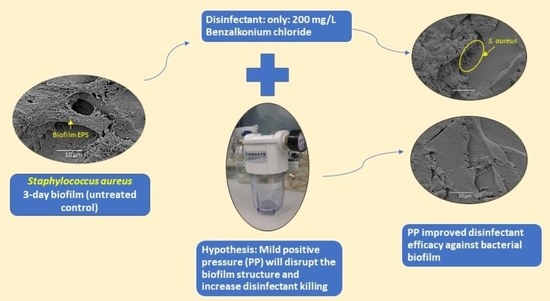
Mild Positive Pressure Improves the Efficacy of Benzalkonium Chloride against Staphylococcus aureus Biofilm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Staphylococcus aureus Biofilm Production
2.2. Strategy for Proof of Concept
Preliminary Titration of Disinfectants
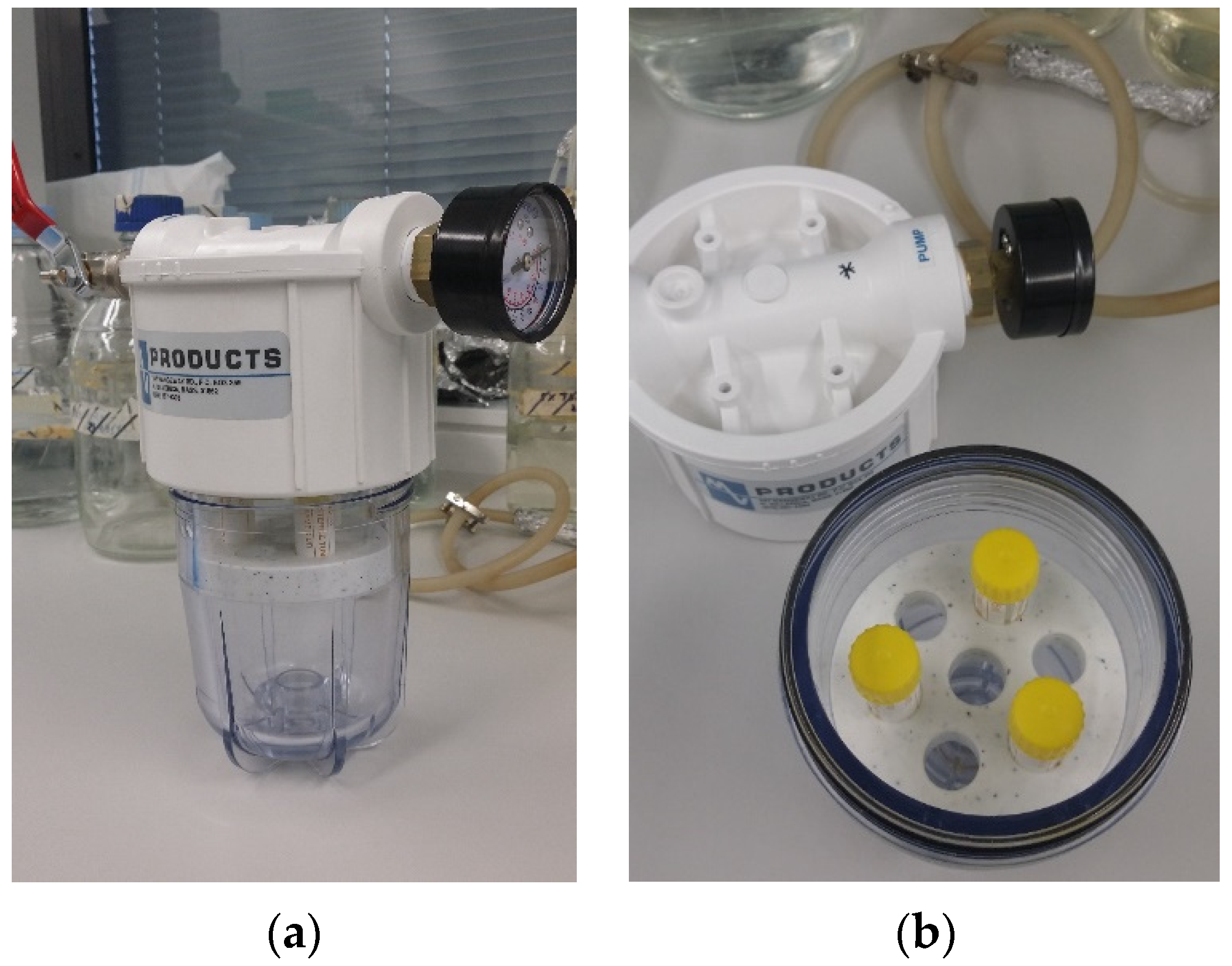
2.3. Combined Positive Pressure and Disinfectant Efficacy Testing
- (1)
- Diluted test disinfectant only without PP (1 atm).
