Normothermic Ex Vivo Liver Platform Using Porcine Slaughterhouse Livers for Disease Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Liver and Blood Procurement
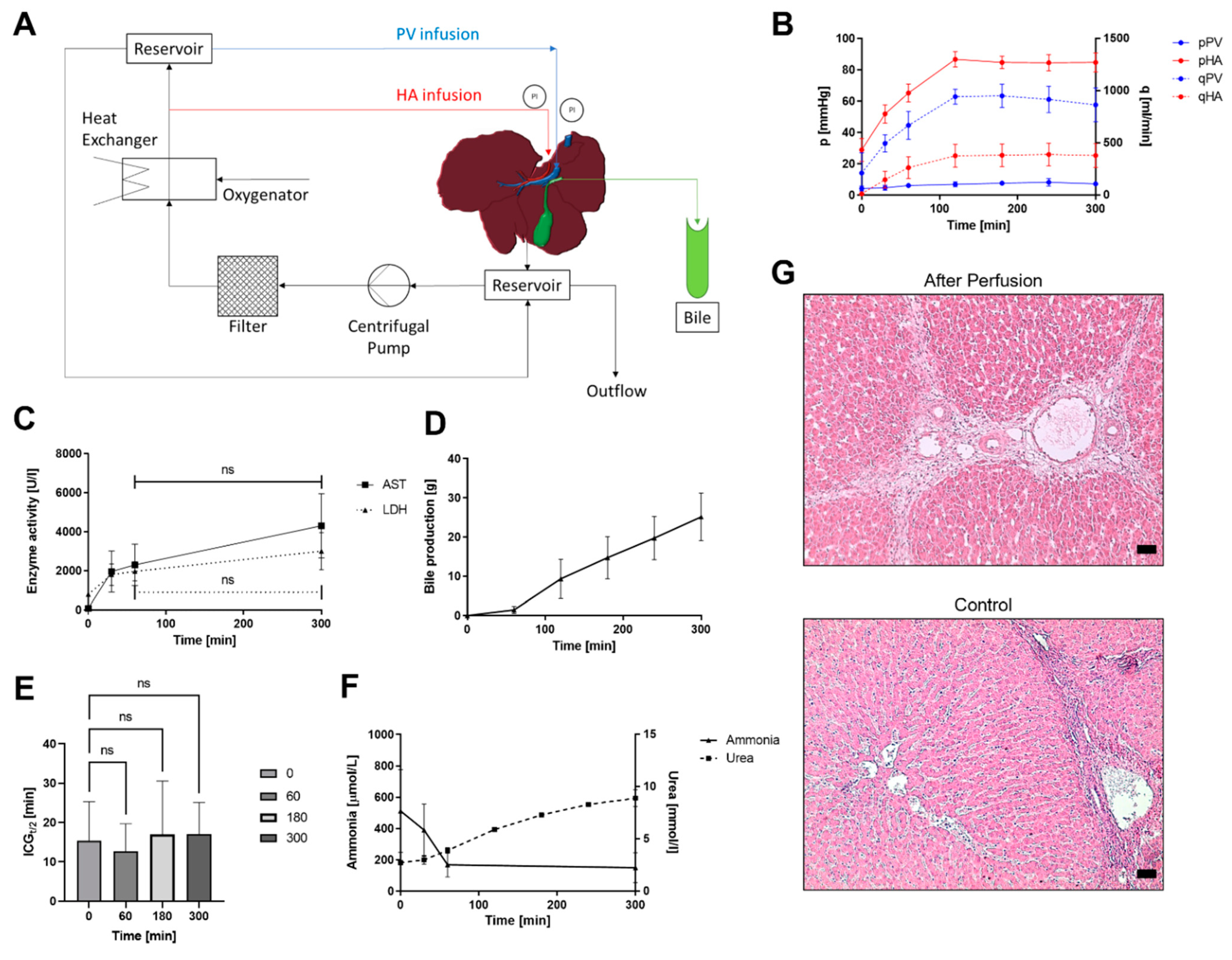
2.2. Circuit Design
2.3. Reperfusion
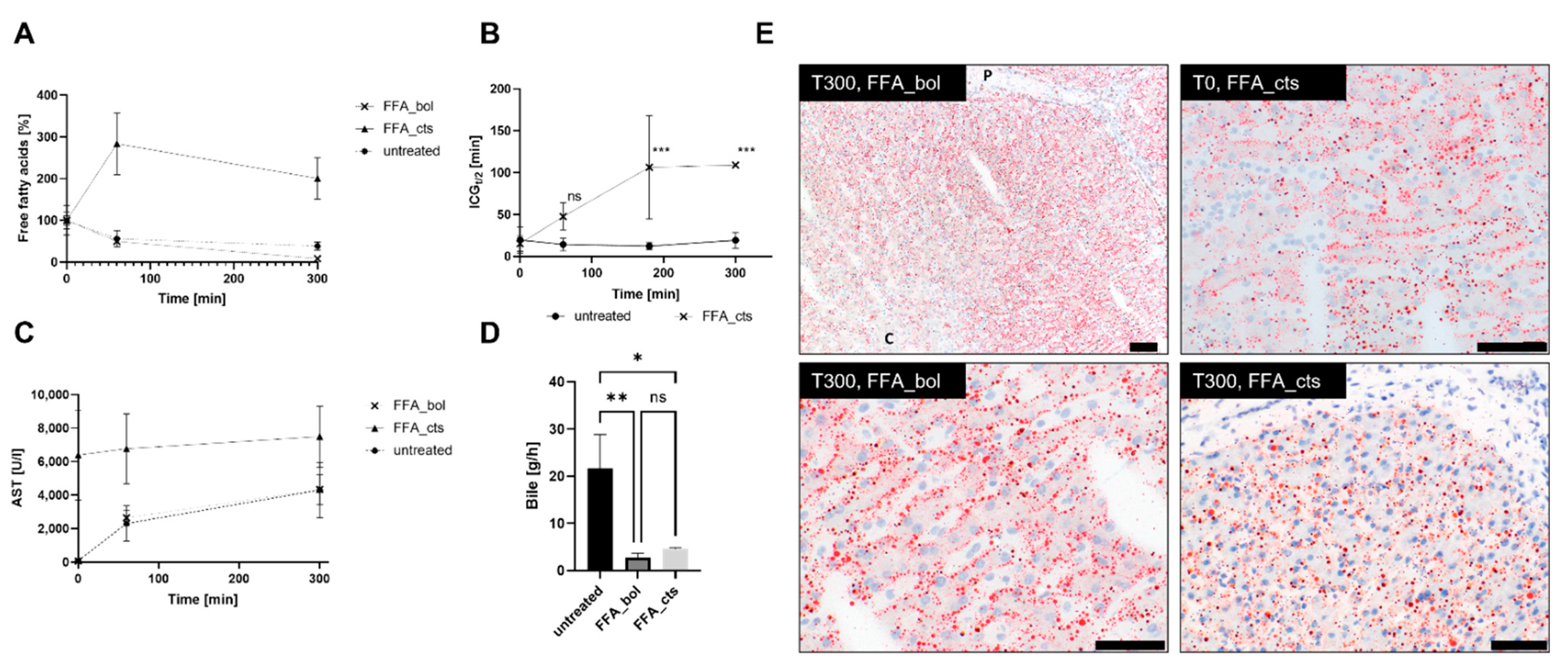
2.4. Free Fatty Acid Treatment
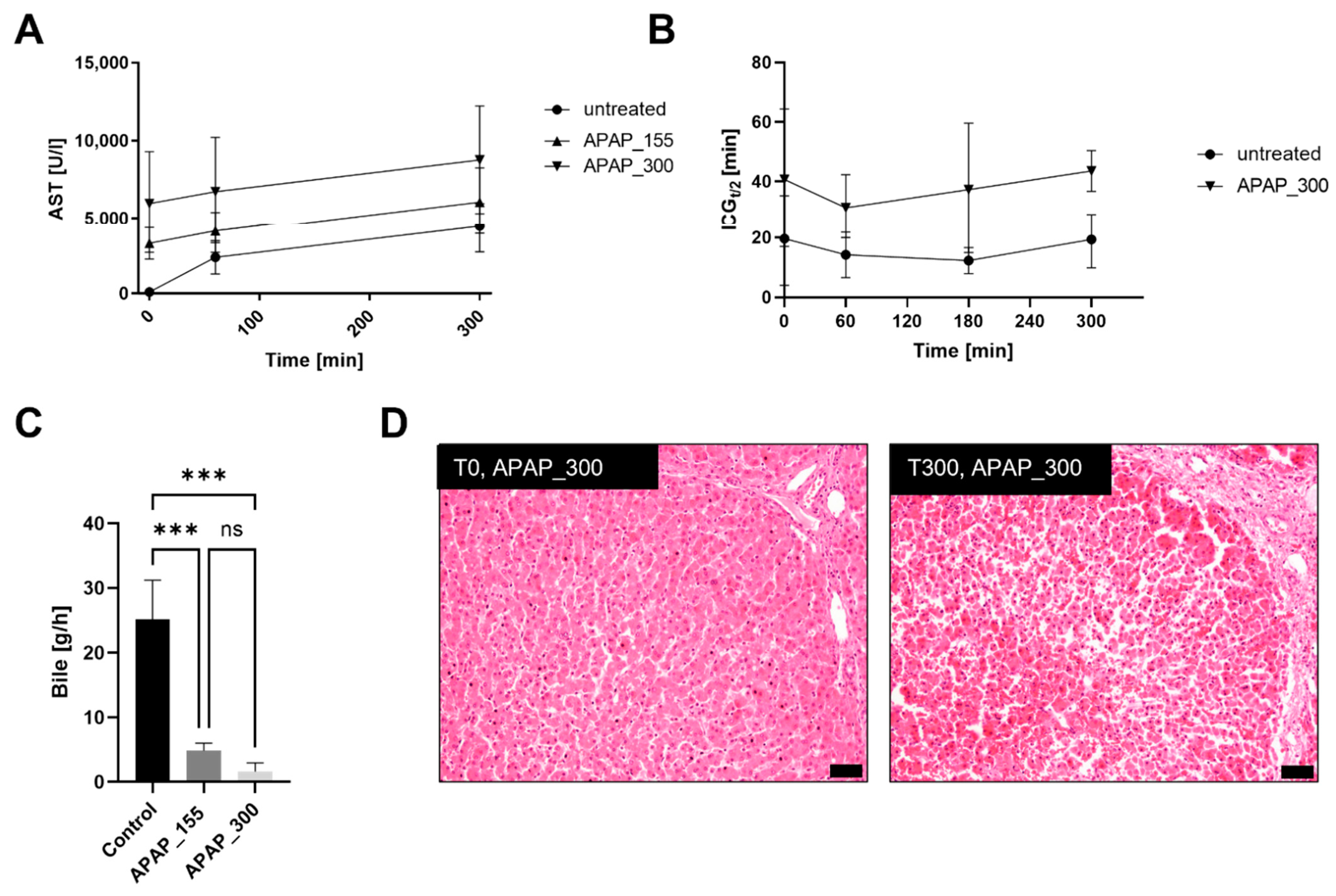
2.5. Acetaminophen Treatment
2.6. Analysis
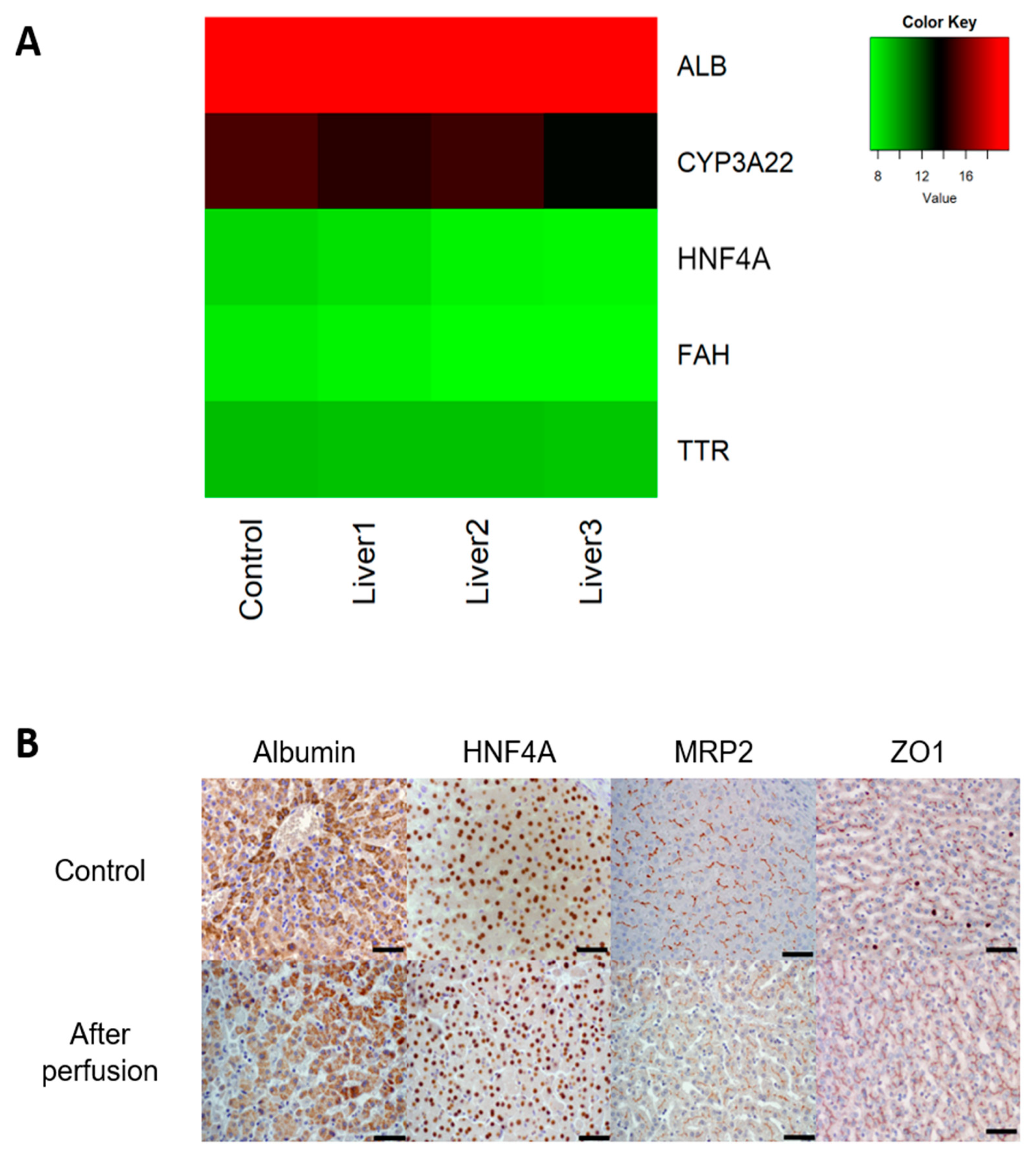
2.7. Gene Expression
2.8. Histology
2.9. Indocyanine Green Functionality Assay
2.10. Data Analysis
3. Results
3.1. Untreated Livers
3.2. Free Fatty Acid Treatment
3.3. Acetaminophen Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gola, A.; Davis, S.; Greenslade, L.; Hopkins, K.; Low, J.; Marshall, A.; Thorburn, D.; Vickerstaff, V.; Jones, L. Economic Analysis of Costs for Patients with End Stage Liver Disease over the Last Year of Life. BMJ Support. Palliat. Care 2015, 5, 110. [Google Scholar] [CrossRef]
- Pimpin, L.; Cortez-Pinto, H.; Negro, F.; Corbould, E.; Lazarus, J.V.; Webber, L.; Sheron, N. Burden of Liver Disease in Europe: Epidemiology and Analysis of Risk Factors to Identify Prevention Policies. J. Hepatol. 2018, 69, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of Liver Diseases in the World. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Blachier, M.; Leleu, H.; Peck-Radosavljevic, M.; Valla, D.C.; Roudot-Thoraval, F. The Burden of Liver Disease in Europe: A Review of Available Epidemiological Data. J. Hepatol. 2013, 58, 593–608. [Google Scholar] [CrossRef]
- Bale, S.S.; Vernetti, L.; Senutovitch, N.; Jindal, R.; Hegde, M.; Gough, A.; McCarty, W.J.; Bakan, A.; Bhushan, A.; Shun, T.Y.; et al. In Vitro Platforms for Evaluating Liver Toxicity. Exp. Biol. Med. 2014, 239, 1180–1191. [Google Scholar] [CrossRef]
- Kaplan, W.; Wirtz, V.J.; Mantel-Teeuwisse, A.; Stolk, P.; Duthey, B.; Laing, R. Priority Medicines for Europe and the World 2013 Update Chapter 6.14. Harmful use of Alcohol, Alcohol Use Disorders and Alcoholic Liver Diseases. In WHO Library Cataloguing-in-Publication Data Technical Report; WHO: Geneva, Switzerland, 2013; pp. 132–134. [Google Scholar] [CrossRef]
- Bruix, J.; Han, K.; Gores, G.; Llovet, J.M.; Mazzaferro, V. Liver Cancer: Approaching a Personalized Care. J. Hepatol. 2015, 62, S144–S156. [Google Scholar] [CrossRef]
- Scherer, A.; Dufour, J.F. Treatment of Non-Alcoholic Fatty Liver Disease. Dig. Dis. 2016, 34, 27–31. [Google Scholar] [CrossRef]
