suppresses inflammation by promoting the inhibition of mitogen-activated protein kinases and oxidative stress in RAW264.7 cells. Fitoterapia 2012, 83, 1628–1635. [Google Scholar] [CrossRef]
- Yang, J.; Lim, S.Y. Fucoidans and Bowel Health. Mar. Drugs 2021, 19, 436. [Google Scholar] [CrossRef] [PubMed]
- Chantree, P.; Na-Bangchang, K.; Martviset, P. Anticancer activity of fucoidan via apoptosis and cell cycle arrest on cholangiocarcinoma cell. Asian Pacific J. Cancer Prev. 2021, 22, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Banafa, A.M.; Roshan, S.; Liu, Y.Y.; Chen, H.J.; Chen, M.J.; Yang, G.X.; He, G.Y. Fucoidan induces G1 phase arrest and apoptosis through caspases-dependent pathway and ROS induction in human breast cancer MCF-7 cells. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2013, 33, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Teruya, K.; Yoshida, T.; Eto, H.; Shirahata, S. Fucoidan extract enhances the anti-cancer activity of chemotherapeutic agents in MDA-MB-231 and MCF-7 breast cancer cells. Mar. Drugs 2013, 11, 81–98. [Google Scholar] [CrossRef]
- Yang, L.; Wang, P.; Wang, H.; Li, Q.; Teng, H.; Liu, Z.; Yang, W.; Hou, L.; Zou, X. Fucoidan derived from Undaria pinnatifida induces apoptosis in human hepatocellular carcinoma SMMC-7721 cells via the ROS-mediated mitochondrial pathway. Mar. Drugs. 2013, 11, 1961–1976. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, Y.; Zhang, Y.; Zhang, D. Fucoidan induces cancer cell apoptosis by modulating the endoplasmic reticulum stress cascades. PLoS ONE 2014, 9, e108157. [Google Scholar] [CrossRef]
- Ma, D.; Wei, J.; Chen, S.; Wang, H.; Ning, L.; Luo, S.-H.; Liu, C.-L.; Song, G.; Yao, Q. Fucoidan Inhibits the Progression of Hepatocellular Carcinoma via Causing lncRNA LINC00261 Overexpression. Front. Oncol. 2021, 11, 653902. [Google Scholar] [CrossRef]
- Takahashi, H.; Kawaguchi, M.; Kitamura, K.; Narumiya, S.; Kawamura, M.; Tengan, I.; Nishimoto, S.; Hanamure, Y.; Majima, Y.; Tsubura, S.; et al. An Exploratory Study on the Anti-inflammatory Effects of Fucoidan in Relation to Quality of Life in Advanced Cancer Patients. Integr. Cancer Ther. 2018, 17, 282–291. [Google Scholar] [CrossRef] [Green Version]
- Nagamine, T.; Kadena, K.; Tomori, M.; Nakajima, K.; Iha, M. Activation of NK cells in male cancer survivors by fucoidan extracted from Cladosiphon okamuranus. Mol. Clin. Oncol. 2020, 12, 81–88. [Google Scholar] [CrossRef]
- Chen, D.; Wu, X.Z.; Wen, Z.Y. Sulfated polysaccharides and immune response: Promoter or inhibitor? Panminerva Med. 2008, 50, 177–183. [Google Scholar]
- Nakamura, T.; Suzuki, H.; Wada, Y.; Kodama, T.; Doi, T. Fucoidan induces nitric oxide production via p38 mitogen-activated protein kinase and NF-κB-dependent signaling pathways through macrophage scavenger receptors. Biochem. Biophys. Res. Commun. 2006, 343, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, F.; Hayes, A.W.; Karimi, G. The role of ferroptosis in organ toxicity. Hum. Exp. Toxicol. 2021, 40, S851–S860. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Tian, Y.; Sui, Y.; Zhao, H.; Gao, H.; Liang, H.; Qiu, X.; Sun, Z.; Zhang, Y.; Qin, Y. Protective effect of fucoidan against iron overload and ferroptosis-induced liver injury in rats exposed to alcohol. Biomed. Pharmacother. 2022, 153, 113402. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, T.; Wang, Z.; Xu, Y.; Zhang, Q.; Luo, D. Low molecular weight fucoidan attenuates liver injury via SIRT1/AMPK/PGC1 α axis in db/db mice. Int. J. Biol. Macromol. 2018, 112, 929–936. [Google Scholar] [CrossRef]
