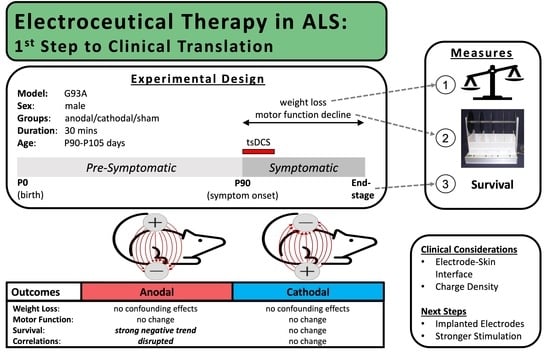
Non-Invasive Transcutaneous Spinal DC Stimulation as a Neurorehabilitation ALS Therapy in Awake G93A Mice: The First Step to Clinical Translation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Outcomes of Parameter and Methodology Exploration to Inform Future Studies
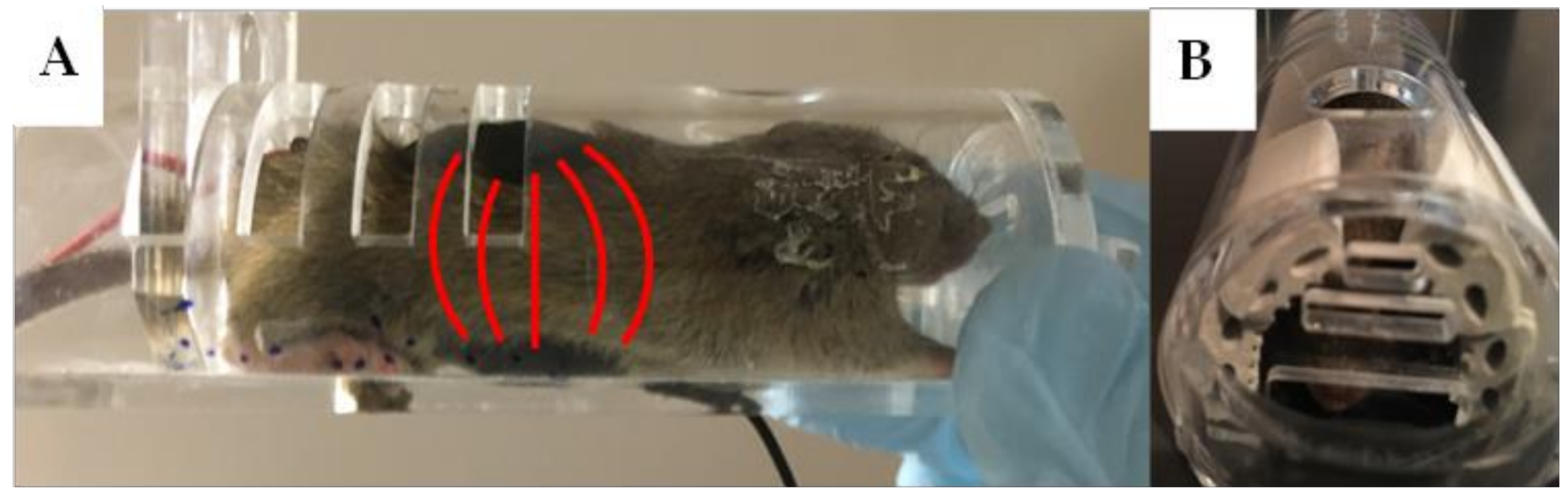
3.2. Method of Restraint
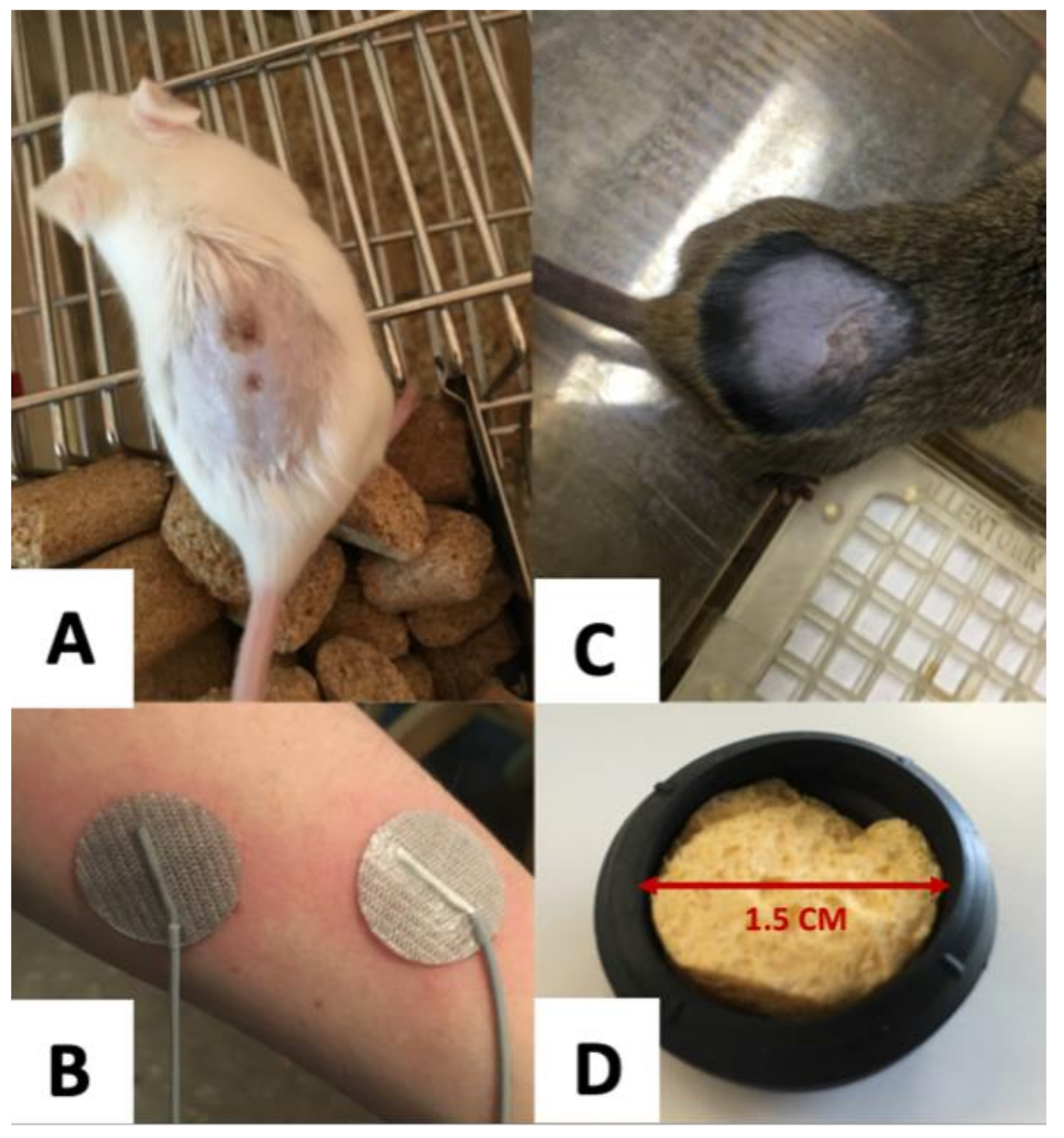
3.3. Electrode Design
3.4. Skin Preparation
3.5. Current Density and Total Charge Delivered
3.6. Effects on Disease Progression: Primary and Secondary Outcomes
3.6.1. Weight
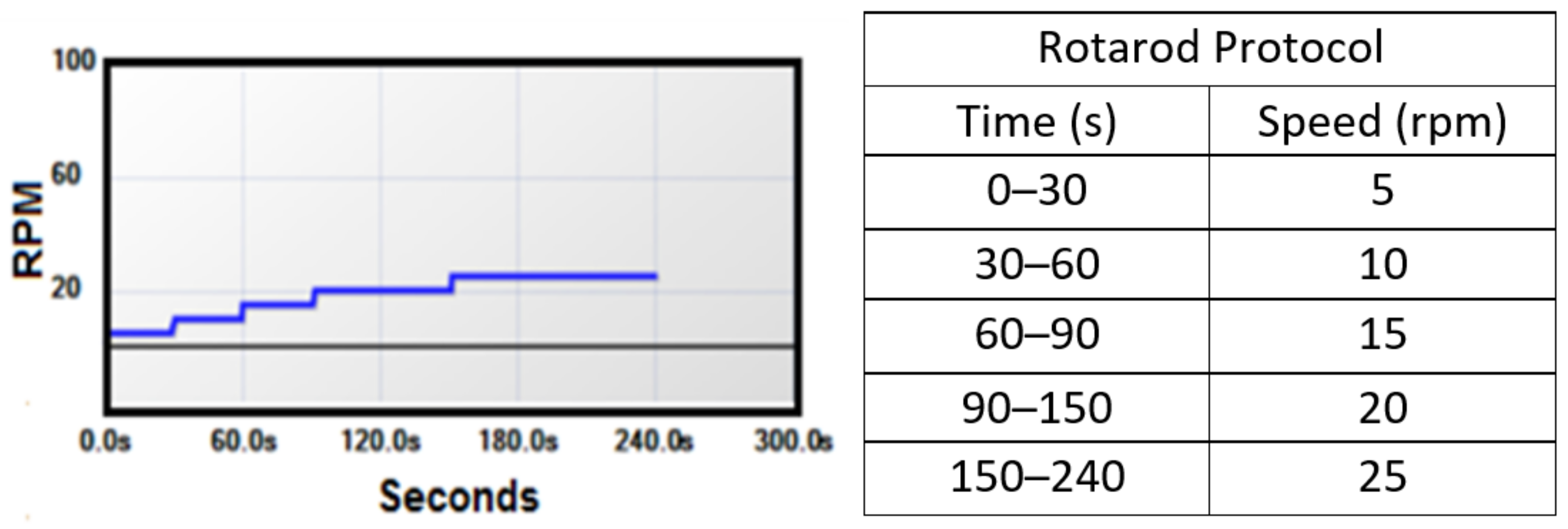
3.6.2. Motor Function
3.6.3. Survival
3.6.4. Correlations
4. Discussion
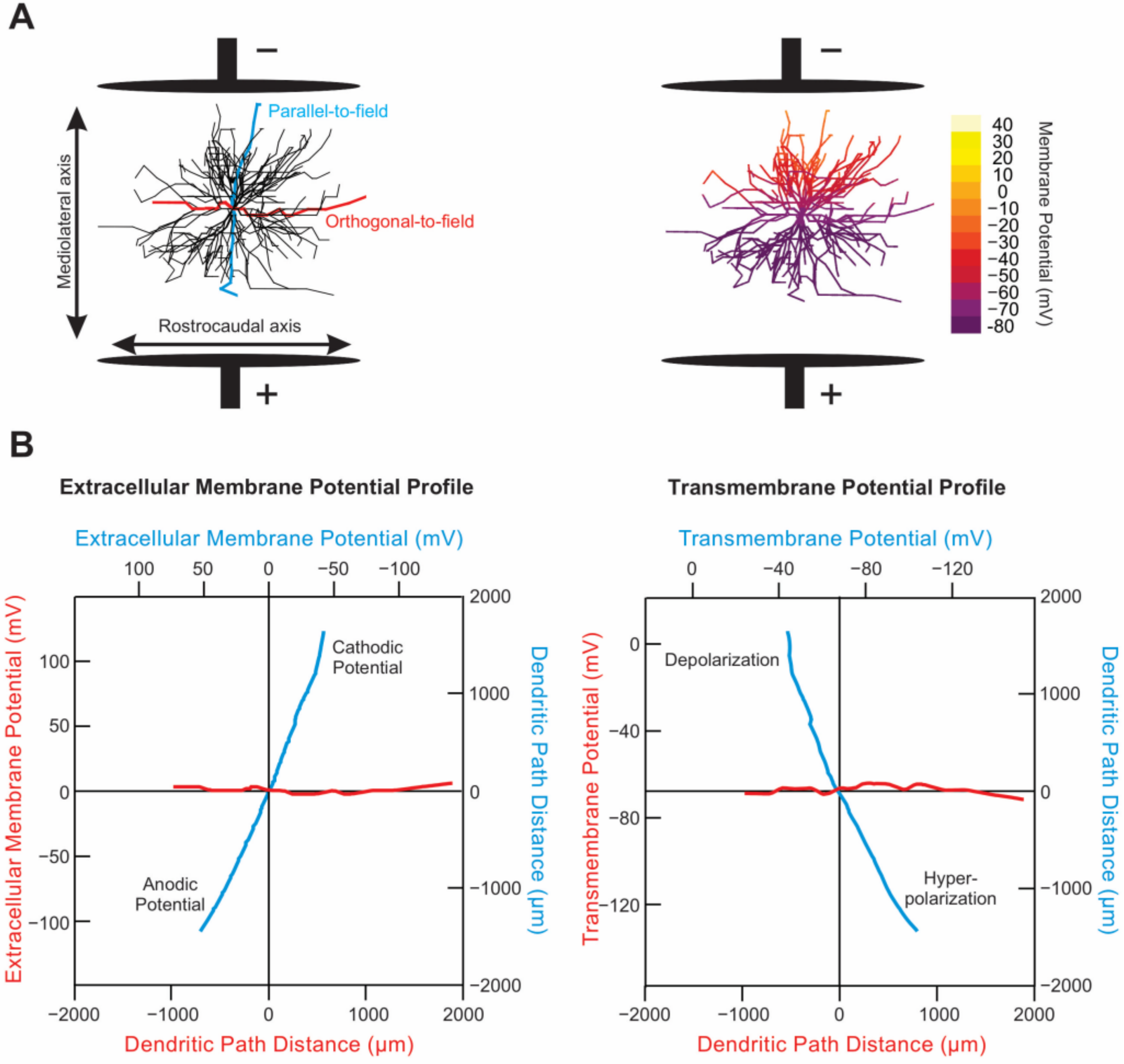
4.1. Physics of tsDCS
4.2. Anodal Stimulation May Be Harmful to Survival
4.3. Weaker Cathodal Effect
4.4. Design Revisions: Secondary Study Outcomes and Clinical Relevance
4.5. Sex Effects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chio, A.; Logroscino, G.; Traynor, B.J.; Collins, J.; Simeone, J.C.; Goldstein, L.A.; White, L.A. Global epidemiology of amyotrophic lateral sclerosis: A systematic review of the published literature. Neuroepidemiology 2013, 41, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Chio, A.; Logroscino, G.; Hardiman, O.; Swingler, R.; Mitchell, D.; Beghi, E.; Traynor, B.G.; Eurals, C. Prognostic factors in ALS: A critical review. Amyotroph. Lateral Scler. 2009, 10, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, K.A.; Schuster, J.E.; Fu, R.; Siddique, T.; Heckman, C.J. Altered postnatal maturation of electrical properties in spinal motoneurons in a mouse model of amyotrophic lateral sclerosis. J. Physiol. 2011, 589, 2245–2260. