The Effect of Mesenchymal Stem Cells, Adipose Tissue Derived Stem Cells, and Cellular Stromal Vascular Fraction on the Repair of Acute Anal Sphincter Injury in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of MSC, ADSC, and SVF
2.2. Characterization of MSC and ADSC
2.3. GFP Labeling of MSC, ADSC and Dil Labeling of CSVF
2.4. Establishment of ASI Rat Model
2.5. Functional Measurements
2.6. Immunohistochemical Staining
2.7. Data Analysis
3. Results
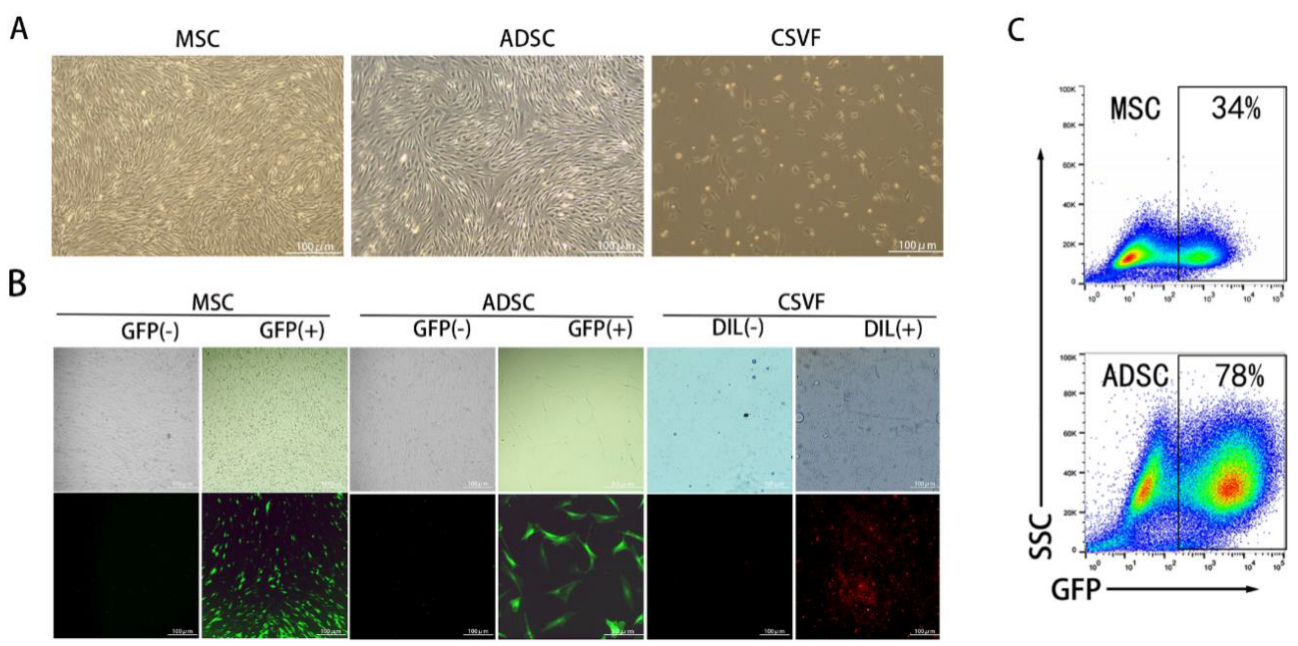
3.1. Characterization of MSC and ADSC
3.1.1. Identification of MSC and ADSC Differentiation
3.1.2. Fluorescent Labeling of ADSC, MSC, and CSVF
3.2. Functional Analysis of the Anal Sphincter
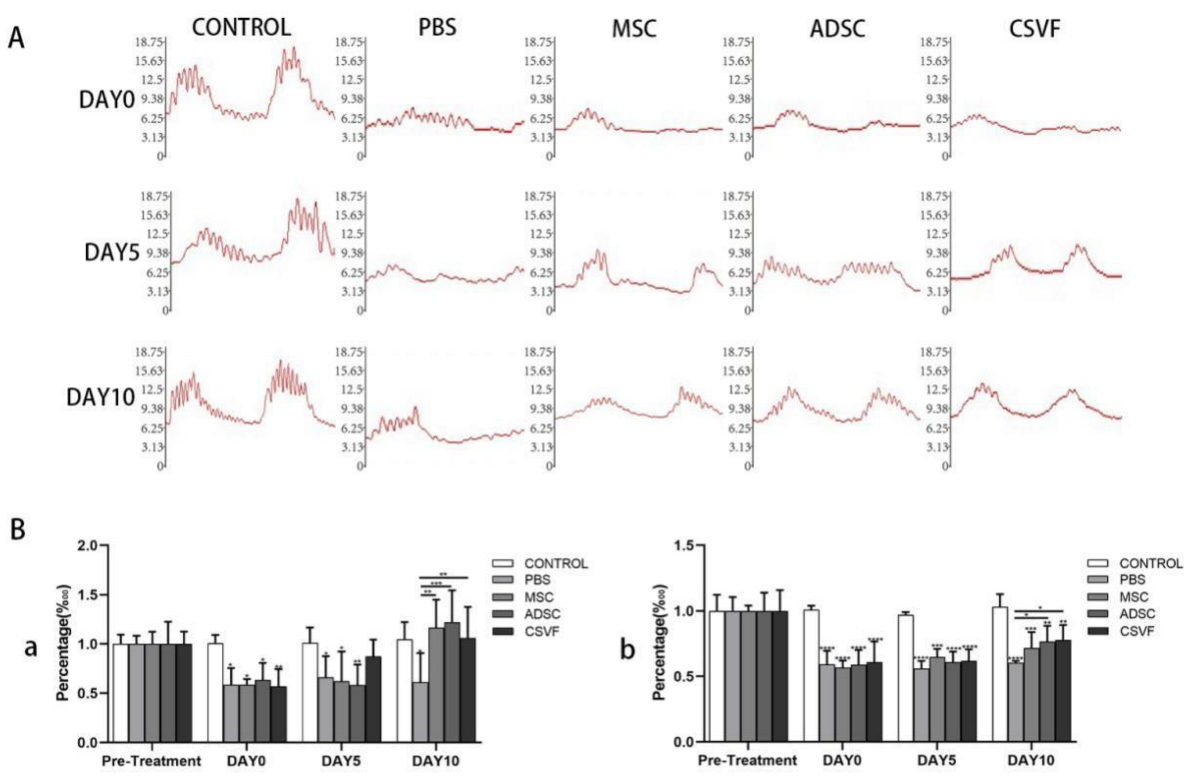
3.2.1. Anal Sphincter Pressure Analysis
