CT-Derived 3D Printing for Coronary Artery Cannulation Simulator Design Manufacturing
Abstract
1. Introduction
- Students are able to learn the coronary artery cannulation with four access points, while seeing and feeling the real physical anatomy of the organ.
- The 3D printing technology gives an advanced and cost-effective medical simulator with simple, readily-available materials for manufacturing.
- The selection of 3D printing materials for the simulator production resembles the proposed organ and can be adjusted for the procedure learning purposes, providing a clear and flexible 3D printout.
2. Materials and Methods
2.1. Three-Dimensional Prototype Design
2.2. Three-Dimensional Design and Prototype Validation
2.3. Analysis
2.4. Ethical Issues
3. Results
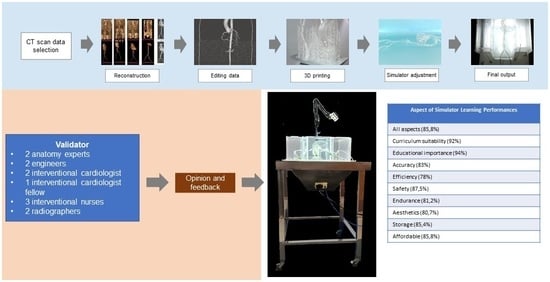
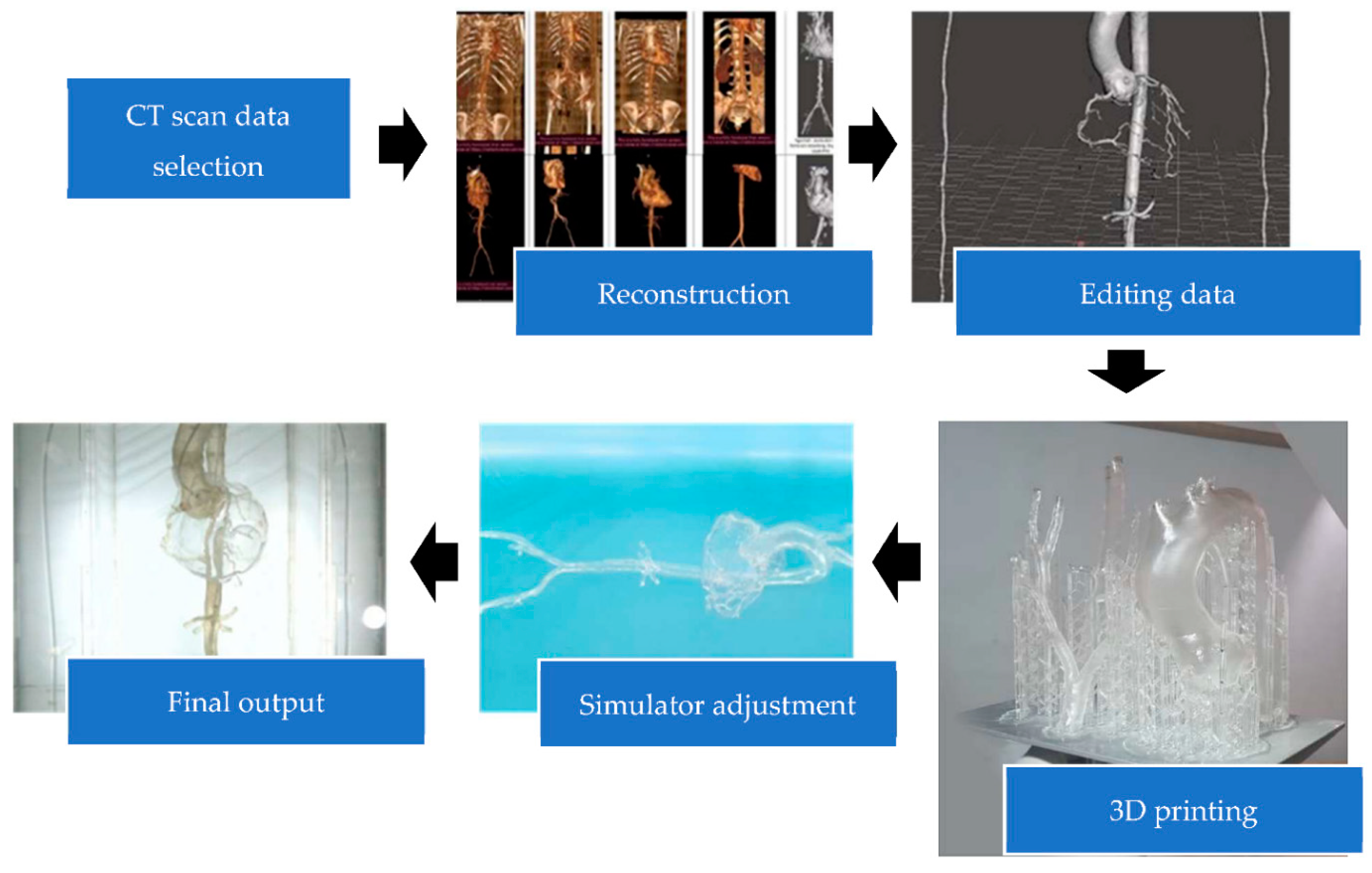
3.1. Simulator Model Making
3.2. Design Validation
3.3. Design Printing
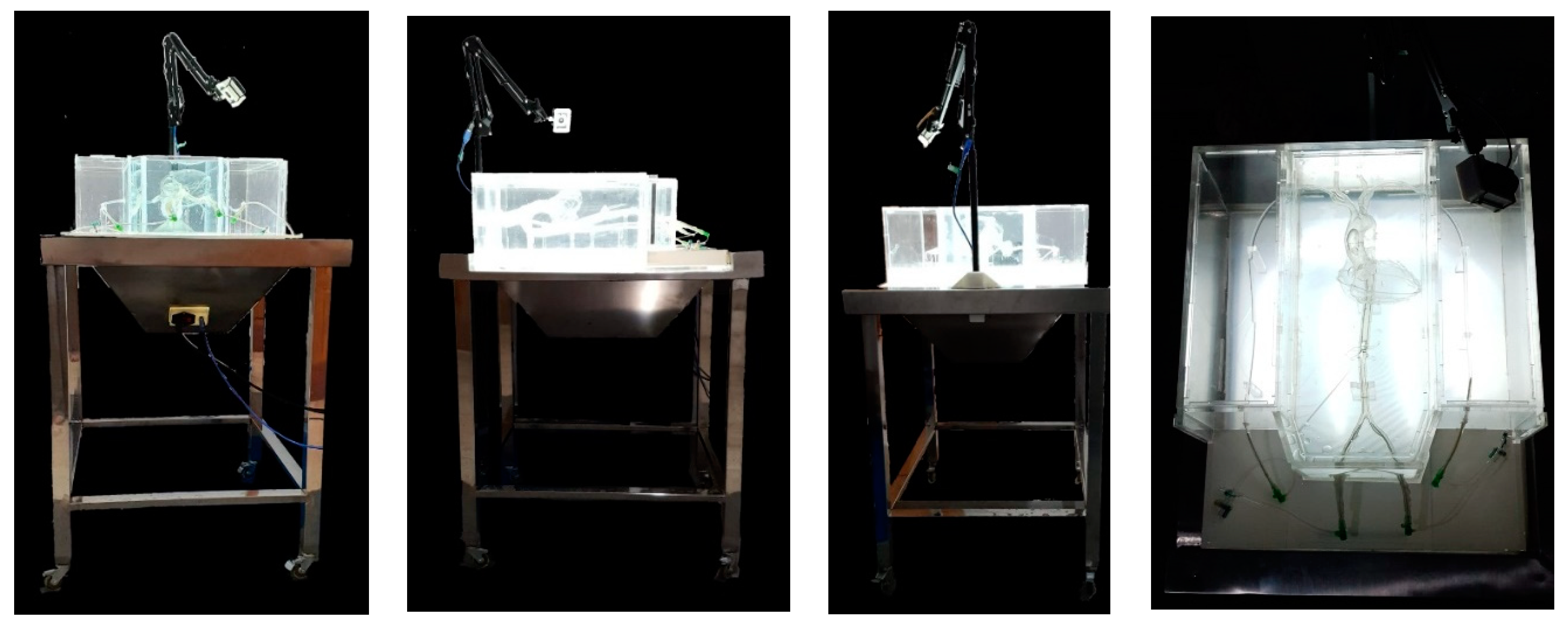
3.4. Simulator Forming
3.5. Prototype Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smoljkic, G.; Gruijthuijsen, C.; Sloten, J.V.; Poorten, E.V. Towards Intraoperative Use of Surgical Simulators: Evaluation of Catheter Insertion Models. In Proceedings of the 3rd Joint Workshop on New Technologies for Computer/Robot Assisted Surgery (CRAS), Verona, Italy, 11–13 September 2013. [Google Scholar]
- Chaer, R.A.; DeRubertis, B.G.; Lin, S.C.; Bush, H.L.; Karwowski, J.K.; Birk, D.; Morrissey, N.J.; Faries, P.L.; McKinsey, J.F.; Kent, K.C. Simulation Improves Resident Performance in Catheter-Based Intervention: Results of a randomized, controlled study. Ann. Surg. 2006, 244, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Sharei, H.; Alderliesten, T.; van den Dobbelsteen, J.J.; Dankelman, J. Navigation of guidewires and catheters in the body during intervention procedures: A review of computer-based models. J. Med. Imaging 2018, 5, 010902. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, P.M.; Aggarwal, S.; Choudhury, E.; Ladha, S.; Kiran, U. Simulation in cardiac catheterization laboratory: Need of the hour to improve the clinical skills. Ann. Card. Anaesth. 2016, 19, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Green, S.M.; Klein, A.J.; Pancholy, S.; Rao, S.V.; Steinberg, D.; Lipner, R.; Marshall, J.; Messenger, J.C. The current state of medical simulation in interventional cardiology: A clinical document from the Society for Cardiovascular Angiography and Intervention’s (SCAI) Simulation Committee. Catheter. Cardiovasc. Interv. 2013, 83, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Dayal, R.; Faries, P.L.; Lin, S.C.; Bernheim, J.; Hollenbeck, S.; DeRubertis, B.; Trocciola, S.; Rhee, J.; McKinsey, J.; Morrissey, N.J.; et al. Computer simulation as a component of catheter-based training. J. Vasc. Surg. 2004, 40, 1112–1117. [Google Scholar] [CrossRef]
- Tejo-Otero, A.; Buj-Corral, I.; Fenollosa-Artés, F. 3D Printing in Medicine for Preoperative Surgical Planning: A Review. Ann. Biomed. Eng. 2019, 48, 536–555. [Google Scholar] [CrossRef]
- Schmauss, D.; Juchem, G.; Weber, S.; Gerber, N.; Hagl, C.; Sodian, R. Three-Dimensional Printing for Perioperative Planning of Complex Aortic Arch Surgery. Ann. Thorac. Surg. 2014, 97, 2160–2163. [Google Scholar] [CrossRef]
- Luo, H.; Meyer-Szary, J.; Wang, Z.; Sabiniewicz, R.; Liu, Y. Three-dimensional printing in cardiology: Current applications and future challenges. Cardiol. J. 2017, 24, 436–444. [Google Scholar] [CrossRef]
- Pleșoianu, F.A.; Pleșoianu, C.E.; Țăruș, A.; Tinică, G. Minimally Invasive, Fully Implantable Left Ventricular Assist Device: Concept, Design, and Early Prototyping. IFMBE Proc. 2022, 87, 200–207. [Google Scholar] [CrossRef]
- Ryan, T.J.; Bauman, W.B.; Kennedy, W.; Kereiaakes, D.J.; Singh, S.B.; McCallister, B.D.; Smith, S.C.; Ullyot, D.J. AHA Medical/Scientific Statement Clinical Competence in Percutaneous Transluminal Coronary Angioplasty. Circulation 1990, 81, 2041–2046. [Google Scholar]