- Kuna, L.; Bozic, I.; Kizivat, T.; Bojanic, K.; Mrso, M.; Kralj, E.; Smolic, R.; Wu, G.Y.; Smolic, M. Models of Drug Induced Liver Injury (DILI)–Current Issues and Future Perspectives. Curr. Drug Metab. 2018, 19, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Kullak-Ublick, G.A.; Andrade, R.J.; Merz, M.; End, P.; Benesic, A.; Gerbes, A.L.; Aithal, G.P. Drug-Induced Liver Injury: Recent Advances in Diagnosis and Risk Assessment. Gut 2017, 66, 1154–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissenbacher, A.; Vrakas, G.; Nasralla, D.; Ceresa, C.D.L. The Future of Organ Perfusion and Re-conditioning. Transpl. Int. 2019, 32, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, R.; Leuvenink, H.; Friend, P.J. Normothermic Liver Preservation: A New Paradigm? Transpl. Int. 2015, 28, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.J.; Rees, M.A.; Wight, D.G.D.; Casey, N.D.; Alexander, G.; White, D.J.G.; Friend, P.J. Successful Extracorporeal Porcine Liver Perfusion for 72 HR. Transplantation 2002, 73, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Hessheimer, A.J.; Fondevila, C.; García-Valdecasas, J.C. Extracorporeal Machine Liver Perfusion: Are We Warming Up? Curr. Opin. Organ Transplant. 2012, 17, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Nassar, A.; Farias, K.; Buccini, L.; Mangino, M.J.; Baldwin, W.; Bennett, A.; O’Rourke, C.; Iuppa, G.; Soliman, B.G.; et al. Comparing Normothermic Machine Perfusion Preservation with Different Perfusates on Porcine Livers from Donors after Circulatory Death. Am. J. Transplant. 2016, 16, 794–807. [Google Scholar] [CrossRef]
- Nassar, A.; Liu, Q.; Farias, K.; D’Amico, G.; Tom, C.; Grady, P.; Bennett, A.; Diago Uso, T.; Eghtesad, B.; Kelly, D.; et al. Ex Vivo Normothermic Machine Perfusion Is Safe, Simple, and Reliable: Results from a Large Animal Model. Surg. Innov. 2015, 22, 61–69. [Google Scholar] [CrossRef]
- Jochmans, I.; Akhtar, M.Z.; Nasralla, D.; Kocabayoglu, P.; Boffa, C.; Kaisar, M.; Brat, A.; O’Callaghan, J.; Pengel, L.H.M.; Knight, S.; et al. Past, Present, and Future of Dynamic Kidney and Liver Preservation and Resuscitation. Am. J. Transplant. 2016, 16, 2545–2555. [Google Scholar] [CrossRef]
- Marecki, H.; Bozorgzadeh, A.; Porte, R.J.; Leuvenink, H.G.; Uygun, K.; Martins, P.N. Liver Ex Situ Machine Perfusion Preservation: A Review of the Methodology and Results of Large Animal Studies and Clinical Trials. Liver Transplant. 2017, 23, 679–695. [Google Scholar] [CrossRef]
- Ceresa, C.D.L.; Nasralla, D.; Jassem, W. Normothermic Machine Preservation of the Liver: State of the Art. Curr. Transplant. Rep. 2018, 5, 104–110. [Google Scholar] [CrossRef]
