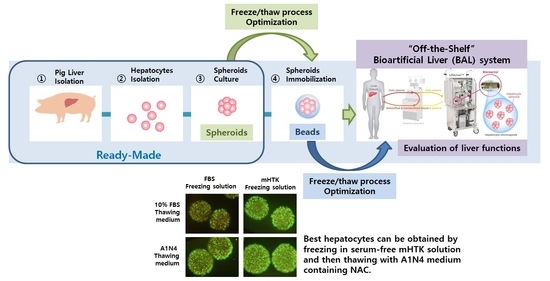
Establishment of a Serum-Free Hepatocyte Cryopreservation Process for the Development of an “Off-the-Shelf” Bioartificial Liver System
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Porcine Hepatocyte Isolation
2.3. Spheroid Culture and Calcium Alginate Immobilization
2.4. Procedures of Cryopreservation and Thawing
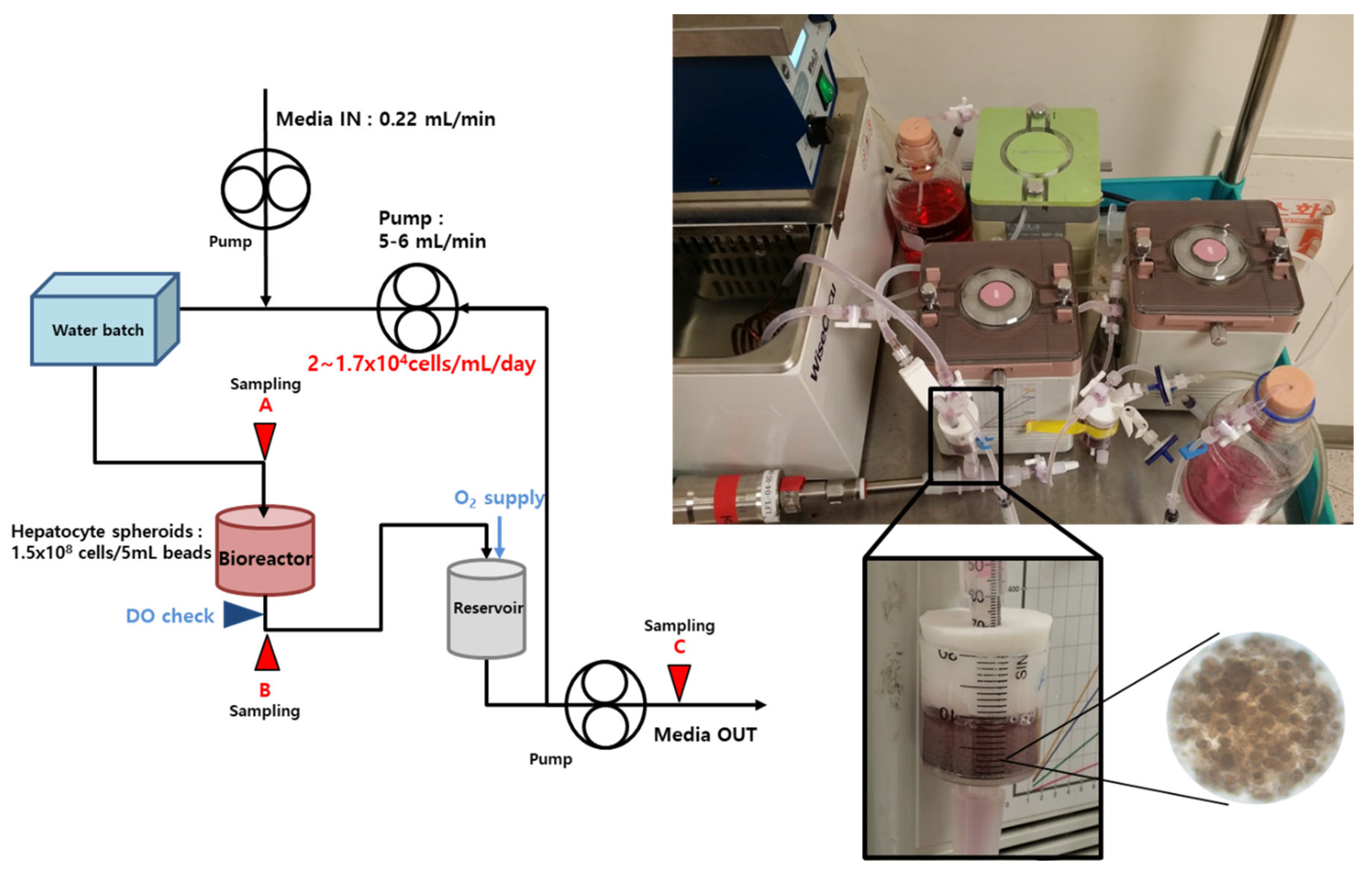
2.5. Bioreactor and BAL System
2.6. Cell Viability Assays
2.7. Measurement of Liver-Specific Functions
2.8. Statistical Analysis
3. Results
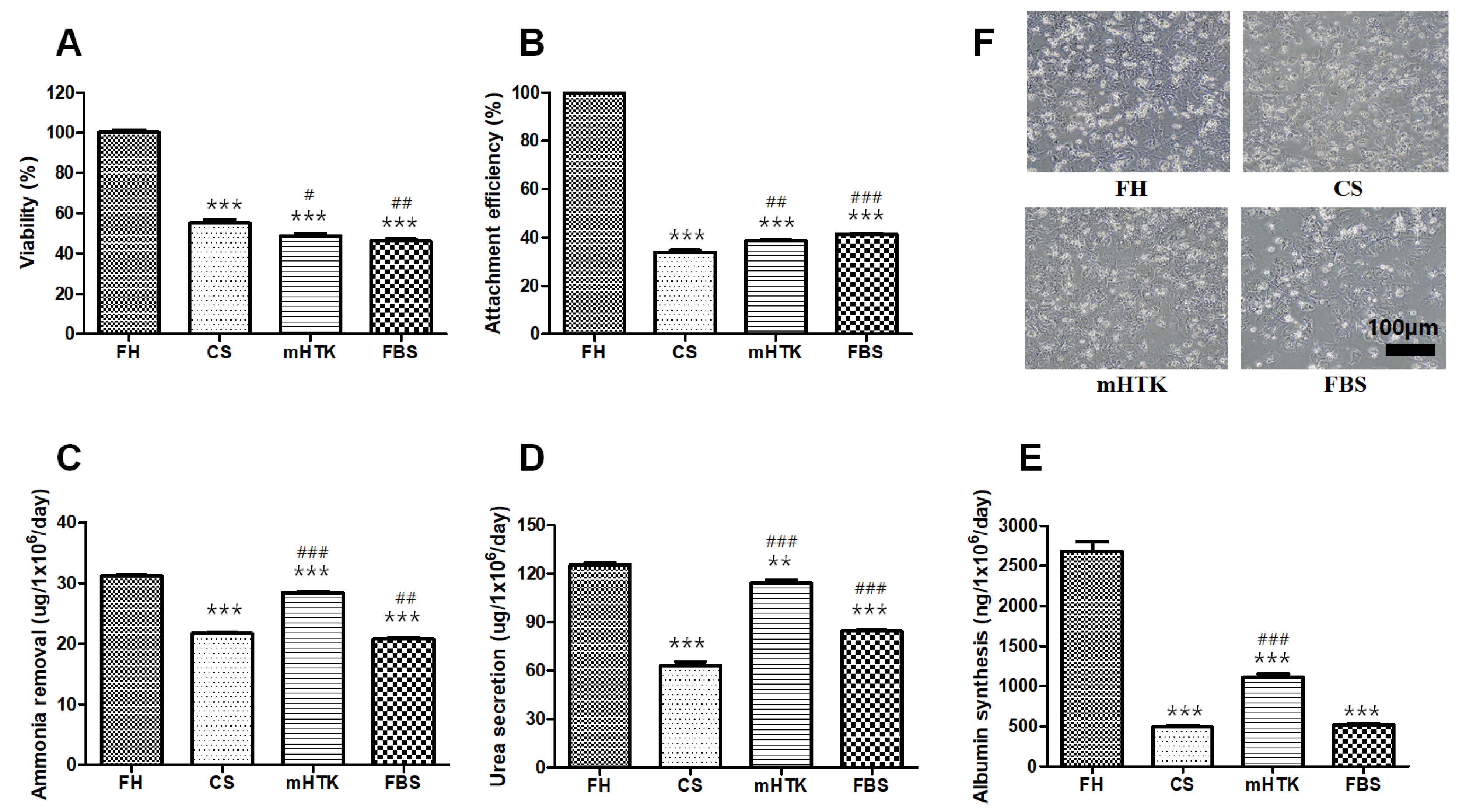
3.1. Viability and Liver-Specific Functions of Cryopreserved Porcine Hepatocytes
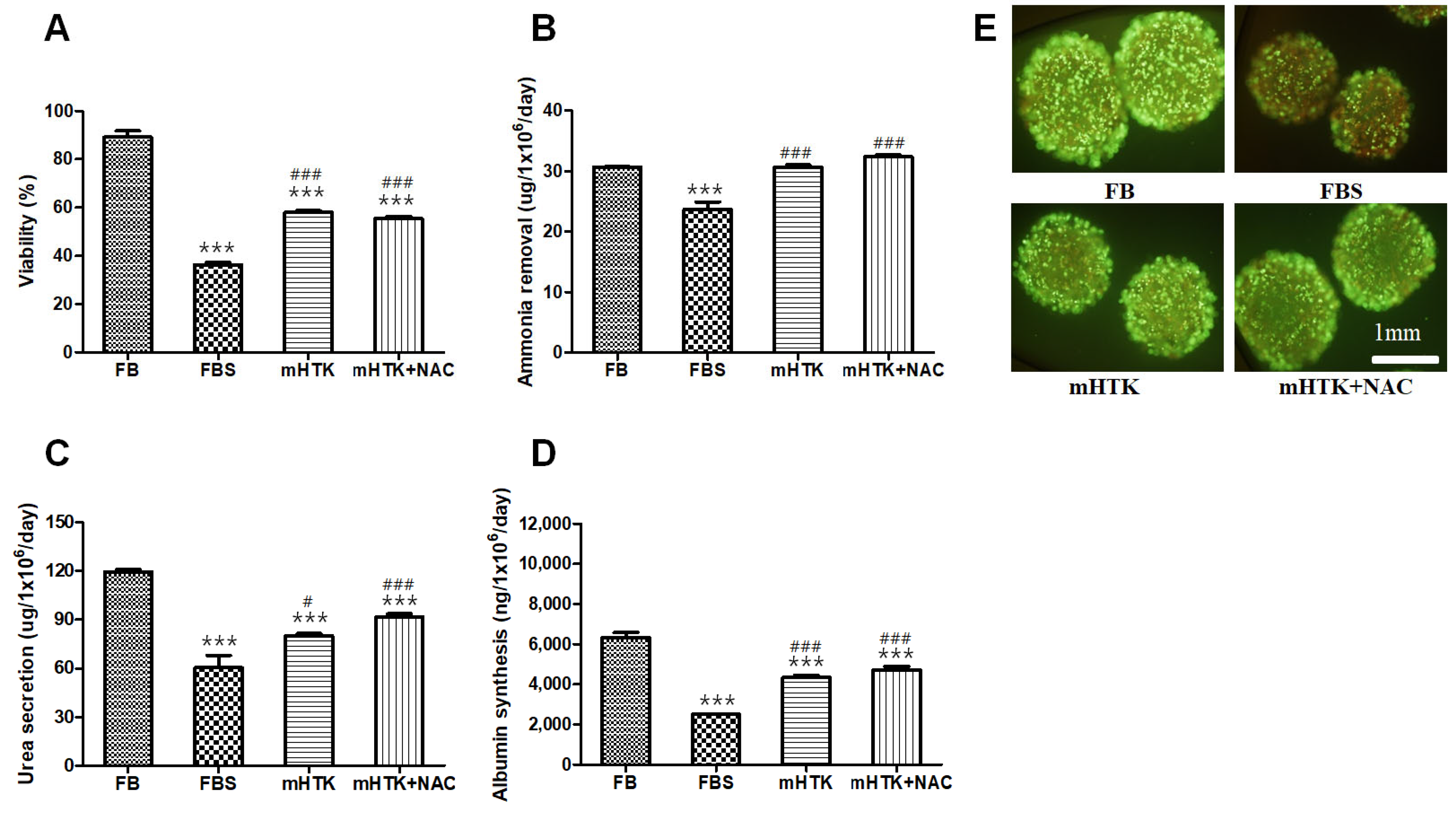
3.2. Viability and Liver-Specific Functions of Cryopreserved Porcine Hepatocyte Spheroids
3.3. Viability and Liver-Specific Functions of Cryopreserved Porcine Hepatocyte Spheroid Beads
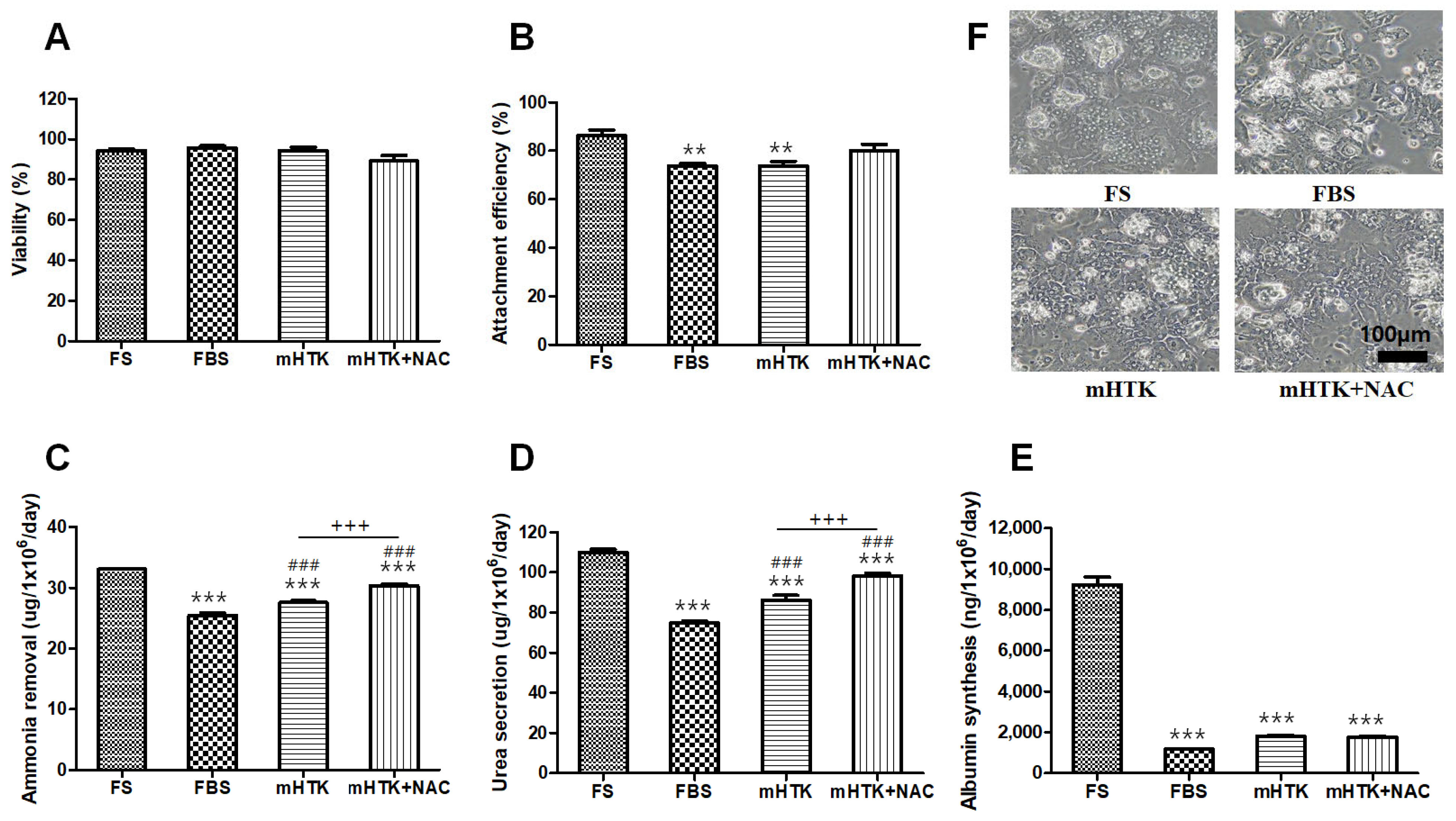
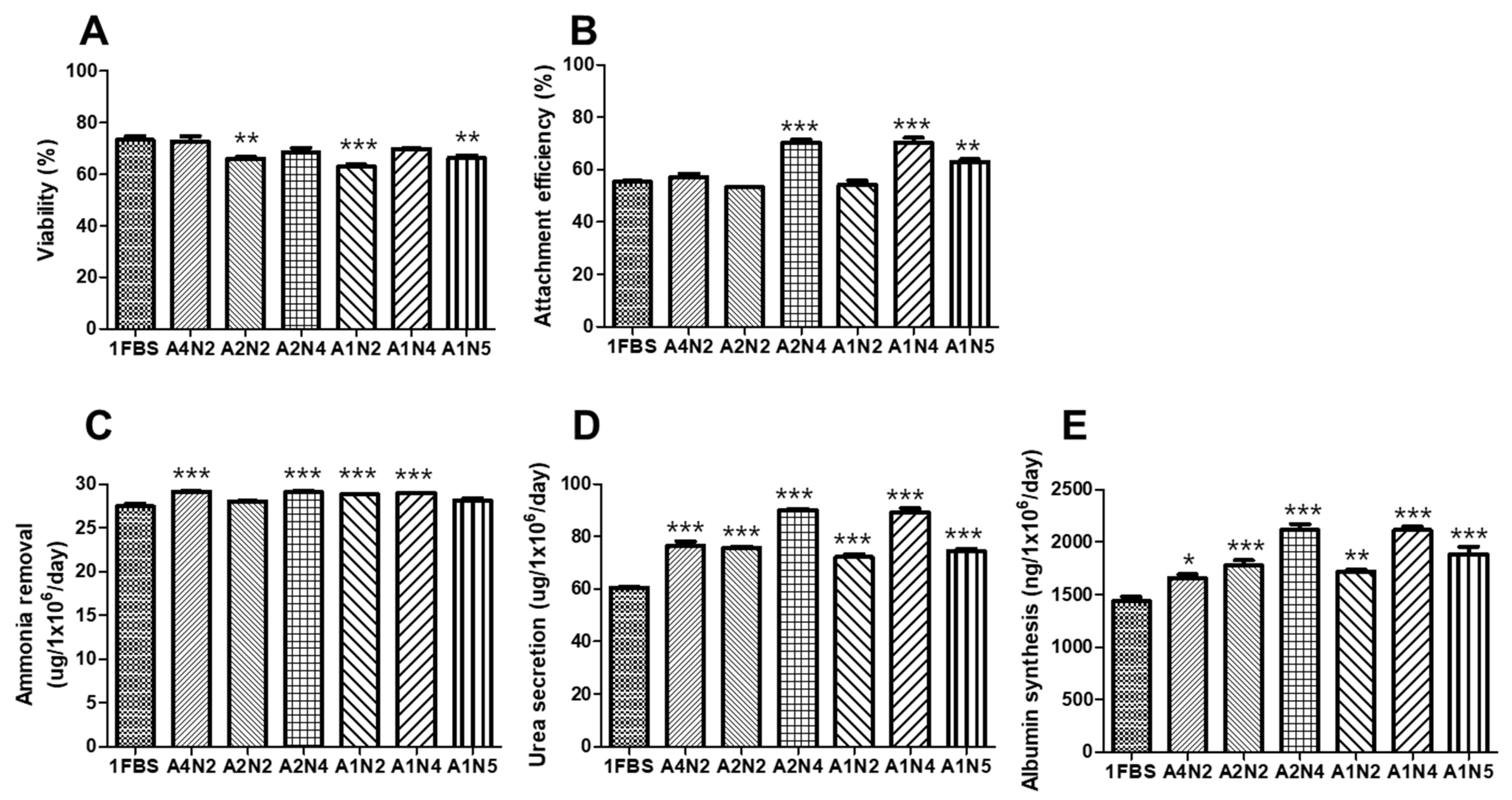
3.4. Optimal Concentration of Human Serum Albumin (HSA) and N-Acetylcysteine (NAC) for Serum-Free Thawing Medium
3.5. Viability and Liver-Specific Functions of Porcine Hepatocyte Spheroids Thawed Using Serum-Free Medium
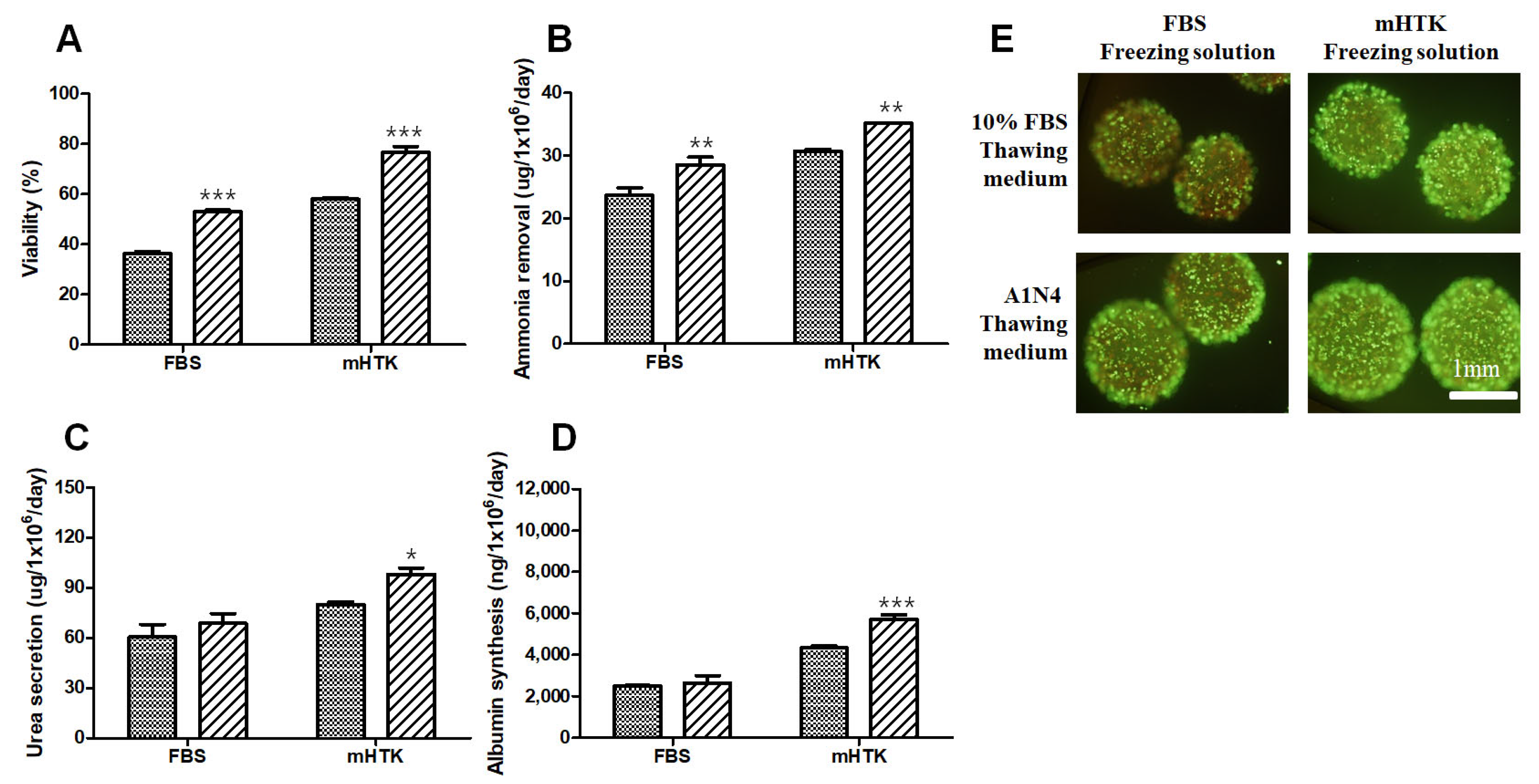
3.6. Viability and Liver-Specific Functions of Porcine Hepatocyte Spheroid Beads Thawed Using Serum-Free Medium
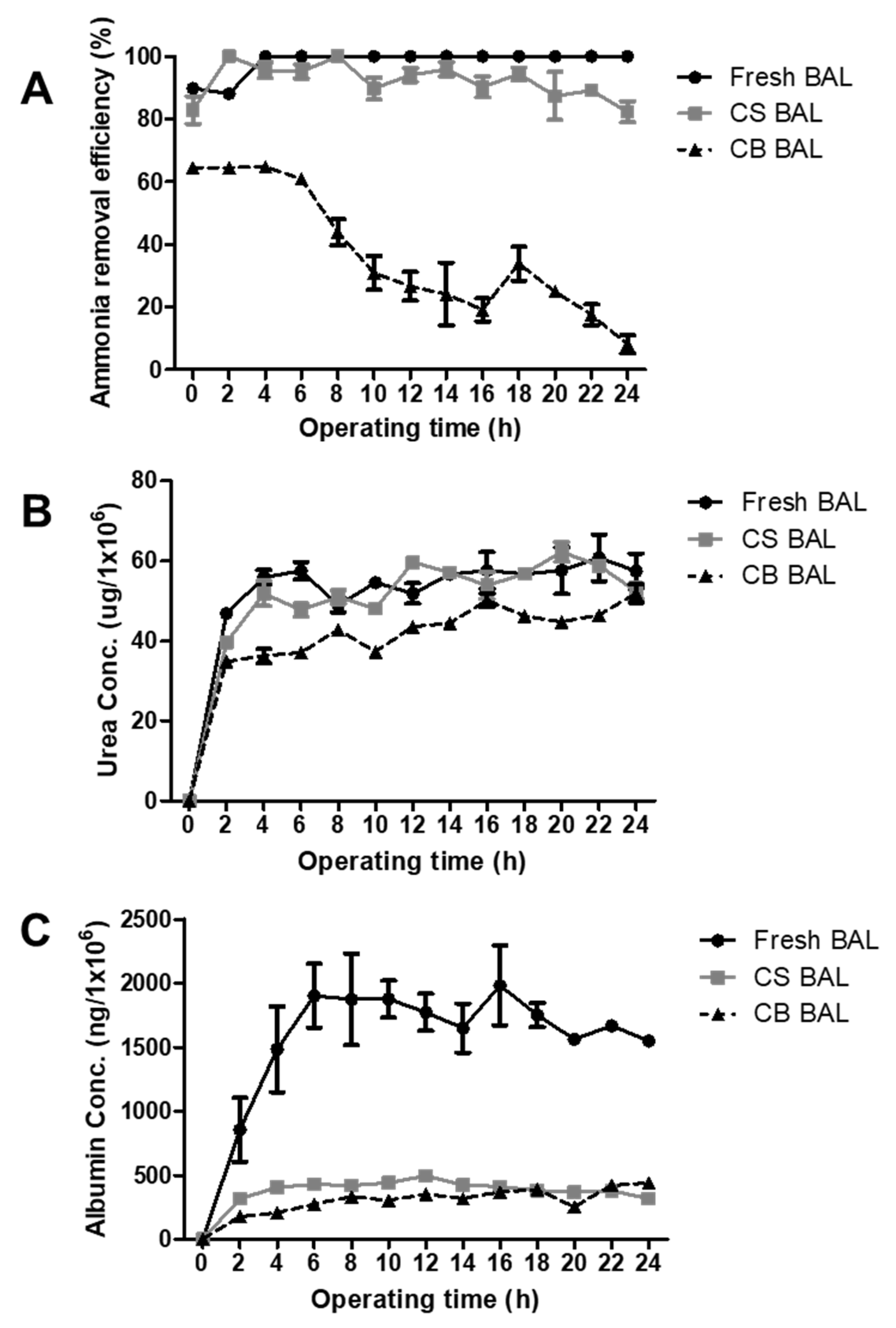
3.7. Liver-Specific Functions of Cryopreserved Porcine Hepatocyte Spheroids in a BAL System
