Green Production and Interaction of Carboxylated CNTs/Biogenic ZnO Composite for Antibacterial Activity
Abstract
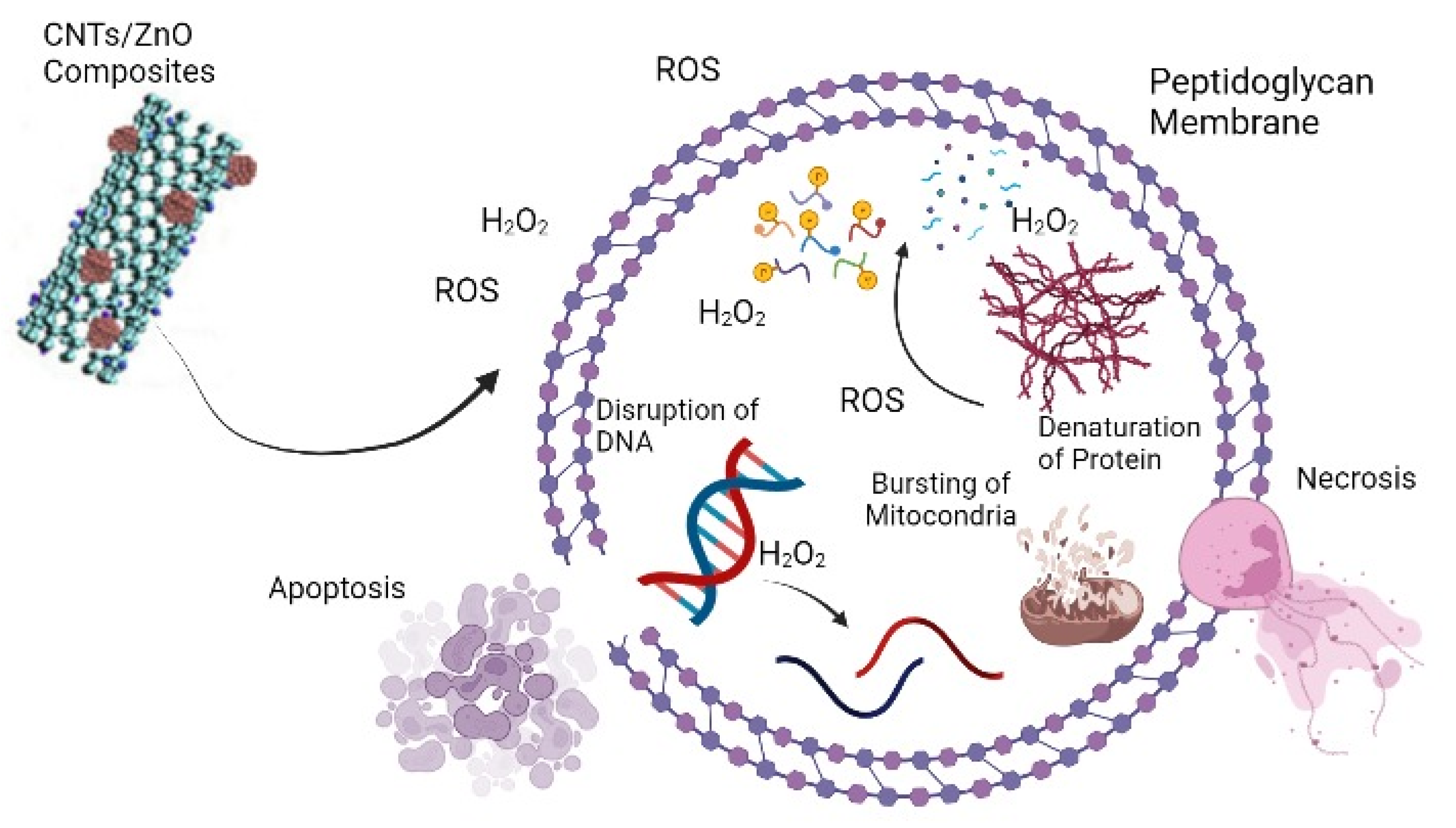
:1. Introduction
2. Materials and Methods
2.1. Research Methods
2.2. Collection of Moringa oleifera Extract
2.3. Synthesis of ZnO−NPs
2.4. Preparation of the CNTs−COOH/ZnO Composite
2.5. Antibacterial Action
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of CarboxylatedCNTs/ZnOComposite
3.2. Antibacterial Activity of ZnO and CNTs/ZnOComposite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, A.; Sabeeh, H.; Zulfiqar, S.; Agboola, P.O.; Shakir, I.; Warsi, M.F. Structural, optical and photocatalytic studies of trimetallic oxides nanostructures prepared via wet chemical approach. Synth. Met. 2020, 259, 116228. [Google Scholar] [CrossRef]
- Datta, A.; Patra, C.; Bharadwaj, H.; Kaur, S.; Dimri, N.; Khajuria, R. Green synthesis of zinc oxide nanoparticles using parthenium hysterophorus leaf extract and evaluation of their antibacterial properties. J. Biotechnol. Biomater. 2017, 7, 271–276. [Google Scholar] [CrossRef]
- Farid, T.; Rafiq, M.I.; Ali, A.; Tang, W. Transforming wood as next-generation structural and functional materials for a sustainable future. EcoMat 2022, 4, e12154. [Google Scholar] [CrossRef]
- Matinise, N.; Fuku, X.; Kaviyarasu, K.; Mayedwa, N.; Maaza, M. ZnO nanoparticles via Moringa oleifera green synthesis: Physical properties & mechanism of formation. Appl. Surf. Sci. 2017, 406, 339–347. [Google Scholar]
- Kumar, V.; Pandey, N.; Mohan, N.; Singh, R.P. Antibacterial & antioxidant activity of different extract of Moringa oleifera Leaves–an in vitro study. Int. J. Pharm. Sci. Rev. Res. 2012, 12, 89–94. [Google Scholar]
- Tshabalala, T.; Ndhlala, A.; Ncube, B.; Abdelgadir, H.; Van Staden, J. Potential substitution of the root with the leaf in the use of Moringa oleifera for antimicrobial, antidiabetic and antioxidant properties. S. Afr. J. Bot. 2020, 129, 106–112. [Google Scholar] [CrossRef]
- Singh, R.G.; Negi, P.S.; Radha, C. Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringa oleifera seed flour. J. Funct. Foods 2013, 5, 1883–1891. [Google Scholar] [CrossRef]
- Kashyap, P.; Kumar, S.; Riar, C.S.; Jindal, N.; Baniwal, P.; Guiné, R.P.; Correia, P.M.; Mehra, R.; Kumar, H. Recent advances in Drumstick (Moringa oleifera) leaves bioactive compounds: Composition, health benefits, bioaccessibility, and dietary applications. Antioxidants 2022, 11, 402. [Google Scholar] [CrossRef]
- Al-Ghanayem, A.A.; Alhussaini, M.S.; Asad, M.; Joseph, B. Moringa oleifera Leaf Extract Promotes Healing of Infected Wounds in Diabetic Rats: Evidence of Antimicrobial, Antioxidant and Proliferative Properties. Pharmaceuticals 2022, 15, 528. [Google Scholar] [CrossRef]