- Duriez, C.; Cotin, S.; Lenoir, J.; Neumann, P. New approaches to catheter navigation for interventional radiology simulation. Comput. Aided Surg. 2006, 11, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Mussi, E.; Mussa, F.; Santarelli, C.; Scagnet, M.; Uccheddu, F.; Furferi, R.; Volpe, Y.; Genitori, L. Current Practice in Preoperative Virtual and Physical Simulation in Neurosurgery. Bioengineering 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Yang, Z.; Mongrain, R.; Leask, R.L.; Lachapelle, K. 3D printing materials and their use in medical education: A review of current technology and trends for the future. BMJ Simul. Technol. Enhanc. Learn. 2017, 4, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Brydges, R.; Carnahan, H.; Rose, D.; Rose, L.; Dubrowski, A. Coordinating Progressive Levels of Simulation Fidelity to Maximize Educational Benefit. Acad. Med. 2010, 85, 806–812. [Google Scholar] [CrossRef]
- Mafeld, S.; Nesbitt, C.; McCaslin, J.; Bagnall, A.; Davey, P.; Bose, P.; Williams, R. Three-dimensional (3D) printed endovascular simulation models: A feasibility study. Ann. Transl. Med. 2017, 5, 42. [Google Scholar] [CrossRef]
- Cappello, I.A.; Candelari, M.; Pannone, L.; Monaco, C.; Bori, E.; Talevi, G.; Ramak, R.; La Meir, M.; Gharaviri, A.; Chierchia, G.B.; et al. 3D Printed Surgical Guide for Coronary Artery Bypass Graft: Workflow from Computed Tomography to Prototype. Bioengineering 2022, 9, 179. [Google Scholar] [CrossRef]
- Huang, J.; Nie, X. A simple and novel method to design flexible and transparent epoxy resin with tunable mechanical properties. Polym. Int. 2016, 65, 835–840. [Google Scholar] [CrossRef]
- Valverde, I. Three-dimensional Printed Cardiac Models: Applications in the Field of Medical Education, Cardiovascular Surgery, and Structural Heart Interventions. Rev. Española Cardiol. 2017, 70, 282–291. [Google Scholar] [CrossRef]
- Sugiyono, D. Metode Penelitian Pendidikan: Pendekatan Kuantitatif, Kualitatif, dan R & D; Alfabeta: Bandung, Indonesia, 2013; pp. 407–445. [Google Scholar]