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Cazenave, W.; Cattaert, D.; Branchereau, P. Embryonic alteration of motoneuronal morphology induces hyperexcitability in the mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2013, 54, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.J.; Schonewille, M.; Siddique, T.; Schults, A.N.A.; Fu, R.; Bar, P.R.; Anelli, R.; Heckman, C.J.; Kroese, A.B.A. Hyperexcitability of Cultured Spinal Motoneurons from Presymptomatic ALS Mice. J. Neurophysiol. 2004, 91, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Fujimaki, Y.; Nakatani-Enomoto, S.; Matsubara, S.; Watabe, K.; Rossini, P.M.; Ugawa, Y. Complex fasciculation potentials and survival in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2014, 125, 1059–1064. [Google Scholar] [CrossRef]
- Bellingham, M.C. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: What have we learned in the last decade? CNS Neurosci. Ther. 2011, 17, 4–31. [Google Scholar] [CrossRef]
- Abe, K.; Itoyama, Y.; Sobue, G.; Tsuji, S.; Aoki, M.; Doyu, M.; Hamada, C.; Kondo, K.; Yoneoka, T.; Akimoto, M.; et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph. Lateral Scler. Frontotemporal. Degener. 2014, 15, 610–617. [Google Scholar] [CrossRef]
- Okada, M.; Yamashita, S.; Ueyama, H.; Ishizaki, M.; Maeda, Y.; Ando, Y. Long-term effects of edaravone on survival of patients with amyotrophic lateral sclerosis. eNeurologicalSci 2018, 11, 11–14. [Google Scholar] [CrossRef]
- Lacomblez, L.; Bensimon, G.; Leigh, P.N.; Guillet, P.; Powe, L.; Durrleman, S.; Delumeau, J.C.; Meininger, V. A confirmatory dose-ranging study of riluzole in ALS. ALS/Riluzole Study Group-II. Neurology 1996, 47, S242–S250. [Google Scholar] [CrossRef]
- Mao, Y.-F.; Yan, N.; Xu, H.; Sun, J.-H.; Xiong, Y.-C.; Deng, X.-M. Edaravone, a free radical scavenger, is effective on neuropathic pain in rats. Brain Res. 2009, 1248, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Aum, D.J.; Tierney, T.S. Deep brain stimulation: Foundations and future trends. Front. Biosci. 2018, 23, 162–182. [Google Scholar]