- Akinyeye, A.; Solanke, E.; Adebiyi, I. Phytochemical and antimicrobial evaluation of leaf and seed of Moringa oleifera extracts. Int. J. Res. Med. Health Sci. 2014, 4, 2083–2307. [Google Scholar]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial activity of metal and metal-oxide based nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Chaudhary, K.; Shaheen, N.; Zulfiqar, S.; Sarwar, M.I.; Suleman, M.; Agboola, P.O.; Shakir, I.; Warsi, M.F. Binary WO3-ZnO nanostructures supported rGO ternary nanocomposite for visible light driven photocatalytic degradation of methylene blue. Synth. Met. 2020, 269, 116526. [Google Scholar] [CrossRef]
- Chaudhary, K.; Aadil, M.; Zulfiqar, S.; Ullah, S.; Haider, S.; Agboola, P.O.; Warsi, M.F.; Shakir, I. Graphene oxide and reduced graphene oxide supported ZnO nanochips for removal of basic dyes from the industrial effluents. Fuller. Nanotub. Carbon Nanostructures 2021, 29, 915–928. [Google Scholar] [CrossRef]
- Yusof, N.A.A.; Zain, N.M.; Pauzi, N. Synthesis of ZnO nanoparticles with chitosan as stabilizing agent and their antibacterial properties against Gram-positive and Gram-negative bacteria. Int. J. Biol. Macromol. 2019, 124, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Sumra, A.A.; Aadil, M.; Ejaz, S.R.; Anjum, S.; Saleem, T.; Zain, M.; Alsafari, I.A. Biological synthesis of nanostructured ZnO as a solar-light driven photocatalyst and antimicrobial agent. Ceram. Int. 2022, 48, 14652–14661. [Google Scholar] [CrossRef]
- Agarwal, H.; Menon, S.; Kumar, S.V.; Rajeshkumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem. -Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial effects of carbon nanotubes: Size does matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Dumortier, H.; Lacotte, S.; Pastorin, G.; Marega, R.; Wu, W.; Bonifazi, D.; Briand, J.-P.; Prato, M.; Muller, S.; Bianco, A. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006, 6, 1522–1528. [Google Scholar] [CrossRef]
- Magrez, A.; Kasas, S.; Salicio, V.; Pasquier, N.; Seo, J.W.; Celio, M.; Catsicas, S.; Schwaller, B.; Forró, L. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006, 6, 1121–1125. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Biomolecule-functionalized carbon nanotubes: Applications in nanobioelectronics. ChemPhysChem 2004, 5, 1084–1104. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Shen, Y.; Wang, M.; Li, J. Poly-L-lysine functionalization of single-walled carbon nanotubes. J. Phys. Chem. B 2004, 108, 15343–15346. [Google Scholar] [CrossRef]
- Lukhele, L.P.; Mamba, B.B.; Momba, M.N.; Krause, R.W. Water disinfection using novel cyclodextrin polyurethanes containing silver nanoparticles supported on carbon nanotubes. J. Appl. Sci. 2010, 10, 65–70. [Google Scholar] [CrossRef]