- Arikunto, S. Prosedur Penelitian Suatu Pendekatan Praktik; Rineka Cipta: Jakarta, Indonesia, 2014; pp. 53–55. [Google Scholar]
- Kern, M.J.; Skelding, K.A. Arterial and Venous Access. In The Cardiac Catheterization Handbook, 5th ed.; Kern, M.J., Andjelkovic, N., Mcllwain, B., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2011; Chapter 2; pp. 37–90. [Google Scholar]
- Shaaruddin, J.; Mohamad, M. Identifying the Effectiveness of Active Learning Strategies and Benefits in Curriculum and Pedagogy Course for Undergraduate TESL Students. Creat. Educ. 2017, 8, 2312–2324. [Google Scholar] [CrossRef][Green Version]
- Bagai, A.; O’Brien, S.; Al Lawati, H.; Goyal, P.; Ball, W.; Grantcharov, T.; Fam, N. Mentored Simulation Training Improves Procedural Skills in Cardiac Catheterization. Circ. Cardiovasc. Interv. 2012, 5, 672–679. [Google Scholar] [CrossRef]
- Kurenov, S.N.; Ionita, C.; Sammons, D.; Demmy, T.L. Three-dimensional printing to facilitate anatomic study, device development, simulation, and planning in thoracic surgery. J. Thorac. Cardiovasc. Surg. 2015, 149, 973–979.e1. [Google Scholar] [CrossRef]
- Caluk, J. Procedural Techniques of Coronary Angiography, Advances in the Diagnosis of Coronary Atherosclerosis; Kirac, S.F., Bakic, S., Smiljanic, T., Eds.; IntechOpen: Rijeka, Croatia, 2011; Volume 6, pp. 95–122. [Google Scholar] [CrossRef]
- Chen, S.Y.J.; Carroll, J.D. Coronary Angiography, Practical Signal and Image Processing in Clinical Cardiology; Goldberger, J.J., Ng, J., Eds.; Springer: White County, IL, USA, 2010; Volume 13, pp. 157–185. [Google Scholar] [CrossRef]
- Vukicevic, M.; Puperi, D.S.; Grande-Allen, K.J.; Little, S.H. 3D Printed Modeling of the Mitral Valve for Catheter-Based Structural Interventions. Ann. Biomed. Eng. 2016, 45, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Lölsberg, J. Rapid Prototyping of Microfluidic Systems Using Two Photon Lithography. Student Report, Third/Fourth Semester. Master’s Thesis, Aalborg University, Aalborg, Denmark, 3 June 2020; pp. 1–27. [Google Scholar]