- Fridriksson, J.; Rorden, C.; Elm, J.; Sen, S.; George, M.S.; Bonilha, L. Transcranial Direct Current Stimulation vs. Sham Stimulation to Treat Aphasia after Stroke: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Hubli, M.; Dietz, V.; Schrafl-Altermatt, M.; Bolliger, M. Modulation of spinal neuronal excitability by spinal direct currents and locomotion after spinal cord injury. Clin. Neurophysiol. 2013, 124, 1187–1195. [Google Scholar] [CrossRef]
- Elbasiouny, S.M.; Mushahwar, V.K. Suppressing the excitability of spinal motoneurons by extracellularly applied electrical fields: Insights from computer simulations. J. Appl. Physiol. 2007, 103, 1824–1836. [Google Scholar] [CrossRef]
- Baczyk, M.; Drzymala-Celichowska, H.; Mrowczynski, W.; Krutki, P. Motoneuron firing properties are modified by trans-spinal direct current stimulation in rats. J. Appl. Physiol. 2019, 126, 1232–1241. [Google Scholar] [CrossRef]
- Baczyk, M.; Drzymala-Celichowska, H.; Mrowczynski, W.; Krutki, P. Long-lasting modifications of motoneuron firing properties by trans-spinal direct current stimulation in rats. Eur. J. Neurosci. 2020, 51, 1743–1755. [Google Scholar] [CrossRef]
- Baczyk, M.; Drzymala-Celichowska, H.; Mrowczynski, W.; Krutki, P. Polarity-dependent adaptations of motoneuron electrophysiological properties after 5-wk transcutaneous spinal direct current stimulation in rats. J. Appl. Physiol. 2020, 129, 646–655. [Google Scholar] [CrossRef]
- Baczyk, M.; Krutki, P. In Vivo Intracellular Recording of Type-Identified Rat Spinal Motoneurons during Trans-Spinal Direct Current Stimulation. J. Vis. Exp. 2020, 159, e61439. [Google Scholar] [CrossRef]
- Baczyk, M.; Krutki, P.; Zytnicki, D. Is there hope that transpinal direct current stimulation corrects motoneuron excitability and provides neuroprotection in amyotrophic lateral sclerosis? Physiol. Rep. 2021, 9, e14706. [Google Scholar] [CrossRef]