- (2)
- PP only, at 3, 5, 7, and 10 atm in a positive pressure chamber designed and custom made by Dr. David Inglis (Figure 1).
- (3)
- Combined disinfectant and PP at 3, 5, 7, and 10 atm.
2.4. Confocal Laser Scanning Microscopy
2.5. Scanning Electron Microscopy
2.6. Statistical Analysis
3. Results
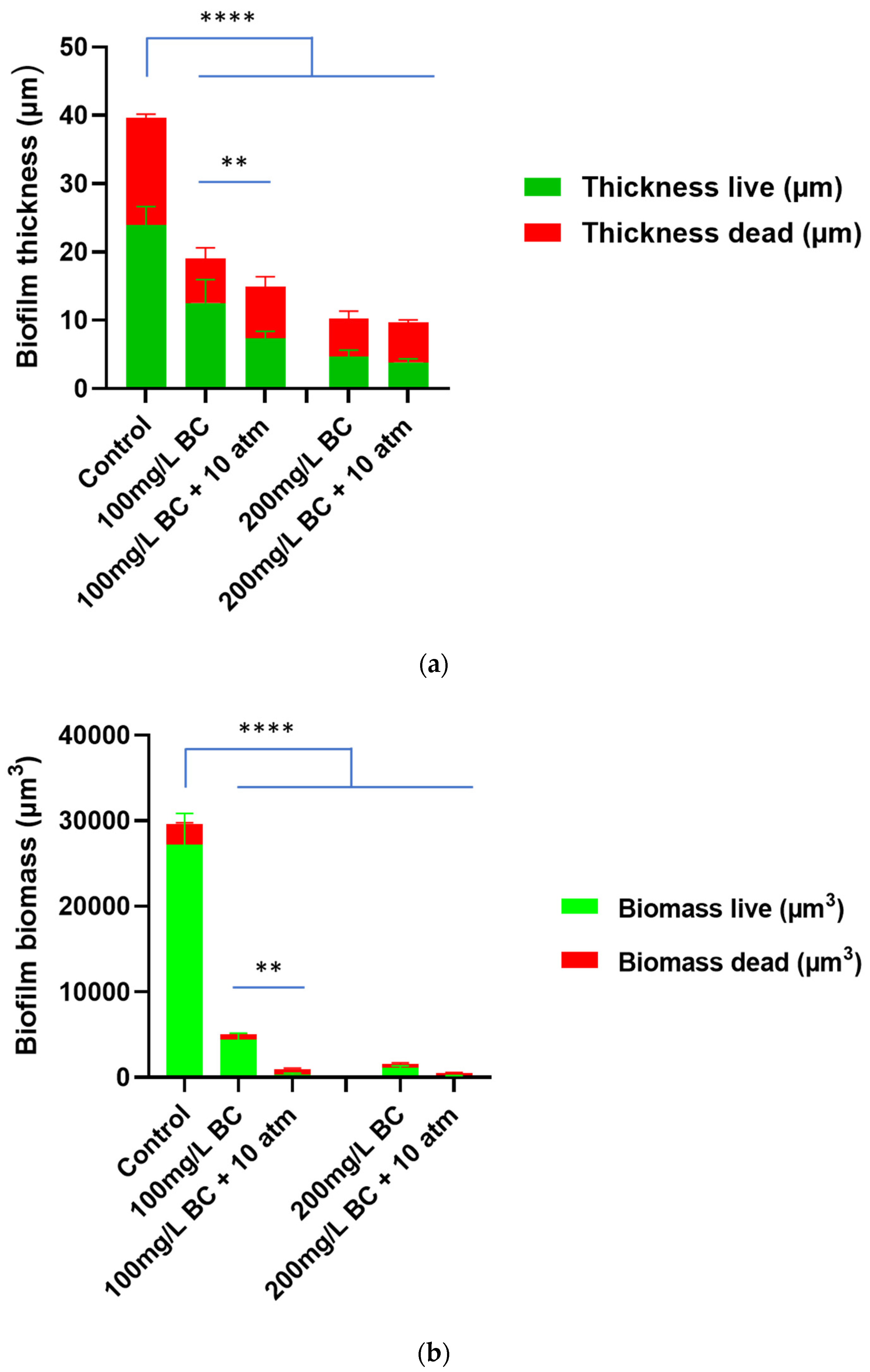
3.1. Effect of Positive Pressure on Benzalkonium Chloride Treatment against Staphylococcus aureus Biofilm
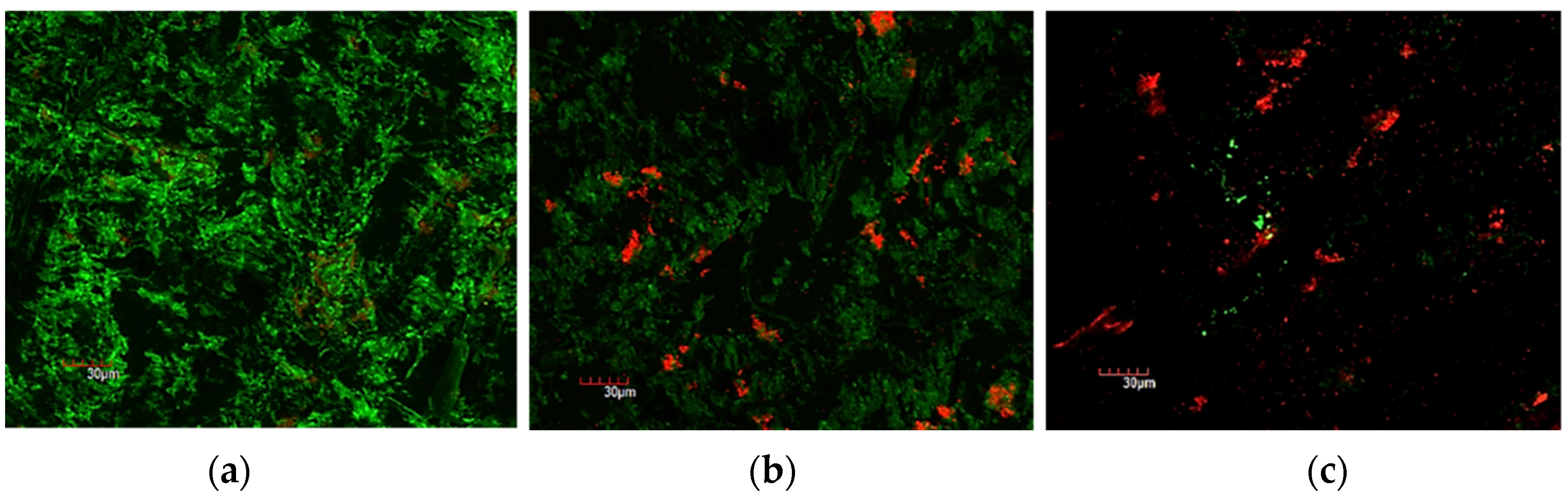
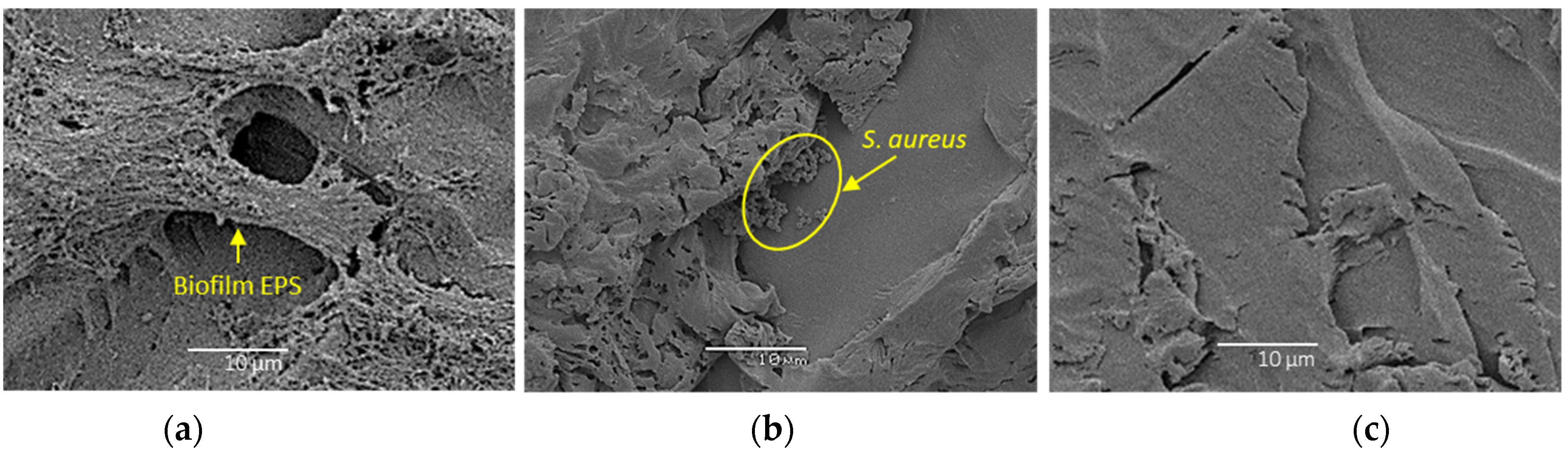
3.2. Confocal Laser Scanning Microscopy and Scanning Electron Microscopy
4. Discussion
4.1. Key Findings of the Study
4.2. How Does the Positive Pressure Improve the Disinfectant Killing of Biofilm Cells?
4.3. Practical Implications and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, H.; Johani, K.; Gosbell, I.B.; Jacombs, A.S.; Almatroudi, A.; Whiteley, G.S.; Deva, A.K.; Jensen, S.; Vickery, K. Intensive care unit environmental surfaces are contaminated by multidrug-resistant bacteria in biofilms: Combined results of conventional culture, pyrosequencing, scanning electron microscopy, and confocal laser microscopy. J. Hosp. Infect. 2015, 91, 35–44. [Google Scholar] [CrossRef]
- Costa, D.; Johani, K.; Melo, D.; Lopes, L.; Lima, L.L.; Tipple, A.; Hu, H.; Vickery, K. Biofilm contamination of high-touched surfaces in intensive care units: Epidemiology and potential impacts. Lett. Appl. Microbiol. 2019, 68, 269–276. [Google Scholar] [CrossRef]
- Lopes, L.K.O.; Costa, D.M.; Tipple, A.F.V.; Watanabe, E.; Castillo, R.B.; Hu, H.; Deva, A.; Vickery, K. Surgical instruments complex design as a barrier for cleaning effectiveness, favouring biofilm formation. J. Hosp. Infect. 2019, 103, e53–e60. [Google Scholar] [CrossRef] [PubMed]
- Hensley, D.M.; Krauland, K.J.; McGlasson, D.L. Acinetobacter baumannii and MRSA contamination on reusable phlebotomy tourniquets. Clin. Lab. Sci. 2010, 23, 151–156. [Google Scholar] [CrossRef]