- Eshmuminov, D.; Becker, D.; Bautista Borrego, L.; Hefti, M.; Schuler, M.J.; Hagedorn, C.; Muller, X.; Mueller, M.; Onder, C.; Graf, R.; et al. An Integrated Perfusion Machine Preserves Injured Human Livers for 1 Week. Nat. Biotechnol. 2020, 38, 189–198. [Google Scholar] [CrossRef]
- Jayant, K.; Reccia, I.; Shapiro, A.M.J. Normothermic Ex-Vivo Liver Perfusion: Where Do We Stand and Where to Reach? Expert Rev. Gastroenterol. Hepatol. 2018, 12, 1045–1058. [Google Scholar] [CrossRef]
- Maione, F.; Gilbo, N.; Lazzaro, S.; Friend, P.; Camussi, G.; Romagnoli, R.; Pirenne, J.; Jochmans, I.; Monbaliu, D. Porcine Isolated Liver Perfusion for the Study of Ischemia Reperfusion Injury: A Systematic Review. Transplantation 2018, 102, 1039. [Google Scholar] [CrossRef] [PubMed]
- Vogel, T.; Brockmann, J.G.; Friend, P.J. Ex-Vivo Normothermic Liver Perfusion: An Update. Curr. Opin. Organ Transplant. 2010, 15, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.P.; Bhattacharjya, S.; Maniakin, N.; Greenwood, J.; Guerreiro, D.; Hughes, D.; Imber, C.J.; Pigott, D.W.; Fuggle, S.; Taylor, R.; et al. Preservation of Porcine Non-Heart-Beating Donor Livers by Sequential Cold Storage and Warm Perfusion. Transplantation 2004, 77, 1328–1332. [Google Scholar] [CrossRef]
- Imber, C.J.; Peter, S.D.; De Cenarruzabeitia, I.L.; Pigott, D.; James, T.; Taylor, R.; Mcguire, J.; Butler, A.; Hughes, D.; Rees, M.; et al. Advantages of normothermic perfusion over cold storage in liver preservation. Transplantation 2002, 73, 701–709. [Google Scholar] [CrossRef]
- Grosse-Siestrup, C.; Nagel, S.; Unger, V.; Meissler, M.; Pfeffer, J.; Fischer, A.; Groneberg, D.A. The Isolated Perfused Liver: A New Model Using Autologous Blood and Porcine Slaughterhouse Organs. J. Pharmacol. Toxicol. Methods 2001, 46, 163–168. [Google Scholar] [CrossRef]
- Grosse-Siestrup, C.; Pfeffer, J.; Unger, V.; Nagel, S.; Witt, C.; Fischer, A.; David, A. Isolated Hemoperfused Slaughterhouse Livers as a Valid Model to Study Hepatotoxicity. Toxicol. Pathol. 2002, 30, 749–754. [Google Scholar] [CrossRef]
- Thewes, S.; Reed, H.K.; Grosse-Siestrup, C.; Groneberg, D.A.; Meissler, M.; Schaller, M.; Hube, B. Haemoperfused Liver as an Ex Vivo Model for Organ Invasion of Candida Albicans. J. Med. Microbiol. 2007, 56, 266–270. [Google Scholar] [CrossRef]
- Izamis, M.L.; Efstathiades, A.; Keravnou, C.; Leen, E.L.; Averkiou, M.A. Dynamic Contrast-Enhanced Ultrasound of Slaughterhouse Porcine Livers in Machine Perfusion. Ultrasound Med. Biol. 2014, 40, 2217–2230. [Google Scholar] [CrossRef]