- Wei, H.; Gao, Z.; Zheng, L.; Zhang, C.; Liu, Z.; Yang, Y.; Teng, H.; Hou, L.; Yin, Y.; Zou, X. Protective Effects of Fucoidan on Aβ 25–35 and D-Gal-Induced Neurotoxicity in PC12 Cells and D-Gal-Induced Cognitive Dysfunction in Mice. Mar. Drugs 2017, 15, 77. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, J.; Zheng, Y.; Su, R.; Liao, Y.; Gong, X.; Liu, L.; Wang, X. Fucoidan protects dopaminergic neurons by enhancing the mitochondrial function in a rotenone-induced rat model of Parkinson’s disease. Aging Dis. 2018, 9, 590–604. [Google Scholar] [CrossRef]
- Subaraja, M.; Krishnan, D.A.; Hillary, V.E.; Raja, T.R.W.; Mathew, P.; RaviKumar, S.; Paulraj, M.G.; Ignacimuthu, S. Fucoidan Serves a Neuroprotective Effect in an Alzheimer’s Disease Model. Front. Biosci. Elite Ed. 2020, 12, 1–34. [Google Scholar]
- Gao, Y.; Dong, C.; Yin, J.; Shen, J.; Tian, J.; Li, C. Neuroprotective effect of fucoidan on H2O2- induced apoptosis in PC12 cells via activation of PI3K/Akt pathway. Cellular Mol. Neurobiol. 2012, 32, 523–529. [Google Scholar] [CrossRef]
- Meenakshi, S.; Umayaparvathi, S.; Saravanan, R.; Manivasagam, T.; Balasubraminan, T. Neuroprotective effect of fucoidan from Turbinaria decurrens in MPTP intoxicated Parkinsonic mice. Int. J. Biol. Macromol. 2016, 86, 425–433. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, Z.; Feng, X.; Deng, J.; He, C.; Li, R.; Zhao, Y.; Ge, Y.; Zhang, Y.; Song, C.; et al. The Emerging Evidence for a Protective Role of Fucoidan from Laminaria japonica in Chronic Kidney Disease-Triggered Cognitive Dysfunction. Mar. Drugs 2022, 20, 258. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, X.; Yuan, H.; Huang, S.; Park, S. Mitigation of Memory Impairment with Fermented Fucoidan and λ-Carrageenan Supplementation through Modulating the Gut Microbiota and Their Metagenome Function in Hippocampal Amyloid-β Infused Rats. Cells 2022, 11, 2301. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Teng, X.; Liang, H.; Zhao, J.; Jiang, Y.; Qiu, X.; Zhang, Z.; Pei, Z.; Zhang, N.; Qin, Y. Neuroprotective effect of fucoidan by regulating gut-microbiota-brain axis in alcohol withdrawal mice. J. Funct. Foods 2021, 86, 104726. [Google Scholar] [CrossRef]
- Vo, T.; Kim, S. Fucoidans as a natural bioactive ingredient for functional foods. J. Funct. Foods 2012, 5, 16–27. [Google Scholar] [CrossRef]
- Rocha, G.A.; Ferreira, R.B.R. Antimicrobial polysaccharides obtained from natural sources. Future Microbiol. 2022, 17, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.; Pinto, E.; Kijjoa, A.; Pinto, M.; Sousa, E. Targeting antimicrobial drug resistance with marine natural products. Int. J. Antimicrob. Agents 2020, 56, 106005. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Antibacterial Derivatives of Marine Algae: An Overview of Pharmacological Mechanisms and Applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef]
- Silva, A.; Silva, S.A.; Carpena, M.; Gull, P.; Barroso, M.F.; Prieto, M.A. Macroalgae as a Source of Valuable Antimicrobial Compounds: Extraction and Applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Jeong, M.-R.; Choi, S.-M.; Na, S.-S.; Cha, J.-D. Synergistic effect of fucoidan with antibiotics against oral pathogenic bacteria. Arch. Oral Biol. 2013, 58, 482–492. [Google Scholar] [CrossRef]
- Hans, N.; Malik, A.; Naik, S. Antiviral activity of sulfated polysaccharides from marine algae and its application in combating COVID-19: Mini review. Bioresour. Technol. Rep. 2021, 13, 100623. [Google Scholar] [CrossRef]