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terry, C.; Dhawan, A.; Mitry, R.R.; Hughes, R.D. Cryopreservation of isolated human hepatocytes for transplantation: State of the art. Cryobiology 2006, 53, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Strain, A.J.; Neuberger, J.M. A bioartificial liver--state of the art. Science 2002, 295, 1005–1009. [Google Scholar] [CrossRef]
- Guha, C.; Deb, N.J.; Sappal, B.S.; Ghosh, S.S.; Roy-Chowdhury, N. Amplification of engrafted hepatocytes by preparative manipulation of the host liver. Artif. Organs 2001, 25, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Lee, J.-H.; Shin, S.W.; Kim, S.J.; Joh, J.-W.; Lee, D.-H.; Kim, J.-W.; Park, H.-Y.; Lee, S.-Y.; Lee, H.H.; et al. Hepatocyte transplantation for glycogen storage disease type Ib. Cell Transplant. 2007, 16, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lechon, M.J.; Lahoz, A.; Jimenez, N.; Vicente Castell, J.; Donato, M.T. Cryopreservation of rat, dog and human hepatocytes: Influence of preculture and cryoprotectants on recovery, cytochrome P450 activities and induction upon thawing. Xenobiotica 2006, 36, 457–472. [Google Scholar] [CrossRef]
- Stephenne, X.; Najimi, M.; Ngoc, D.K.; Smets, F.; Hue, L.; Guigas, B.; Sokal, E.M. Cryopreservation of human hepatocytes alters the mitochondrial respiratory chain complex 1. Cell Transplant. 2007, 16, 409–419. [Google Scholar] [CrossRef]
- Terry, C.; Hughes, R.D.; Mitry, R.R.; Lehec, S.C.; Dhawan, A. Cryopreservation-induced nonattachment of human hepatocytes: Role of adhesion molecules. Cell Transplant. 2007, 16, 639–647. [Google Scholar] [CrossRef]