- Maki, D.G. Mayo Clinic: Proceedings: Stethoscopes and health care-associated infection. Mayo Clin. Proc. 2014, 89, 277. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef]
- Leung, C.Y.; Chan, Y.C.; Samaranayake, L.P.; Seneviratne, C.J. Biocide resistance of Candida and Escherichia coli biofilms is associated with higher antioxidative capacities. J. Hosp. Infect. 2012, 81, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Vickery, K.; Pajkos, A.; Cossart, Y. Removal of biofilm from endoscopes: Evaluation of detergent efficiency. Am. J. Infect. Control. 2004, 32, 170–176. [Google Scholar] [CrossRef]
- Da Costa Luciano, C.; Olson, N.; Tipple, A.F.; Alfa, M. Evaluation of the ability of different detergents and disinfectants to remove and kill organisms in traditional biofilm. Am. J. Infect. Control. 2016, 44, e243–e249. [Google Scholar] [CrossRef]
- Otter, J.A.; Vickery, K.; Walker, J.T.; deLancey Pulcini, E.; Stoodley, P.; Goldenberg, S.D.; Salkeld, J.A.; Chewins, J.; Yezli, S.; Edgeworth, J.D. Surface-attached cells, biofilms and biocide susceptibility: Implications for hospital cleaning and disinfection. J. Hosp. Infect. 2015, 89, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Vickery, K.; Ngo, Q.D.; Zou, J.; Cossart, Y.E. The effect of multiple cycles of contamination, detergent washing, and disinfection on the development of biofilm in endoscope tubing. Am. J. Infect. Control. 2009, 37, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Luciano, C.; Olson, N.; DeGagne, P.; Franca, R.; Tipple, A.F.V.; Alfa, M. A new buildup biofilm model that mimics accumulation of material in flexible endoscope channels. J. Microbiol. Methods 2016, 127, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Parvin, F.; Hu, H.; Whiteley, G.S.; Glasbey, T.; Vickery, K. Difficulty in removing biofilm from dry surfaces. J. Hosp. Infect. 