- Queiroz, K.C.S.; Medeiros, V.P.; Queiroz, L.S.; Abreu, L.R.D.; Rocha, H.A.O.; Ferreira, C.V.; Jucá, M.B.; Aoyama, H.; Leite, E.L. Inhibition of reverse transcriptase activity of HIV by polysaccharides of brown algae. Biomed. Pharmacother. 2008, 62, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.T.; Ly, B.M.; Van, T.T.T.; Quang, N.V.; Tu, H.C.; Zheng, Y.; Seguin-Devaux, C.; Mi, B.; Ai, U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015, 115, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.J.; Dow, A.A. Clinical Studies of the Safety and Efficacy of Macroalgae Extracts in Cosmeceuticals. J. Clin. Aesthet. Dermatol. 2021, 14, 37–41. [Google Scholar] [PubMed]
- Brunt, E.G.; Burgess, J.G. The promise of marine molecules as cosmetic active ingredients. Int. J. Cosmet. Sci. 2018, 40, 1–15. [Google Scholar] [CrossRef]
- Fujimura, T.; Tsukahara, K.; Moriwaki, S.; Kitahara, T.; Sano, T.; Takema, Y. Treatment of human skin with an extract of Fucus vesiculosus changes its thickness and mechanical properties. J. Cosmet. Sci. 2002, 53, 1–9. [Google Scholar]
- Agatonovic-Kustrin, S.; Morton, D.W. Cosmeceuticals Derived from Bioactive Substances Found in Marine Algae. J. Oceanogr. Mar. Res. 2013, 1, 106. [Google Scholar] [CrossRef]
- Hirose, K.; Sasatsu, M.; Toraishi, T.; Onishi, H. Novel Xyloglucan Sheet for the Treatment of Deep Wounds: Preparation, Physicochemical Characteristics, and in vivo Healing Effects. Biol. Pharm. Bull. 2019, 42, 1409–1414. [Google Scholar] [CrossRef]
- Kim, B.-S.; Yang, S.-S.; You, H.-K.; Shin, H.-I.; Lee, J. Fucoidan-induced osteogenic differentiation promotes angiogenesis by inducing vascular endothelial growth factor secretion and accelerates bone repair. J. Tissue Eng. Regen. Med. 2018, 12, e1311–e1324. [Google Scholar] [CrossRef]
- Ahn, T.Y.; Kang, J.H.; Kang, D.J.; Venkatesan, J.; Chang, H.K.; Bhatnagar, I.; Chang, K.Y.; Hwang, J.H.; Salameh, Z.; Kim, S.K.; et al. Interaction of stem cells with nano hydroxyapatite-fucoidan bionanocomposites for bone tissue regeneration. Int. J. Biol. Macromol. 2016, 93, 1488–1491. [Google Scholar] [CrossRef]
- Pawar, V.K.; Singh, Y.; Sharma, K.; Shrivastav, A.; Sharma, A.; Singh, A.; Gopal, J.; Singh, P.; Raval, K.; Kumar, A.; et al. Improved chemotherapy against breast cancer through immunotherapeutic activity of fucoidan decorated electrostatically assembled nanoparticles bearing doxorubicin. Int. J. Biol. Macromol. 2019, 122, 1100–1114. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.J.; Costa, A.; Afonso, C.M.M.; Reis, S. Mucoadhesive and pH responsive fucoidan-chitosan nanoparticles for the oral delivery of methotrexate. Int. J. Biol. Macromol. 2020, 158, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Ramos-de-la-Peña, A.M.; Contreras-esquivel, J.C.; Aguilar, O.; Gonz’alez-Valdez, J. Structural and bioactive roles of fucoidan in nanogel delivery systems. A review. Carbohydr. Polym. Technol. Appl. 2022, 4, 100235. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.I.; Costa, A.; Reis, S. Development of methotrexate loaded fucoidan/chitosan nanoparticles with anti-in fl ammatory potential and enhanced skin permeation. Int. J. Biol. Macromol. 2019, 124, 1115–1122. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, Y.; Jiang, B.; Chen, J.; Zhang, T. Development of self-assembled zein-fucoidan complex nanoparticles as a delivery system for resveratrol. Colloids Surf. B Biointerfaces 2022, 216, 112529. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Michalak, I.; Tiwari, R.; Dhawan, M.; Alagawany, M.; Farag, M.R.; Sharun, K.; Emran, T.B.; Dhama, K. Antioxidant effects of seaweeds and their active compounds on animal health and production—A Review. Vet. Q. 2022, 42, 48–67. [Google Scholar] [CrossRef]