- Yuan, W.; Jiang, G.; Che, J.; Qi, X.; Xu, R.; Chang, M.W.; Chen, Y.; Lim, S.Y.; Dai, J.; Chan-Park, M.B. Deposition of silver nanoparticles on multiwalled carbon nanotubes grafted with hyperbranched poly (amidoamine) and their antimicrobial effects. J. Phys. Chem. C 2008, 112, 18754–18759. [Google Scholar] [CrossRef]
- Liu, T.; Tang, H.; Cai, X.; Zhao, J.; Li, D.; Li, R.; Sun, X. A study on bactericidal properties of Ag coated carbon nanotubes. Nucl. Instrum. Methods Phys. Res. Sect. B BeamInteract. Mater. At. 2007, 264, 282–286. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Cao, A.; Jiang, Y.; Zhang, X.; Liu, J.-H.; Liu, Y.; Wang, H. Superior antibacterial activity of zinc oxide/graphene oxide composites originating from high zinc concentration localized around bacteria. ACS Appl. Mater. Interfaces 2014, 6, 2791–2798. [Google Scholar] [CrossRef]
- Khashan, K.S.; Jabir, M.S.; Abdulameer, F.A. Carbon Nanoparticles prepared by laser ablation in liquid environment. Surf. Rev. Lett. 2019, 26, 1950078. [Google Scholar] [CrossRef]
- Khashan, K.S.; Jabir, M.S.; Abdulameer, F.A. Carbon Nanoparticles decorated with cupric oxide Nanoparticles prepared by laser ablation in liquid as an antibacterial therapeutic agent. Mater. Res. Express 2018, 5, 035003. [Google Scholar] [CrossRef]
- Saleh, T.A. The influence of treatment temperature on the acidity of MWCNT oxidized by HNO3 or a mixture of HNO3/H2SO4. Appl. Surf. Sci. 2011, 257, 7746–7751. [Google Scholar] [CrossRef]
- Sathappan, S.; Kirubakaran, N.; Gunasekaran, D.; Gupta, P.K.; Verma, R.S.; Sundaram, J. Green synthesis of zinc oxide nanoparticles (ZnO NPs) using cissus quadrangularis: Characterization, antimicrobial and anticancer studies. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2021, 91, 289–296. [Google Scholar] [CrossRef]
- Tripathi, R.; Bhadwal, A.S.; Gupta, R.K.; Singh, P.; Shrivastav, A.; Shrivastav, B. ZnO nanoflowers: Novel biogenic synthesis and enhanced photocatalytic activity. J. Photochem. Photobiol. B Biol. 2014, 141, 288–295. [Google Scholar] [CrossRef]
- Musić, S.; Popović, S.; Maljković, M.; Dragčević, Đ. Influence of synthesis procedure on the formation and properties of zinc oxide. J. Alloys Compd. 2002, 347, 324–332. [Google Scholar] [CrossRef]
- Bhuyan, T.; Mishra, K.; Khanuja, M.; Prasad, R.; Varma, A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater. Sci. Semicond. Process. 2015, 32, 55–61. [Google Scholar] [CrossRef]
- Narendhran, S.; Sivaraj, R. Biogenic ZnO nanoparticles synthesized using L. aculeata leaf extract and their antifungal activity against plant fungal pathogens. Bull. Mater. Sci. 2016, 39, 1–5. [Google Scholar] [CrossRef]