- Rosu, D.; Rosu, L.; Cascaval, C.N. IR-change and yellowing of polyurethane as a result of UV irradiation. Polym. Degrad. Stab. 2009, 94, 591–596. [Google Scholar] [CrossRef]
- Understanding Ultraviolet LED Wavelength. Available online: https://phoseon.com/wp-content/uploads/2019/08/Understanding-Ultraviolet-LED-Wavelenght_UVEB-Technology_June-2016.pdf (accessed on 1 July 2022).
- Nikafshar, S.; Zabihi, O.; Ahmadi, M.; Mirmohseni, A.; Taseidifar, M.; Naebe, M. The Effects of UV Light on the Chemical and Mechanical Properties of a Transparent Epoxy-Diamine System in the Presence of an Organic UV Absorber. Materials 2017, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Alshehri, S.Z.; Alhamid, S.A.; Albarrak, A.; Khan, S.Q.; Alshahrani, F.A.; Alqarawi, F.K. Water Sorption, Solubility, and Translucency of 3D-Printed Denture Base Resins. Dent. J. 2022, 10, 42. [Google Scholar] [CrossRef]
- Liu, C.Z.; Sun, M.M.; Jian, J.X.; Xue, G.; Song, C.Y.; Li, J.H.; Zhang, X.G.; Wang, L.; Zhao, M.; Zhang, B. Preparation of Flexible Epoxy Resin Containing Polyurethane Segments. In Proceedings of the International Workshop on Materials, Chemistry and Engineering (No. Iwmce 2018), Xiamen, China, 16–18 June 2018; pp. 565–570. [Google Scholar] [CrossRef]
- King, S.B. The Development of Interventional Cardiology. J. Am. Coll. Cardiol. 1998, 31, 64B–88B. [Google Scholar] [CrossRef][Green Version]
- Zahr, F.; Cigarroa, J.E. Introduction of Fluoroscopy and Radiation Safety. In Transcatheter Heart Valve Handbook: A Sur-geons and Interventional Council Review; Iribarne, A., Schmidt, A.C.S., Nguyen, T.C., Eds.; American College of Cardiology: Washington, DC, USA, 2010; Chapter 3; Available online: https://www.acc.org//-/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/Membership/TAVR-Handbook/Chapter-3-Fluoroscopy-and-contrast-angles-doses-and-radiation-safety-March-2-2018.pdf (accessed on 28 May 2021).