- Schreiter, T.; Sowa, J.P.; Schlattjan, M.; Treckmann, J.; Paul, A.; Strucksberg, K.H.; Baba, H.A.; Odenthal, M.; Gieseler, R.K.; Gerken, G.; et al. Human Ex-Vivo Liver Model for Acetaminophen-Induced Liver Damage. Sci. Rep. 2016, 6, 31916. [Google Scholar] [CrossRef] [Green Version]
- Newsome, P.N.; Henderson, N.C.; Nelson, L.J.; Dabos, C.; Filippi, C.; Bellamy, C.; Howie, F.; Clutton, R.E.; Lee, A.; King, T.; et al. Development of an Invasively Monitored Porcine Model of Acetaminophen-Induced Acute Liver Failure. BMC Gastroenterol. 2010, 10, 34. [Google Scholar] [CrossRef]
- Jamieson, R.W.; Zilvetti, M.; Roy, D.; Hughes, D.; Morovat, A.; Coussios, C.C.; Friend, P.J. Hepatic Steatosis and Normothermic Perfusion—Preliminary Experiments in a Porcine Model. Transplantation 2011, 92, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Westra, I.M. Precision-Cut Liver Slices: An Ex Vivo Model for the Early Onset and End-Stage of Liver Fibrosis; University of Groningen: Groningen, The Netherlands, 2014. [Google Scholar]
- Van der Laan, L.J.W.; Geijsen, N.; Spee, B.; Huch, M.; Schotanus, B.A.; Verstegen, M.M.A.; Grinwis, G.C.M.; Vernooij, I.G.W.H.; Oosterhoff, L.A.; Roesch, C.; et al. Long-Term Adult Feline Liver Organoid Cultures for Disease Modeling of Hepatic Steatosis. Stem Cell Rep. 2017, 8, 822–830. [Google Scholar] [CrossRef]
- Gómez-Lechón, M.J.; Donato, M.T.; Martínez-Romero, A.; Jiménez, N.; Castell, J.V.; O’Connor, J.E. A Human Hepatocellular in Vitro Model to Investigate Steatosis. Chem. Biol. Interact. 2007, 165, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Winek, C.L.; Wahba, W.W.; Winek, C.L.; Balzer, T.W. Drug and Chemical Blood-Level Data 2001. Forensic Sci. Int. 2001, 122, 107–123. [Google Scholar] [CrossRef]
- Brüggenwirth, I.M.A.; van Leeuwen, O.B.; de Vries, Y.; Bodewes, S.B.; Adelmeijer, J.; Wiersema-Buist, J.; Lisman, T.; Martins, P.N.; de Meijer, V.E.; Porte, R.J. Extended Hypothermic Oxygenated Machine Perfusion Enables Ex Situ Preservation of Porcine Livers for up to 24 Hours. JHEP Rep. 2020, 2, 100092. [Google Scholar] [CrossRef]
- Pool, M.B.F.; Hartveld, L.; Leuvenink, H.G.D.; Moers, C. Normothermic Machine Perfusion of Ischaemically Damaged Porcine Kidneys with Autologous, Allogeneic Porcine and Human Red Blood Cells. PLoS ONE 2020, 15, e0229566. [Google Scholar] [CrossRef]
- Eshmuminov, D.; Mueller, M.; Brugger, S.D.; Bautista Borrego, L.; Becker, D.; Hefti, M.; Hagedorn, C.; Duskabilova, M.; Tibbitt, M.W.; Dutkowski, P.; et al. Sources and Prevention of Graft Infection during Long-Term Ex Situ Liver Perfusion. Transpl. Infect. Dis. 2021, 23, e13623. [Google Scholar] [CrossRef]