- Grant, M.H.; Stevenson, D. Cryopreservation of Hepatocytes for Bioartificial Liver Devices. In Bioreactors for Tissue Engineering; Springer: Berlin, Germany, 2006; pp. 353–372. [Google Scholar]
- Dunne, J.B.; Davenport, M.; Williams, R.; Tredger, J.M. Evidence that S-adenosylmethionine and N-acetylcysteine reduce injury from sequential cold and warm ischemia in the isolated perfused rat liver. Transplantation 1994, 57, 1161–1168. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Emre, S.; Senyuz, O.; Acarli, K.; Schwartz, M.E.; Miller, C.M. N-acetylcysteine ameliorates reperfusion injury after warm hepatic ischemia. Transplantation 1995, 59, 6–9. [Google Scholar] [CrossRef]
- Fusai, G.; Glantzounis, G.K.; Hafez, T.; Yang, W.; Quaglia, A.; Sheth, H.; Kanoria, S.; Parkes, H.; Seifalian, A.; Davidson, B.R. N-Acetylcysteine ameliorates the late phase of liver ischaemia/reperfusion injury in the rabbit with hepatic steatosis. Clin. Sci. 2005, 109, 465–473. [Google Scholar] [CrossRef]
- Sagias, F.G.; Mitry, R.R.; Hughes, R.D.; Lehec, S.C.; Patel, A.G.; Rela, M.; Mieli-Vergani, G.; Heaton, N.D.; Dhawan, A. N-acetylcysteine improves the viability of human hepatocytes isolated from severely steatotic donor liver tissue. Cell Transplant. 2010, 19, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lechon, M.J.; Lahoz, A.; Jimenez, N.; Bonora, A.; Castell, J.V.; Donato, M.T. Evaluation of drug-metabolizing and functional competence of human hepatocytes incubated under hypothermia in different media for clinical infusion. Cell Transplant. 2008, 17, 887–897. [Google Scholar] [CrossRef] [PubMed]