- Prabhala, V.A.; Baddipadiga, B.P.; Fajri, P.; Ferdowsi, M. An Overview of Direct Current Distribution System Architectures & Benefits. Energies 2018, 11, 2463. [Google Scholar] [CrossRef]
- Fish, R.; Geddes, L. Educación Popular en la elaboración de materiales para capacitación en TICs para el desarrollo social. J. Plast. Surg. 2009, 9, 407–421. [Google Scholar]
- Krishnasamy, S.; Mokhtar, R.A.R.; Singh, R.; Sivallingam, S.; Aziz, Y.F.A.; Mathaneswaran, V. 3D Rapid Prototyping Heart Model Validation for Teaching and Training—A Pilot Project in a Teaching Institution. Braz. J. Cardiovasc. Surg. 2021, 36, 707–716. [Google Scholar] [CrossRef]
- Dankowski, R.; Baszko, A.; Sutherland, M.; Firek, L.; Kałmucki, P.; Wróblewska, K.; Szyszka, A.; Groothuis, A.; Siminiak, T. 3D heart model printing for preparation of percutaneous structural interventions: Description of the technology and case report. Kardiol. Pol. 2014, 72, 546–551. [Google Scholar] [CrossRef]
- Goudie, C.; Kinnin, J.; Bartellas, M.; Gullipalli, R.; Dubrowski, A. The Use of 3D Printed Vasculature for Simulation-based Medical Education Within Interventional Radiology. Cureus 2019, 11, e4381. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-H.; Jo, G.; Koo, J.-H.; Woo, S.-Y.; Kim, J.U.; Kim, Y.-M. A compact pulsatile simulator based on cam-follower mechanism for generating radial pulse waveforms. Biomed. Eng. Online 2019, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Abuouf, Y.; AlBadawi, M.; Ookawara, S.; Ahmed, M. Effect of guidewire insertion in fractional flow reserve procedure for real geometry using computational fluid dynamics. Biomed. Eng. Online 2021, 20, 95. [Google Scholar] [CrossRef] [PubMed]