- Mendes-Braz, M.; Elias-Miró, M.; Jiménez-Castro, M.B.; Casillas-Ramírez, A.; Ramalho, F.S.; Peralta, C. The Current State of Knowledge of Hepatic Ischemia-Reperfusion Injury Based on Its Study in Experimental Models. J. Biomed. Biotechnol. 2012, 2012, 298657. [Google Scholar] [CrossRef]
- Siniscalchi, A. Post Reperfusion Syndrome during Liver Transplantation: From Pathophysiology to Therapy and Preventive Strategies. World J. Gastroenterol. 2016, 22, 1551. [Google Scholar] [CrossRef]
- Banan, B.; Xiao, Z.; Watson, R.; Xu, M.; Jia, J.; Upadhya, G.A.; Mohanakumar, T.; Lin, Y.; Chapman, W. Novel Strategy to Decrease Reperfusion Injuries and Improve Function of Cold-Preserved Livers Using Normothermic Ex Vivo Liver Perfusion Machine. Liver Transplant. 2016, 22, 333–343. [Google Scholar] [CrossRef]
- Fujita, S.; Roerig, D.L.; Bosnjak, Z.J.; Stowe, D.F. Effects of Vasodilators and Perfusion Pressure on Coronary Flow and Simultaneous Release of Nitric Oxide from Guinea Pig Isolated Hearts. Cardiovasc. Res. 1998, 38, 655–667. [Google Scholar] [CrossRef]
- Grosse-Siestrup, C.; Unger, V.; Pfeffer, J.; Dinh, Q.T.; Nagel, S.; Springer, J.; Witt, C.; Wussow, A.; Groneberg, D.A. Hepatotoxic Effects of Polidocanol in a Model of Autologously Perfused Porcine Livers. Arch. Toxicol. 2004, 78, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Schön, M.R.; Kollmar, O.; Akkoc, N.; Matthes, M.; Wolf, S.; Schrem, H.; Tominaga, M.; Keech, G.; Neuhaus, P. Cold Ischemia Affects Sinusoidal Endothelial Cells While Warm Ischemia Affects Hepatocytes in Liver Transplantation. Transplant. Proc. 1998, 30, 2318–2320. [Google Scholar] [CrossRef]
- Gilbo, N.; Wylin, T.; Heedfeld, V.; Jochmans, I.; Pirenne, J.; Friend, P.; Monbaliu, D. Porcine Liver Normothermic Machine Perfusion: Methodological Framework and Potential Pitfalls. Transplant. Direct 2022, 8, e1276. [Google Scholar] [CrossRef]
- Boteon, Y.L.; Laing, R.W.; Schlegel, A.; Wallace, L.; Smith, A.; Attard, J.; Bhogal, R.H.; Neil, D.A.H.; Hübscher, S.; Thamara, M.; et al. Combined Hypothermic and Normothermic Machine Perfusion Improves Functional Recovery of Extended Criteria Donor Livers. Liver Transplant. 2018, 24, 1699–1715. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, R.L.; Anderson, V. A Comprehensive Tissue Properties Database Provided for the Thermal Assessment of a Human at Rest. Biophys. Rev. Lett. 2010, 05, 129–151. [Google Scholar] [CrossRef]