- de Rougemont, O.; Lehmann, K.; Clavien, P.A. Preconditioning, organ preservation, and postconditioning to prevent ischemia-reperfusion injury to the liver. Liver Transpl. 2009, 15, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Stephenne, X.; Najimi, M.; Sokal, E.M. Hepatocyte cryopreservation: Is it time to change the strategy? World J. Gastroenterol. 2010, 16, 1–14. [Google Scholar] [CrossRef]
- Mazur, P. Principles of Cryobiology. In Life in the Frozen State; CRC Press: Boca Raton, FL, USA, 2004; pp. 3–65. ISBN 9780429212482. [Google Scholar]
- Hewitt, N.J.; Fischer, T.; Zuehlke, U.; Oesch, F.; Utesch, D. Metabolic activity of fresh and cryopreserved cynomolgus monkey (Macaca fascicularis) hepatocytes. Xenobiotica 2000, 30, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Guo, D.; Huang, X.; O’Gorman, M.R.G.; Huang, L.; Crawford, S.E.; Soriano, H.E. Apoptosis Occurs in Isolated and Banked Primary Mouse Hepatocytes. Cell Transplant. 2001, 10, 59–66. [Google Scholar] [CrossRef]
- Cotgreave, I.A. N-acetylcysteine: Pharmacological considerations and experimental and clinical applications. Adv. Pharmacol. 1996, 38, 205–227. [Google Scholar] [CrossRef]
- Zwingmann, C.; Bilodeau, M. Metabolic insights into the hepatoprotective role of N-acetylcysteine in mouse liver. Hepatology 2006, 43, 454–463. [Google Scholar] [CrossRef]
- Zafarullah, M.; Li, W.Q.; Sylvester, J.; Ahmad, M. Molecular mechanisms of N-acetylcysteine actions. Cell. Mol. Life Sci. 2003, 60, 6–20. [Google Scholar] [CrossRef]