2019, 103, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Almatroudi, A.; Gosbell, I.B.; Hu, H.; Jensen, S.O.; Espedido, B.A.; Tahir, S.; Glasbey, T.O.; Legge, P.; Whiteley, G.; Deva, A.; et al. Staphylococcus aureus dry-surface biofilms are not killed by sodium hypochlorite: Implications for infection control. J. Hosp. Infect. 2016, 93, 263–270. [Google Scholar] [CrossRef]
- Almatroudi, A.; Hu, H.; Deva, A.; Gosbell, I.B.; Jacombs, A.; Jensen, S.O.; Whiteley, G.; Glasbey, T.; Vickery, K. A new dry-surface biofilm model: An essential tool for efficacy testing of hospital surface decontamination procedures. J. Microbiol. Methods 2015, 117, 171–176. [Google Scholar] [CrossRef]
- Ngo, Q.D.; Vickery, K.; Deva, A.K. The effect of topical negative pressure on wound biofilms using an in vitro wound model. Wound Repair Regen. 2012, 20, 83–90. [Google Scholar] [CrossRef]
- Tahir, S.; Malone, M.; Hu, H.; Deva, A.; Vickery, K. The Effect of Negative Pressure Wound Therapy with and without Instillation on Mature Biofilms In Vitro. Materials 2018, 11, 811. [Google Scholar] [CrossRef]
- Bello, E.F.; Martínez, G.G.; Ceberio, B.F.; Rodrigo, D.; López, A.M. High Pressure Treatment in Foods. Foods 2014, 3, 476–490. [Google Scholar] [CrossRef]
- Zmantar, T.; Kouidhi, B.; Miladi, H.; Bakhrouf, A. Detection of macrolide and disinfectant resistance genes in clinical Staphylococcus aureus and coagulase-negative staphylococci. BMC Res. Notes 2011, 4, 453. [Google Scholar] [CrossRef]
- Treangen, T.J.; Maybank, R.A.; Enke, S.; Friss, M.B.; Diviak, L.F.; Karaolis, D.K.; Koren, S.; Ondov, B.; Phillippy, A.M.; Bergman, N.H.; et al. Complete Genome Sequence of the Quality Control Strain Staphylococcus aureus subsp. aureus ATCC 25923. Genome Announc. 