- Jankowiak, T.; Cholewiński, M.; Bączyk, M. Differential effects of invasive anodal trans-spinal direct current stimulation on monosynaptic EPSPs, Ia afferents excitability, and motoneuron intrinsic properties between SOD1 G93A and WT mice. Neuroscience 2022, in press. [Google Scholar]
- Alves, C.J.; de Santana, L.P.; dos Santos, A.J.D.; de Oliveira, G.P.; Duobles, T.; Scorisa, J.M.; Martins, R.S.; Maximino, J.R.; Chadi, G. Early motor and electrophysiological changes in transgenic mouse model of amyotrophic lateral sclerosis and gender differences on clinical outcome. Brain Res. 2011, 1394, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.; Pulecchi, F.; Dilena, R.; Oliviero, A.; Priori, A.; Foffani, G. Spinal direct current stimulation modulates the activity of gracile nucleus and primary somatosensory cortex in anaesthetized rats. J. Physiol. 2011, 589, 4981–4996. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z. Trans-spinal direct current stimulation modulates motor cortex-induced muscle contraction in mice. J. Appl. Physiol. 2011, 110, 1414–1424. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Nitsche, M.S.; Klein, C.C.; Tergau, F.; Rothwell, J.C.; Paulus, W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin. Neurophysiol. 2003, 114, 600–604. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Seeber, A.; Frommann, K.; Klein, C.C.; Rochford, C.; Nitsche, M.S.; Fricke, K.; Liebetanz, D.; Lang, N.; Antal, A.; et al. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J. Physiol. 2005, 568, 291–303. [Google Scholar] [CrossRef]
- Lang, N.; Nitsche, M.A.; Paulus, W.; Rothwell, J.C.; Lemon, R.N. Effects of transcranial direct current stimulation over the human motor cortex on corticospinal and transcallosal excitability. Exp. Brain Res. 2004, 156, 439–443. [Google Scholar] [CrossRef]
- Ardolino, G.; Bossi, B.; Barbieri, S.; Priori, A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J. Physiol. 2005, 568, 653–663. [Google Scholar] [CrossRef]