- Farid, T.; Wang, Y.; Rafiq, M.I.; Ali, A.; Tang, W. Porous Flexible Wood Scaffolds Designed for High-Performance Electrochemical Energy Storage. ACS Sustain. Chem. Eng. 2022, 10, 7078–7090. [Google Scholar] [CrossRef]
- AL-Anbari, R.H. Synthesis of multi-walled carbon nanotubes decorated with zinc oxide nanoparticles for removal of pathogenic bacterial. Eng. Technol. J. 2018, 36, 1075–1080. [Google Scholar]
- Suresh, D.; Shobharani, R.; Nethravathi, P.; Kumar, M.P.; Nagabhushana, H.; Sharma, S. Artocarpus gomezianus aided green synthesis of ZnO nanoparticles: Luminescence, photocatalytic and antioxidant properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 141, 128–134. [Google Scholar] [CrossRef]
- Ngoepe, N.; Mbita, Z.; Mathipa, M.; Mketo, N.; Ntsendwana, B.; Hintsho-Mbita, N. Biogenic synthesis of ZnO nanoparticles using Monsonia burkeana for use in photocatalytic, antibacterial and anticancer applications. Ceram. Int. 2018, 44, 16999–17006. [Google Scholar] [CrossRef]
- Verma, R.; Chauhan, A.; Shandilya, M.; Li, X.; Kumar, R.; Kulshrestha, S. Antimicrobial potential of Ag-doped ZnO nanostructure synthesized by the green method using Moringa oleifera extract. J. Environ. Chem. Eng. 2020, 8, 103730. [Google Scholar]
- David, M.E.; Ion, R.-M.; Grigorescu, R.M.; Iancu, L.; Holban, A.M.; Nicoara, A.I.; Alexandrescu, E.; Somoghi, R.; Ganciarov, M.; Vasilievici, G. Hybrid materials based on multi-walled carbon nanotubes and nanoparticles with antimicrobial properties. Nanomaterials 2021, 11, 1415. [Google Scholar] [CrossRef]
- Tahir, T.; Chaudhary, K.; Warsi, M.F.; Saif, M.S.; Alsafari, I.A.; Shakir, I.; Agboola, P.O.; Haider, S.; Zulfiqar, S. Synthesis of sponge like Gd3+ doped vanadium oxide/2D MXene composites for improved degradation of industrial effluents and pathogens. Ceram. Int. 2022, 48, 1969–1980. [Google Scholar] [CrossRef]
- Umair, M.; Sultana, T.; Xiaoyu, Z.; Senan, A.M.; Jabbar, S.; Khan, L.; Abid, M.; Murtaza, M.A.; Kuldeep, D.; Al-Areqi, N.A. LC-ESI-QTOF/MS characterization of antimicrobial compounds with their action mode extracted from vine tea (Ampelopsis grossedentata) leaves. Food Sci. Nutr. 2022, 10, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Senan, A.M.; Yin, B.; Zhang, Y.; Nasiru, M.M.; Lyu, Y.M.; Umair, M.; Bhat, J.A.; Zhang, S.; Liu, L. Efficient and selective catalytic hydroxylation of unsaturated plant oils: A novel method for producing anti-pathogens. BMC Chem. 2021, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Jabbar, S.; Sultana, T.; Ayub, Z.; Abdelgader, S.A.; Xiaoyu, Z.; Chong, Z.; Fengxia, L.; Xiaomei, B.; Zhaoxin, L. Chirality of the biomolecules enhanced its stereospecific action of dihydromyricetin enantiomers. Food Sci. Nutr. 2020, 8, 4843–4856. [Google Scholar] [CrossRef]