2014, 2, e01110-14. [Google Scholar] [CrossRef] [Green Version]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Bridier, A.; Briandet, R.; Thomas, V.; Dubois-Brissonnet, F. Resistance of bacterial biofilms to disinfectants: A review. Biofouling 2011, 27, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, M.; Morris, D.; De Lappe, N.; O’Connor, J.; Lalor, P.; Dockery, P.; Cormican, M. Commonly used disinfectants fail to eradicate Salmonella enterica biofilms from food contact surface materials. Appl. Environ. Microbiol. 2014, 80, 1507–1514. [Google Scholar] [CrossRef]
- Valente, P.M.; Deva, A.; Ngo, Q.; Vickery, K. The increased killing of biofilms in vitro by combining topical silver dressings with topical negative pressure in chronic wounds. Int. Wound J. 2016, 13, 130–136. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahir, S.; Emanuel, S.; Inglis, D.W.; Vickery, K.; Deva, A.K.; Hu, H. Mild Positive Pressure Improves the Efficacy of Benzalkonium Chloride against Staphylococcus aureus Biofilm. Bioengineering 2022, 9, 461. https://doi.org/10.3390/bioengineering9090461
Tahir S, Emanuel S, Inglis DW, Vickery K, Deva AK, Hu H. Mild Positive Pressure Improves the Efficacy of Benzalkonium Chloride against Staphylococcus aureus Biofilm. Bioengineering. 2022; 9(9):461. https://doi.org/10.3390/bioengineering9090461
Chicago/Turabian StyleTahir, Shamaila, Sarah Emanuel, David W. Inglis, Karen Vickery, Anand K. Deva, and Honghua Hu. 2022. "Mild Positive Pressure Improves the Efficacy of Benzalkonium Chloride against Staphylococcus aureus Biofilm" Bioengineering 9, no. 9: 461. https://doi.org/10.3390/bioengineering9090461
APA StyleTahir, S., Emanuel, S., Inglis, D. W., Vickery, K., Deva, A. K., & Hu, H. (2022). Mild Positive Pressure Improves the Efficacy of Benzalkonium Chloride against Staphylococcus aureus Biofilm. Bioengineering, 9(9), 461. https://doi.org/10.3390/bioengineering9090461