- Quartarone, A.; Lang, N.; Rizzo, V.; Bagnato, S.; Morgante, F.; Sant’angelo, A.; Crupi, D.; Battaglia, F.; Messina, C.; Girlanda, P. Motor cortex abnormalities in amyotrophic lateral sclerosis with transcranial direct-current stimulation. Muscle Nerve 2007, 35, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Monte-Silva, K.; Kuo, M.F.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS). J. Neurophysiol. 2010, 103, 1735–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Lazzaro, V.; Manganelli, F.; Dileone, M.; Notturno, F.; Esposito, M.; Capasso, M.; Dubbioso, R.; Pace, M.; Ranieri, F.; Minicuci, G.; et al. The effects of prolonged cathodal direct current stimulation on the excitatory and inhibitory circuits of the ipsilateral and contralateral motor cortex. J. Neural Transm. 2012, 119, 1499–1506. [Google Scholar] [CrossRef]
- Jankowska, E.; Kaczmarek, D.; Bolzoni, F.; Hammar, I. Long-lasting increase in axonal excitability after epidurally applied DC. J. Neurophysiol. 2017, 118, 1210–1220. [Google Scholar] [CrossRef]
- Jensen, D.B.; Kadlecova, M.; Allodi, I.; Meehan, C.F. Spinal motoneurones are intrinsically more responsive in the adult G93A SOD1 mouse model of amyotrophic lateral sclerosis. J. Physiol. 2020, 598, 4385–4403. [Google Scholar] [CrossRef]
- Eccles, J.C.; Kostyuk, P.G.; Schmidt, R.F. The effect of electric polarization of the spinal cord on central afferent fibres and on their excitatory synaptic action. J. Physiol. 1962, 162, 138–150. [Google Scholar] [CrossRef]
- Chan, C.Y.; Hounsgaard, J.; Nicholson, C. Effects of electric fields on transmembrane potential and excitability of turtle cerebellar Purkinje cells in vitro. J. Physiol. 1988, 402, 751–771. [Google Scholar] [CrossRef] [PubMed]
- Hounsgaard, J.; Kiehn, O. Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurones in vitro. J. Physiol. 1993, 468, 245–259. [Google Scholar] [CrossRef]
- Bikson, M.; Inoue, M.; Akiyama, H.; Deans, J.K.; Fox, J.E.; Miyakawa, H.; Jefferys, J.G. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J. Physiol. 2004, 557, 175–190. [Google Scholar] [CrossRef]