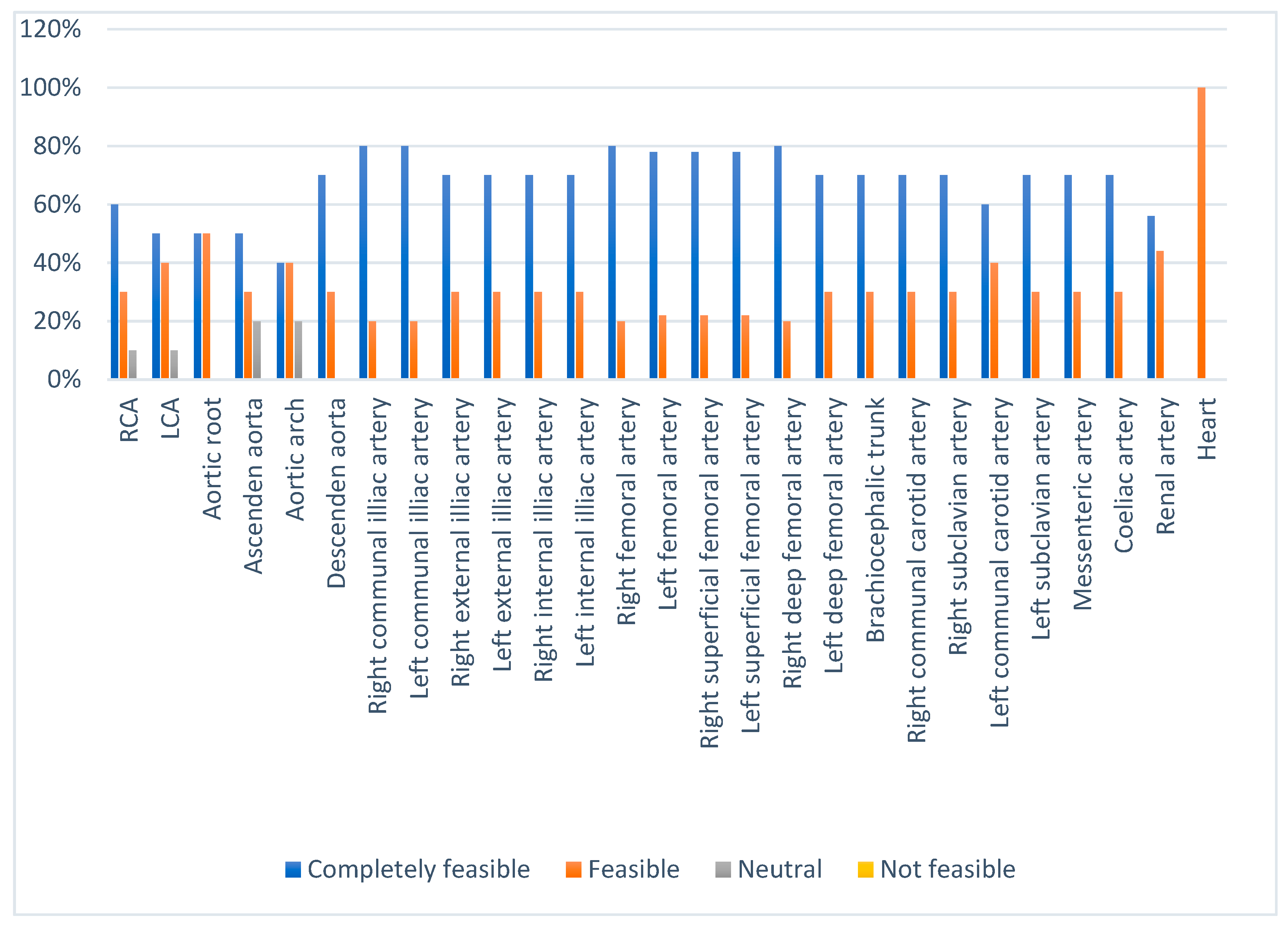

| Score | Category |
|---|---|
| <40% | Not feasible |
| 40–55% | Neutral |
| 56–75% | Feasible |
| 76–100% | Completely feasible |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etami, H.V.; Rismawanti, R.I.; Hanifah, V.A.N.; Herianto, H.; Yanuar, Y.; Kuswanto, D.; Anggrahini, D.W.; Gharini, P.P.R. CT-Derived 3D Printing for Coronary Artery Cannulation Simulator Design Manufacturing. Bioengineering 2022, 9, 338. https://doi.org/10.3390/bioengineering9080338
Etami HV, Rismawanti RI, Hanifah VAN, Herianto H, Yanuar Y, Kuswanto D, Anggrahini DW, Gharini PPR. CT-Derived 3D Printing for Coronary Artery Cannulation Simulator Design Manufacturing. Bioengineering. 2022; 9(8):338. https://doi.org/10.3390/bioengineering9080338
Chicago/Turabian StyleEtami, Helvina Vika, Rochmi Isnaini Rismawanti, Vita Arfiana Nur Hanifah, Herianto Herianto, Yarabisa Yanuar, Djoko Kuswanto, Dyah Wulan Anggrahini, and Putrika Prastuti Ratna Gharini. 2022. "CT-Derived 3D Printing for Coronary Artery Cannulation Simulator Design Manufacturing" Bioengineering 9, no. 8: 338. https://doi.org/10.3390/bioengineering9080338
APA StyleEtami, H. V., Rismawanti, R. I., Hanifah, V. A. N., Herianto, H., Yanuar, Y., Kuswanto, D., Anggrahini, D. W., & Gharini, P. P. R. (2022). CT-Derived 3D Printing for Coronary Artery Cannulation Simulator Design Manufacturing. Bioengineering, 9(8), 338. https://doi.org/10.3390/bioengineering9080338