- Eshmuminov, D.; Leoni, F.; Schneider, M.A.; Becker, D.; Muller, X.; Onder, C.; Hefti, M.; Schuler, M.J.; Dutkowski, P.; Graf, R.; et al. Perfusion Settings and Additives in Liver Normothermic Machine Perfusion with Red Blood Cells as Oxygen Carrier. A Systematic Review of Human and Porcine Perfusion Protocols. Transpl. Int. 2018, 31, 956–969. [Google Scholar] [CrossRef]
- Reiling, J.; Lockwood, D.S.R.; Simpson, A.H.; Campbell, C.M.; Bridle, K.R.; Santrampurwala, N.; Britton, L.J.; Crawford, D.H.G.; Dejong, C.H.C.; Fawcett, J. Urea Production during Normothermic Machine Perfusion: Price of Success? Liver Transplant. 2015, 21, 700–703. [Google Scholar] [CrossRef]
- Bruinsma, B.G.; Sridharan, G.V.; Weeder, P.D.; Avruch, J.H.; Saeidi, N.; Özer, S.; Geerts, S.; Porte, R.J.; Heger, M.; Van Gulik, T.M.; et al. Metabolic Profiling during Ex Vivo Machine Perfusion of the Human Liver. Sci. Rep. 2016, 6, 22415. [Google Scholar] [CrossRef] [Green Version]
- Sutton, M.E.; Op Den Dries, S.; Karimian, N.; Weeder, P.D.; De Boer, M.T.; Wiersema-Buist, J.; Gouw, A.S.H.; Leuvenink, H.G.D.; Lisman, T.; Porte, R.J. Criteria for Viability Assessment of Discarded Human Donor Livers during Ex Vivo Normothermic Machine Perfusion. PLoS ONE 2014, 9, e110642. [Google Scholar] [CrossRef]
- Matton, A.P.M.; De Vries, Y.; Burlage, L.C.; Van Rijn, R.; Fujiyoshi, M.; De Meijer, V.E.; De Boer, M.T.; De Kleine, R.H.J.; Verkade, H.J.; Gouw, A.S.H.; et al. Biliary Bicarbonate, PH, and Glucose Are Suitable Biomarkers of Biliary Viability During Ex Situ Normothermic Machine Perfusion of Human Donor Livers. Transplantation 2019, 103, 1405. [Google Scholar] [CrossRef] [PubMed]
- Matton, A.P.M.; Burlage, L.C.; van Rijn, R.; de Vries, Y.; Karangwa, S.A.; Nijsten, M.W.; Gouw, A.S.H.; Wiersema-Buist, J.; Adelmeijer, J.; Westerkamp, A.C.; et al. Normothermic Machine Perfusion of Donor Livers without the Need for Human Blood Products. Liver Transplant. 2018, 24, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Eshmuminov, D.; Keller, R.; Mueller, M.; Bautista Borrego, L.; Hagedorn, C.; Duskabilova, M.; Tibbitt, M.W.; Onder, C.; Clavien, P.A.; et al. Automated Insulin Delivery-Continuous Blood Glucose Control during Ex Situ Liver Perfusion. IEEE Trans. Biomed. Eng. 2021, 68, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Izamis, M.L.; Tolboom, H.; Uygun, B.; Berthiaume, F.; Yarmush, M.L.; Uygun, K. Resuscitation of Ischemic Donor Livers with Normothermic Machine Perfusion: A Metabolic Flux Analysis of Treatment in Rats. PLoS ONE 2013, 8, e69758. [Google Scholar] [CrossRef]