- Gonzalez, R.; Ferrín, G.; Hidalgo, A.B.; Ranchal, I.; López-Cillero, P.; Santos-Gónzalez, M.; López-Lluch, G.; Briceño, J.; Gómez, M.A.; Poyato, A.; et al. N-acetylcysteine, coenzyme Q10 and superoxide dismutase mimetic prevent mitochondrial cell dysfunction and cell death induced by d-galactosamine in primary culture of human hepatocytes. Chem. Biol. Interact. 2009, 181, 95–106. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.-H.; Park, H.-J.; General, T.; Cho, M.-G.; Lee, S.-K. Cryopreservation of immobilized rat hepatocytes for the development of a bioartificial liver system. Transplant. Proc. 2012, 44, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, D.-H.; Lee, S.; Kwon, C.H.D.; Ryu, J.-N.; Noh, J.-K.; Jang, I.K.; Park, H.-J.; Yoon, H.-H.; Park, J.-K.; et al. Functional Evaluation of a Bioartificial Liver Support System Using Immobilized Hepatocyte Spheroids in a Porcine Model of Acute Liver Failure. Sci. Rep. 2017, 7, 3804–3810. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, D.H.; Son, J.H.; Park, J.K.; Kim, S.K. Optimization of chitosan-alginate encapsulation process using pig hepatocytes for development of bioartificial liver. J. Microbiol. Biotechnol. 2005, 15, 7–13. [Google Scholar]
- Dou, M.; de Sousa, G.; Lacarelle, B.; Placidi, M.; de La Porte, P.; Domingo, M.; Lafont, H.; Rahmani, R. Thawed human hepatocytes in primary culture. Cryobiology 1992, 29, 454–469. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, S.L.; Hardin, J.; Amiot, B.; Argikar, U.A.; Remmel, R.P.; Rinaldo, P. Rapid, large-scale formation of porcine hepatocyte spheroids in a novel spheroid reservoir bioartificial liver. Liver Transplant. 2005, 11, 901–910. [Google Scholar] [CrossRef]