- Muncke, J.; Andersson, A.-M.; Backhaus, T.; Boucher, J.M.; Carney Almroth, B.; Castillo, A.; Chevrier, J.; Demeneix, B.A.; Emmanuel, J.A.; Fini, J.-B.; et al. Impacts of food contact chemicals on human health: A consensus statement. Environ. Health 2020, 19, 25. [Google Scholar] [CrossRef]
- Carina, D.; Sharma, S.; Jaiswal, A.K.; Jaiswal, S. Seaweeds polysaccharides in active food packaging: A review of recent progress. Trends Food Sci. Technol. 2021, 110, 559–572. [Google Scholar] [CrossRef]
- Perera, K.Y.; Sharma, S.; Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Seaweed Polysaccharide in Food Contact Materials (Active Packaging, Intelligent Packaging, Edible Films, and Coatings). Foods 2021, 10, 2088. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Doh, H.; Dunno, K.D.; Whiteside, W.S. Preparation of novel seaweed nanocomposite film from brown seaweeds Laminaria japonica and Sargassum natans. Food Hydrocoll. 2020, 105, 105744. [Google Scholar] [CrossRef]
- García-Soto, B.; Miranda, J.M.; Rodríguez-Bernaldo de Quirós, A.; Sendón, R.; Rodríguez-Martínez, A.V.; Barros-Velázquez, J.; Aubourg, S.P. Effect of biodegradable film (lyophilised alga Fucus spiralis and sorbic acid) on quality properties of refrigerated megrim (Lepidorhombus whiffiagonis). Int. J. Food Sci. Technol. 2015, 50, 1891–1900. [Google Scholar] [CrossRef]
- Gomaa, M.; Hifney, A.F.; Fawzy, M.A.; Abdel-Gawad, K.M. Use of seaweed and filamentous fungus derived polysaccharides in the development of alginate-chitosan edible films containing fucoidan: Study of moisture sorption, polyphenol release and antioxidant properties. Food Hydrocoll. 2018, 82, 239–247. [Google Scholar] [CrossRef]
- Sebök, S.; Herppich, W.B.; Hanelt, D. Development of an innovative ring-shaped cultivation system for a land-based cultivation of marine macroalgae. Aquac. Eng. 2017, 77, 33–41. [Google Scholar] [CrossRef]
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]

| Marine Macroalga | Chemical Composition/Structure | Reference |
|---|---|---|
| Fucus evanescens | ([→3)-α-L-Fucp(2,4O SO3−)-(1→4)-α-L-Fucp(2OSO3−)-(1→])n | [18] |
| Sargassum horneri | repeating →3-α-l-Fucp(2 SO3−)-1→4-α-l-Fucp(2,3SO(3)(-))-1→ fragment, with insertions of →3-α-l-Fucp(2,4SO(3)(-))-1→ fragment | [19] |
| Laminaria longipes | [→3)-α-l-Fucp-(2SO(3)-)-(1→4)-α-l-Fucp-(1→2)-α-l-Fucp-(4 SO3−)-(1→]n | [20] |
| Laminaria hyperborea | (1→3)-α-L-fuco-pyranose (31.9%) to be the dominant residue, followed by 1→2-linked (13.2%) and 1→4-linked (7.7%) fuco-pyranose | [21] |
| Fucus evanescens | ([→3)-α-L-Fucp(2,4O SO3−)-(1→4)-α-L-Fucp(2OSO3-)-(1→]n) | [18] |
| Ascophyllum nodosum | [→3)-α-l-Fuc(2SO3−)-(1→4)-α-l-Fuc(2,3diSO3−)-(1]n | [14] |
| Fucus evanescens | [→3)-α-l-Fucp(2SO3−)-(1→4)-α-l-Fucp(2SO3−)-(1→]n | [22] |
| Fucus distichus | [→3)-α-l-Fucp-(2,4-di-SO3−)-(1→4)-α-l-Fucp-(2SO3−)-(1→]n | [23] |
| Macroalgal Species | Extraction Method | Extraction Yield/Efficiency | Reference |
|---|---|---|---|
| Ascophyllum nodosum | Microwave-assisted extraction | 16.08% | [50] |
| Fucus vesiculosus | Pressurized liquid extraction at high temperature | 25.99 ± 2.22% | [51] |