- Ott, P.; Keiding, S.; Bass, L. Intrinsic Hepatic Clearance of Indocyanine Green in the Pig: Dependence on Plasma Protein Concentration. Eur. J. Clin. Investig. 1992, 22, 347–357. [Google Scholar] [CrossRef]
- De Gasperi, A.; Mazza, E.; Prosperi, M. Indocyanine Green Kinetics to Assess Liver Function: Ready for a Clinical Dynamic Assessment in Major Liver Surgery? World J. Hepatol. 2016, 8, 355–367. [Google Scholar] [CrossRef]
- Lamesch, P.; Raygrotzki, S.; Evers, B.; Pichlmayr, R. ICG Test Als Verlaufsparameter Nach Unterschiedlichen Ischämiebelastungen Der Leber–Eine Tierexperimentelle Studie. In Chirurgisches Forum ’92 Für Experimentelle und Klinische Forschung; Springer: Berlin/Heidelberg, Germany, 1992; pp. 171–176. [Google Scholar]
- Imber, C.J.; Peter, S.D.S.; De Cenarruzabeitia, I.L.; Lemonde, H.; Rees, M.; Butler, A.; Clayton, P.T.; Friend, P.J. Optimisation of Bile Production during Normothermic Preservation of Porcine Livers. Am. J. Transplant. 2002, 2, 593–599. [Google Scholar] [CrossRef]
- Schleicher, J.; Dahmen, U.; Guthke, R.; Schuster, S. Zonation of Hepatic Fat Accumulation: Insights from Mathematical Modelling of Nutrient Gradients and Fatty Acid Uptake. J. R. Soc. Interface 2017, 14, 20170443. [Google Scholar] [CrossRef]
- Kanuri, G.; Bergheim, I. In Vitro and in Vivo Models of Non-Alcoholic Fatty Liver Disease (NAFLD). Int. J. Mol. Sci. 2013, 14, 11963–11980. [Google Scholar] [CrossRef] [Green Version]
- Seifalian, A.M.; El-Desoky, A.; Davidson, B.R. Hepatic Indocyanine Green Uptake and Excretion in a Rabbit Model of Steatosis. Eur. Surg. Res. 2001, 33, 193–201. [Google Scholar] [CrossRef]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Thiel, C.; Thiel, K.; Etspueler, A.; Morgalla, M.H.; Rubitschek, S.; Schmid, S.; Steurer, W.; Königsrainer, A.; Schenk, M. A Reproducible Porcine Model of Acute Liver Failure Induced by Intrajejunal Acetaminophen Administration. Eur. Surg. Res. 2011, 46, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Manibur Rahman, T.; Humphrey, J.F. Hodgson Animal Models of Acute Hepatic Failure. Int. J. Exp. Pathol. 2000, 81, 145–157. [Google Scholar] [CrossRef] [PubMed]
- He, G.-L.; Feng, L.; Cai, L.; Zhou, C.-J.; Cheng, Y.; Jiang, Z.-S.; Pan, M.-X.; Gao, Y. Artificial Liver Support in Pigs with Acetaminophen-Induced Acute Liver Failure. World J. Gastroenterol. 2017, 23, 3262–3268. [Google Scholar] [CrossRef] [PubMed]