- Mahler, S.; Desille, M.; Frémond, B.; Chesné, C.; Guillouzo, A.; Campion, J.-P.; Clément, B. Hypothermic storage and cryopreservation of hepatocytes: The protective effect of alginate gel against cell damages. Cell Transplant. 2003, 12, 579–592. [Google Scholar] [CrossRef]
- Aoki, T.; Koizumi, T.; Kobayashi, Y.; Yasuda, D.; Izumida, Y.; Jin, Z.; Nishino, N.; Shimizu, Y.; Kato, H.; Murai, N.; et al. A novel method of cryopreservation of rat and human hepatocytes by using encapsulation technique and possible use for cell transplantation. Cell Transplant. 2005, 14, 609–620. [Google Scholar] [CrossRef]
- Kusano, T.; Aoki, T.; Yasuda, D.; Matsumoto, S.; Jin, Z.; Nishino, N.; Hayashi, K.; Odaira, M.; Yamada, K.; Koizumi, T.; et al. Microencapsule technique protects hepatocytes from cryoinjury. Hepatol. Res. 2008, 38, 593–600. [Google Scholar] [CrossRef]
- Zeisberger, S.M.; Schulz, J.C.; Mairhofer, M.; Ponsaerts, P.; Wouters, G.; Doerr, D.; Katsen-Globa, A.; Ehrbar, M.; Hescheler, J.; Hoerstrup, S.P.; et al. Biological and physicochemical characterization of a serum- and xeno-free chemically defined cryopreservation procedure for adult human progenitor cells. Cell Transplant. 2011, 20, 1241–1257. [Google Scholar] [CrossRef]