- Radman, T.; Ramos, R.L.; Brumberg, J.C.; Bikson, M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul. 2009, 2, 215–228. [Google Scholar] [CrossRef]
- Andreasen, M.; Nedergaard, S. Dendritic electrogenesis in rat hippocampal CA1 pyramidal neurons: Functional aspects of Na+ and Ca2+ currents in apical dendrites. Hippocampus 1996, 6, 79–95. [Google Scholar] [CrossRef]
- Bikson, M.; Reato, D.; Rahman, A. Cellular and Network Effects of Transcranial Direct Current Stimulation. In Transcranial Brain Stimulation; CRC Press: Boca Raton, FL, USA, 2012; pp. 55–92. [Google Scholar]
- Dukkipati, S.S.; Garrett, T.L.; Elbasiouny, S.M. The vulnerability of spinal motoneurons and soma size plasticity in a mouse model of amyotrophic lateral sclerosis. J. Physiol. 2018, 596, 1723–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, S.; Heckman, C.J.; Manuel, M. Time Course of Alterations in Adult Spinal Motoneuron Properties in the SOD1(G93A) Mouse Model of ALS. eNeuro 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Lamy, J.C.; Ho, C.; Badel, A.; Arrigo, R.T.; Boakye, M. Modulation of soleus H reflex by spinal DC stimulation in humans. J. Neurophysiol. 2012, 108, 906–914. [Google Scholar] [CrossRef]
- Tomofumi, Y.; Fujimoto, S.; Otaka, Y.; Tanaka, S. Effects of transcutaneous spinal dc stimulation on plasticity of the spinal circuits and corticospinal tracts in humans. Neural Eng. 2013, 275–278. [Google Scholar] [CrossRef]
- Ahmed, Z. Effects of Cathodal Trans-Spinal Direct Current Stimulation on Mouse Spinal Network and Complex Multijoint Movements. J. Neurosci. 2013, 33, 14949–14957. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z. Trans-Spinal Direct Current Stimulation Alters Muscle Tone in Mice with and without Spinal Cord Injury with Spasticity. J. Neurosci. 2014, 34, 1701–1709. [Google Scholar] [CrossRef]