| Fucus vesiculosus | Microwave-assisted extraction | 18.2 ± 1.4% | [52] |
| Fucus vesiculosus | Autohydrolysis process | 16.5 ± 1.2% | [52] |
| Fucus vesiculosus | Microwave-assisted extraction | 18.22% | [53] |
| Nizamuddinia zanardinii | Ultrasound-assisted extraction | 3.51% | [54] |
| Sargassum myriocystum | Enzyme-assisted extraction | 6.2% | [46] |
| Turbinaria decurrens | Soaking in chloroform/methanol, sequential extraction in CaCl2, HCl | 5.58% (crude) 1.28% (purified) | [47] |
| Sargassum ilicifolium | Probe sonication–microwave assisted extraction method Hot water extraction method | 8 ± 0.9% 6 ± 0.5% | [55] |
| Macroalgal Source | Biological Action | Mechanism of Action | Application | Reference |
|---|---|---|---|---|
| Ascophyllum nodosum | Dermatological action | Inhibition of gelatinase A secretion and stromelysin 1 induction by interleukin-1β on dermal fibroblasts Increasing the association of MMPs with their specific inhibitors, namely TIMPs Minimize human leukocyte elastase activity Protection of human skin elastic fiber network against proteolysis by serine proteinase | Treating inflammatory pathologies with uncontrolled extracellular matrix degradation | [75] |
| Cladosiphon okamuranus | Antiviral action | Inhibition of viral entry into host cell, formation of syncytia and plaque forming units by blocking F protein | Antiviral to prevent New Castle Disease Virus infection in poultry | [15] |
| Fucus evanescens | Antiviral action | Preventive effect, virucidal effect and inhibition of virus adsorption and early stages of virus replication | Broad spectrum antiviral against DNA and RNA viruses, such as herpes simplex viruses (HSV-1, HSV-2), enterovirus (ECHO-1), and human immunodeficiency virus (HIV-1) | [18] |
| Fucus vesiculosus | Anti-adipogenic action | Decrease the expression of key proteins of adipogenic differentiation (C/EBPα, C/EBPβ, and PPARγ) | Treatment of obesity | [73] |
| Fucus vesiculosus | Antioxidant action Dermatological action | Inhibition of skin aging by increasing the expression of SIRTI. Improve skin immunity, soothing and protection, age spot reduction | Topical application for skin brightening | [42] |
| Laminaria hyperborea | Anticoagulant action | Inhibition of coagulation proteins Inhibition of complement activation by monocytes Inhibition of platelets | Potential alternative to heparin | [21] |
| Laminaria japonica | Antibacterial | Bactericidal action through destruction of cytomembranes targeting the membrane proteins, which can result in changed membrane fluidity and/or activated autophagocytosis. | Potential for partly or totally replacing antibiotics against Escherichia coli and Staphylococcus aureus | [76] |
| Laminaria longipes | Anticancer action | Prevent growth of cancer cells Sensitization of cancer cells to X-ray radiation | Effective against melanoma and colon cancer cells | [20] |
| Saccharina cichorioides | Anticancer action | Prevent growth of cancer cells Sensitization of cancer cells to X-ray radiation | Effective against melanoma and colon cancer cells | [20] |
| Sargassum binderi | Antioxidant | Free-radical scavenging activity (DPPH), reducing power, superoxide anion scavenging activity (SOA) and hydroxyl radical scavenging activity (OH) | Attenuation of inflammatory cytokines, such as IL-1β, IL-1 and TNF-α, and the degradation of phosphorylated p38 MAPK, ERK1/2 and JNK. Inhibition of iNOS and COX-2 expression induced by lipopolysaccharides | [77] |