- Hreinsson, J.; Zhang, P.; Swahn, M.L.; Hultenby, K.; Hovatta, O. Cryopreservation of follicles in human ovarian cortical tissue. Comparison of serum and human serum albumin in the cryoprotectant solutions. Hum. Reprod. 2003, 18, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.W.; Hassanein, T.; Bhatia, S.N. Advances in bioartificial liver devices. Hepatology 2001, 34, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Illouz, S.; Alexandre, E.; Pattenden, C.; Mark, L.; Bachellier, P.; Webb, M.; Berry, D.; Dennison, A.; Richert, L. Differential effects of curcumin on cryopreserved versus fresh primary human hepatocytes. Phytother. Res. 2008, 22, 1688–1691. [Google Scholar] [CrossRef]
- Muldrew, K.; Acker, J.P.; Elliott, J.A.; McGann, L.E. The water to ice transition: Implications for living cells. In Life in the Frozen State; CRC Press: Boca Raton, FL, USA, 2004; pp. 67–108. ISBN 9780429212482. [Google Scholar]
- Terry, C.; Dhawan, A.; Mitry, R.R.; Lehec, S.C.; Hughes, R.D. Optimization of the cryopreservation and thawing protocol for human hepatocytes for use in cell transplantation. Liver Transpl. 2010, 16, 229–237. [Google Scholar] [CrossRef]
- Sosef, M.N.; Baust, J.M.; Sugimachi, K.; Fowler, A.; Tompkins, R.G.; Toner, M. Cryopreservation of isolated primary rat hepatocytes: Enhanced survival and long-term hepatospecific function. Ann. Surg. 2005, 241, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ding, Y.; Zhang, H. Cryopreservation of suckling pig hepatocytes. Ann. Clin. Lab. Sci. 2001, 31, 391–398. [Google Scholar]
- Hewitt, N.J. Optimisation of the cryopreservation of primary hepatocytes. Methods Mol. Biol. 2010, 640, 83–105. [Google Scholar]