- Sharma, N.; Kumar, J.; Thakur, S.; Sharma, S.; Shrivastava, V. Antibacterial study of silver doped zinc oxide nanoparticles against Staphylococcus aureus and Bacillus subtilis. Drug Inventig. Today 2013, 5, 50–54. [Google Scholar] [CrossRef]
- Rahman, A.; Afzal, R.; Zulfiqar, S.; Alsafari, I.A.; Khan, M.A.; Agboola, P.O.; Haider, S.; Warsi, M.F.; Shakir, I. Superior photodegradation and antibacterial activity of r-GO supported ternary nanocomposite of doped transition metal compounds. Ceram. Int. 2021, 47, 14569–14578. [Google Scholar] [CrossRef]
- Akter, F.; Tinni, H.H.; Banarjee, P.; Hossain, M.Z. Effects Of heavy metals (Cd, Zn And Cu) on carbon, nitrogen and iron mineralization in soil. Malays. J. Sustain. Agric. 2019, 3, 33–38. [Google Scholar] [CrossRef]
- Ji, X.; Cheng, Y.; Tian, J.; Zhang, S.; Jing, Y.; Shi, M. Structural characterization of polysaccharide from jujube (Ziziphus jujuba Mill.) fruit. Chem. Biol. Technol. Agric. 2021, 8, 54. [Google Scholar] [CrossRef]
- Lai, W.-F. Development of hydrogels with self-healing properties for delivery of bioactive agents. Mol. Pharm. 2021, 18, 1833–1841. [Google Scholar] [CrossRef]
- Xu, P.; Cao, J.; Yin, C.; Wang, L.; Wu, L. Quantum chemical study on the adsorption of megazol drug on the pristine BC3 nanosheet. Supramol. Chem. 2021, 33, 63–69. [Google Scholar] [CrossRef]





| Bacterial Specie | ZOI of ZnO−NPs | ZOI of CNTs/ZnO Composite |
|---|---|---|
| (mm) | (mm) | |
| Escherichia Coli | 18.4 ± 3.7 | 18.8 ± 3.7 |
| Bacillus Cereus | 13.9 ± 0.9 | 16.8 ± 2.1 |
| Pseudomonas aeruginosa | 16.3 ± 1.5 | 17.2 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Khakwani, N.; Faiz, Y.; Zulfiqar, S.; Shafiq, Z.; Faiz, F.; Elhakem, A.; Sami, R.; Aljuraide, N.I.; Farid, T.; et al. Green Production and Interaction of Carboxylated CNTs/Biogenic ZnO Composite for Antibacterial Activity. Bioengineering 2022, 9, 437. https://doi.org/10.3390/bioengineering9090437
Hussain S, Khakwani N, Faiz Y, Zulfiqar S, Shafiq Z, Faiz F, Elhakem A, Sami R, Aljuraide NI, Farid T, et al. Green Production and Interaction of Carboxylated CNTs/Biogenic ZnO Composite for Antibacterial Activity. Bioengineering. 2022; 9(9):437. https://doi.org/10.3390/bioengineering9090437
Chicago/Turabian StyleHussain, Saghir, Noorulain Khakwani, Yasir Faiz, Sonia Zulfiqar, Zahid Shafiq, Faisal Faiz, Abeer Elhakem, Rokayya Sami, N. I. Aljuraide, Tanveer Farid, and et al. 2022. "Green Production and Interaction of Carboxylated CNTs/Biogenic ZnO Composite for Antibacterial Activity" Bioengineering 9, no. 9: 437. https://doi.org/10.3390/bioengineering9090437