| Sargassum cinereum | Anticancer action | Dose-dependent inhibition of growth of colon cancer cells (Caco-2) by induction of apoptosis, increase in ROS production and augmentation of mitochondrial membrane permeability | Promising therapeutic regimen against various cancer cell types | [78] |
| Sargassum crassifolium and Padina australis | Immunomodulation | Intestinal immunomodulating activity via Peyer’s patch cells | Maintenance of bowel health | [79] |
| Sargassum duplicatum | Anticancer action | Prevent growth of cancer cells | Effective against colon cancer | [61] |
| Sargassum hemiphyllum | Anti-inflammatory effect | Reduction of secretion profiles of pro-inflammatory cytokines, including IL-1β, IL-6, TNF-α, and NO Dose-dependent inhibition of lipopolysaccharide-induced mRNA expressions of IL-β, iNOS, and COX-2 Down-regulation of NF-κB | Treatment of inflammation | [80] |
| Sargassum henslowianum | Antiviral action | Reduction of plaque forming units Block virion adsorption to host cells | Treatment of Human Simplex Virus (HSV-1 and HSV-2) infection | [81] |
| Sargassum myriocystum | Antioxidant action | Free radical scavenging activity against hydroxyl and DPPH radical | Treatment for various oxidative stress and age-related diseases | [46] |
| Sargassum swartzii | Antiviral action | Reduction in HIV-1 p24 antigen levels and reverse transcriptase activity | Potential as an anti-HIV-1 agent | [82] |
| Undaria pinnatifida | Antioxidant | Secondary antioxidant capacity | Can replace synthetic antioxidant butylated hydroxyanisole (BHA) in treatment of diseases related to oxidative stress | [60] |
| Undaria pinnatifida | Antiviral action and immunomodulation | Inhibit replication of influenza A virus Stimulate both innate and adaptive immune defense functions in virus-infected host | Development of new therapeutic options, including its combination with neuraminidase inhibitors, such as oseltamivir | [83] |
| Fucus vesiculosus | Antiviral action | Inhibit viral replication Enhance host innate immune response through up-regulation of interferons signaling related genes and interferon-stimulated genes encoding antiviral effectors | Effective against human noroviruses (hNoV) | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anisha, G.S.; Padmakumari, S.; Patel, A.K.; Pandey, A.; Singhania, R.R. Fucoidan from Marine Macroalgae: Biological Actions and Applications in Regenerative Medicine, Drug Delivery Systems and Food Industry. Bioengineering 2022, 9, 472. https://doi.org/10.3390/bioengineering9090472
Anisha GS, Padmakumari S, Patel AK, Pandey A, Singhania RR. Fucoidan from Marine Macroalgae: Biological Actions and Applications in Regenerative Medicine, Drug Delivery Systems and Food Industry. Bioengineering. 2022; 9(9):472. https://doi.org/10.3390/bioengineering9090472
Chicago/Turabian StyleAnisha, Grace Sathyanesan, Savitha Padmakumari, Anil Kumar Patel, Ashok Pandey, and Reeta Rani Singhania. 2022. "Fucoidan from Marine Macroalgae: Biological Actions and Applications in Regenerative Medicine, Drug Delivery Systems and Food Industry" Bioengineering 9, no. 9: 472. https://doi.org/10.3390/bioengineering9090472
APA StyleAnisha, G. S., Padmakumari, S., Patel, A. K., Pandey, A., & Singhania, R. R. (2022). Fucoidan from Marine Macroalgae: Biological Actions and Applications in Regenerative Medicine, Drug Delivery Systems and Food Industry. Bioengineering, 9(9), 472. https://doi.org/10.3390/bioengineering9090472