- Paget-Blanc, A.; Chang, J.L.; Saul, M.; Lin, R.; Ahmed, Z.; Volpe, B.T. Non-invasive treatment of patients with upper extremity spasticity following stroke using paired trans-spinal and peripheral direct current stimulation. Bioelectron. Med. 2019, 5, 11. [Google Scholar] [CrossRef]
- Fernandes, S.R.; Pereira, M.; Elbasiouny, S.M.; Dhaher, Y.Y.; de Carvalho, M.; Miranda, P.C. Modelling Studies of Non-invasive Electric and Magnetic Stimulation of the Spinal Cord. In Brain and Human Body Modeling 2021; Makarov, S.N., Noetscher, G.M., Nummenmaa, A., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Stoney, S.D.; Thompson, W.D.; Asanuma, H. Excitation of pyramidal tract cells by intracortical microstimulation: Effective extent of stimulating current. J. Neurophysiol. 1968, 31, 659–669. [Google Scholar] [CrossRef]
- Tsytsarev, V.; Premachandra, K.; Takeshita, D.; Bahar, S. Imaging cortical electrical stimulation in vivo: Fast intrinsic optical signal versus voltage-sensitive dyes. Opt. Lett. 2008, 33, 1032–1034. [Google Scholar] [CrossRef]
- McCombe, P.A.; Henderson, R.D. Effects of gender in amyotrophic lateral sclerosis. Gend. Med. 2010, 7, 557–570. [Google Scholar] [CrossRef] [PubMed]





| Author | Technique | Density (A/m2) | Stimulation Duration (min) | Total Charge Delivered (C/cm2) | Effect Duration (min) |
|---|---|---|---|---|---|
| Nitsche 2000 [27] | tDCS | 0.29 | 5 | 0.0087 | 5 |
| Nitsche 2003 [26] | tDCS | 0.29 | 9 | 0.01566 | 60 |
| Lang 2004 [29] | tDCS | 0.29 | 10 | 0.0174 | 40 |
| Ardolino 2005 [30] | tDCS | 0.43 | 10 | 0.0258 | 60 |
| Nitsche 2005 [28] | tDCS | 0.29 | 9–13 | 0.02262 | 40 |
| Quartarone 2007 [31] | tDCS | 0.29 | 7 | 0.01218 | 10 |
| Monte 2010 [32] | tDCS | 0.29 | 9 | 0.01566 | 60 |
| Monte 2010 [32] | tDCS | 0.29 | 18 | 0.03132 | 90 |
| Aguilar 2011 [24] | sDCS (implanted) | 12.7 | 15 | 1.15 | N/A |
| Ahmed 2011 [25] | tsDCS | 0.64–38.2 | 3 | 0.6876 | 20 |
| Lazzaro 2012 [33] | tDCS | 0.29 | 20 | 0.0348 | 180 |