- Dargue, R.; Zia, R.; Lau, C.; Nicholls, A.W.; Dare, T.O.; Lee, K.; Jalan, R.; Coen, M.; Wilson, I.D. Metabolism and Effects on Endogenous Metabolism of Paracetamol (Acetaminophen) in a Porcine Model of Liver Failure. Toxicol. Sci. 2020, 175, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Olazábal, A.; Martínez-Galán, P.; Castejón-Moreno, R.; García-Moreno, M.E.; García-Muro, C.; Esteban-Zubero, E. Management of Acetaminophen Toxicity, a Review. Iberoam. J. Med. 2019, 1, 22–28. [Google Scholar] [CrossRef]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; García-Valdecasas, J.C.; Heaton, N.; et al. A Randomized Trial of Normothermic Preservation in Liver Transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef]
- Ghallab, A.; Hassan, R.; Hofmann, U.; Friebel, A.; Hobloss, Z.; Brackhagen, L.; Begher-Tibbe, B.; Myllys, M.; Reinders, J.; Overbeck, N.; et al. Interruption of Bile Acid Uptake by Hepatocytes after Acetaminophen Overdose Ameliorates Hepatotoxicity; European Association for the Study of the Liver (EASL): Marburg, Germany, 2022. [Google Scholar]
- Brüggenwirth, I.M.A.; Porte, R.J.; Martins, P.N. Bile Composition as a Diagnostic and Prognostic Tool in Liver Transplantation. Liver Transplant. 2020, 26, 1177–1187. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, M.; Ruppelt, A.; Kappler, B.; Van Soest, E.; Samsom, R.A.; Grinwis, G.C.M.; Geijsen, N.; Helms, J.B.; Stijnen, M.; Kock, L.M.; et al. Normothermic Ex Vivo Liver Platform Using Porcine Slaughterhouse Livers for Disease Modeling. Bioengineering 2022, 9, 471. https://doi.org/10.3390/bioengineering9090471
Krüger M, Ruppelt A, Kappler B, Van Soest E, Samsom RA, Grinwis GCM, Geijsen N, Helms JB, Stijnen M, Kock LM, et al. Normothermic Ex Vivo Liver Platform Using Porcine Slaughterhouse Livers for Disease Modeling. Bioengineering. 2022; 9(9):471. https://doi.org/10.3390/bioengineering9090471
Chicago/Turabian StyleKrüger, Melanie, Alicia Ruppelt, Benjamin Kappler, Elke Van Soest, Roos Anne Samsom, Guy C. M. Grinwis, Niels Geijsen, J. Bernd Helms, Marco Stijnen, Linda M. Kock, and et al. 2022. "Normothermic Ex Vivo Liver Platform Using Porcine Slaughterhouse Livers for Disease Modeling" Bioengineering 9, no. 9: 471. https://doi.org/10.3390/bioengineering9090471
APA StyleKrüger, M., Ruppelt, A., Kappler, B., Van Soest, E., Samsom, R. A., Grinwis, G. C. M., Geijsen, N., Helms, J. B., Stijnen, M., Kock, L. M., Rasponi, M., Kooistra, H. S., & Spee, B. (2022). Normothermic Ex Vivo Liver Platform Using Porcine Slaughterhouse Livers for Disease Modeling. Bioengineering, 9(9), 471. https://doi.org/10.3390/bioengineering9090471