- Risso, P.S.; Koike, M.K.; Abrahao Mde, S.; Ferreira, N.C.; Montero, E.F. The effect of n-acetylcysteine on hepatic histomorphology during hypothermic preservation. Acta Cir. Bras. 2014, 29 (Suppl. S3), 28–32. [Google Scholar] [CrossRef][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Park, H.-J.; Kim, Y.-A.; Lee, D.-H.; Noh, J.-K.; Jung, J.-G.; Yoon, H.-H.; Lee, S.-K.; Lee, S. Establishment of a Serum-Free Hepatocyte Cryopreservation Process for the Development of an “Off-the-Shelf” Bioartificial Liver System. Bioengineering 2022, 9, 738. https://doi.org/10.3390/bioengineering9120738
Lee J-H, Park H-J, Kim Y-A, Lee D-H, Noh J-K, Jung J-G, Yoon H-H, Lee S-K, Lee S. Establishment of a Serum-Free Hepatocyte Cryopreservation Process for the Development of an “Off-the-Shelf” Bioartificial Liver System. Bioengineering. 2022; 9(12):738. https://doi.org/10.3390/bioengineering9120738
Chicago/Turabian StyleLee, Ji-Hyun, Hey-Jung Park, Young-A Kim, Doo-Hoon Lee, Jeong-Kwon Noh, Jong-Gab Jung, Hee-Hoon Yoon, Suk-Koo Lee, and Sanghoon Lee. 2022. "Establishment of a Serum-Free Hepatocyte Cryopreservation Process for the Development of an “Off-the-Shelf” Bioartificial Liver System" Bioengineering 9, no. 12: 738. https://doi.org/10.3390/bioengineering9120738
APA StyleLee, J.-H., Park, H.-J., Kim, Y.-A., Lee, D.-H., Noh, J.-K., Jung, J.-G., Yoon, H.-H., Lee, S.-K., & Lee, S. (2022). Establishment of a Serum-Free Hepatocyte Cryopreservation Process for the Development of an “Off-the-Shelf” Bioartificial Liver System. Bioengineering, 9(12), 738. https://doi.org/10.3390/bioengineering9120738