| Jankowska 2017 [34] | Epidural sDC | 10 | 60 |
| Treatment Group | Final Completion | Final Run | Survival | |||
|---|---|---|---|---|---|---|
| Sham | 102.33 ± 6.60 | (n = 12) | 118.31 ± 9.09 | (n = 13) | 127.62 ± 11.11 | (n = 13) |
| Anodal | 102.31 ± 8.94 | (n = 13) | 117.38 ± 7.27 | (n = 13) | 123.50 ± 4.87 | (n = 12) |
| Cathodal | 104.18 ± 5.96 | (n = 11) | 118.77 ± 7.24 | (n = 13) | 127.77 ± 9.44 | (n = 13) |
| Group | Correlation Coefficient (r) | p-Value Halt: r ≠ 0 |
|---|---|---|
| Motor Decline and Survival | ||
| S | 0.6328 | 0.0203 |
| A | −0.1923 | 0.5290 |
| C | 0.7631 | 0.0024 |
| Baseline Weight and Survival | ||
| S | −0.2035 | 0.5050 |
| A | 0.3094 | 0.3037 |
| C | 0.1448 | 0.6370 |
| Baseline Weight and Motor Function Decline | ||
| S | −0.1392 | 0.6501 |
| A | −0.4629 | 0.1112 |
| C | 0.3559 | 0.2327 |
| Weight Loss Rate and Survival | ||
| S | 0.3914 | 0.1860 |
| A | 0.3912 | 0.1862 |
| C | 0.5326 | 0.0609 |
| Weight Loss Rate and Motor Function Decline | ||
| S | 0.5005 | 0.0815 |
| A | −0.4040 | 0.1709 |
| C | 0.3408 | 0.2544 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Highlander, M.M.; Elbasiouny, S.M. Non-Invasive Transcutaneous Spinal DC Stimulation as a Neurorehabilitation ALS Therapy in Awake G93A Mice: The First Step to Clinical Translation. Bioengineering 2022, 9, 441. https://doi.org/10.3390/bioengineering9090441
Highlander MM, Elbasiouny SM. Non-Invasive Transcutaneous Spinal DC Stimulation as a Neurorehabilitation ALS Therapy in Awake G93A Mice: The First Step to Clinical Translation. Bioengineering. 2022; 9(9):441. https://doi.org/10.3390/bioengineering9090441
Chicago/Turabian StyleHighlander, Morgan M., and Sherif M. Elbasiouny. 2022. "Non-Invasive Transcutaneous Spinal DC Stimulation as a Neurorehabilitation ALS Therapy in Awake G93A Mice: The First Step to Clinical Translation" Bioengineering 9, no. 9: 441. https://doi.org/10.3390/bioengineering9090441