Anal Pressure Basal Values
Peak anal Pressure
3.2.2. EMG Analysis of the Anal Sphincter
Peak EMG
EMG Frequency
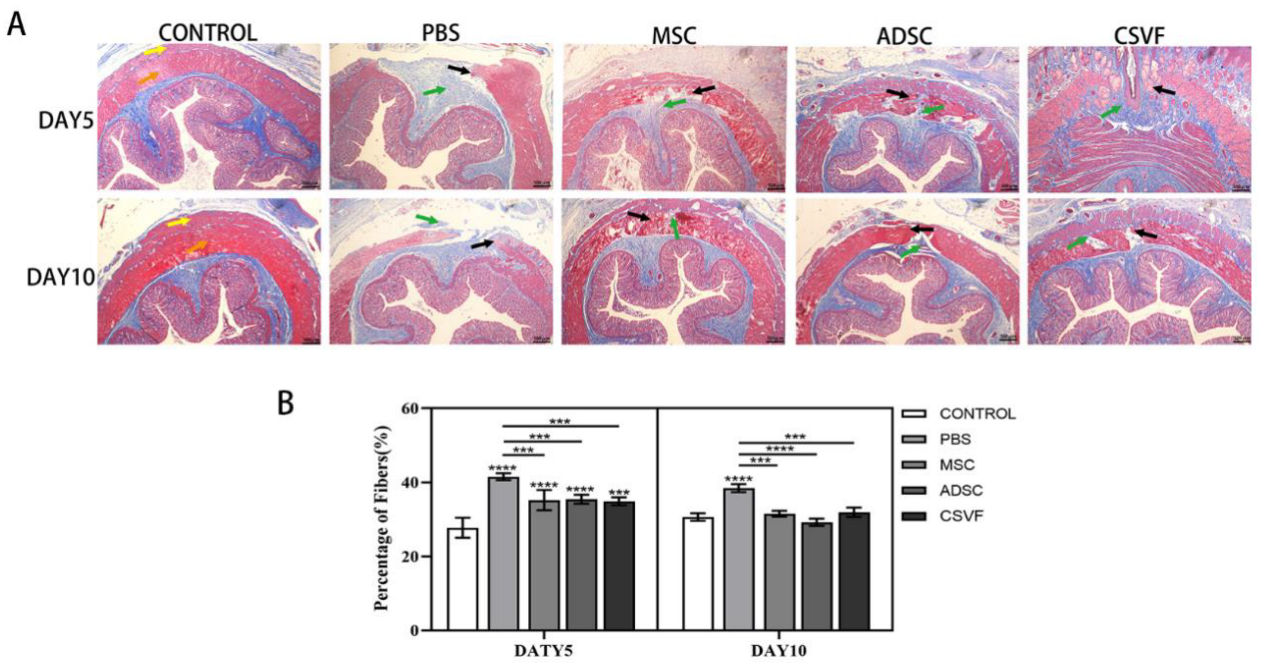
3.3. Gross Healing of the Surgical Sites
3.4. Masson Trichrome Stain
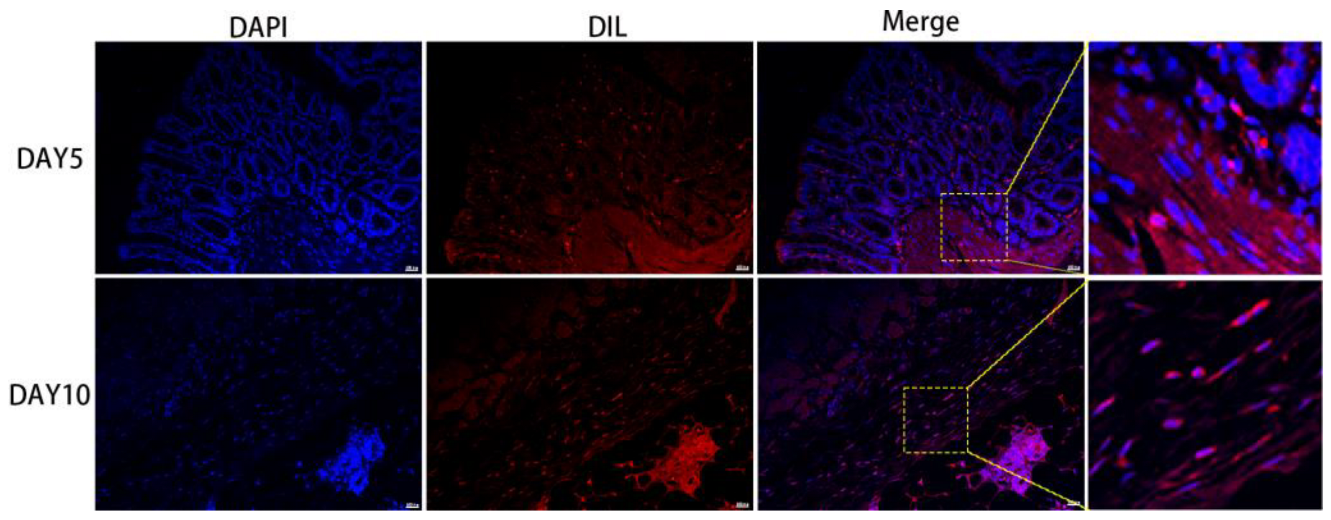
3.5. The Survival, Proliferation and Differentiation of MSC, ADSC, and CSVF In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatoor, D.R.; Taylor, S.J.; Cohen, C.R.; Emmanuel, A.V. Faecal incontinence. Br. J. Surg. 2007, 94, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Meyer, I.; Richter, H.E. Impact of fecal incontinence and its treatment on quality of life in women. Women’s Health 2015, 11, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C. Pathophysiology of adult fecal incontinence. Gastroenterology 2004, 126, S14–S22. [Google Scholar] [CrossRef]
- LaCross, A.; Groff, M.; Smaldone, A. Obstetric Anal Sphincter Injury and Anal Incontinence Following Vaginal Birth: A Systematic Review and Meta-Analysis. J. Midwifery Women’s Health 2015, 60, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Wexner, S.D.; Bleier, J. Current surgical strategies to treat fecal incontinence. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1577–1589. [Google Scholar] [CrossRef]
- Huang, S.; Leung, V.; Peng, S.; Li, L.; Lu, F.; Wang, T.; Lu, W.; Lu, W.; Cheung, K.; Zhou, G. Developmental definition of MSCs: New insights into pending questions. Cell Reprogram 2011, 13, 465–472. [Google Scholar] [CrossRef]
- Deng, Z.; Jin, J.; Wang, S.; Wang, S.; Qi, F.; Chen, X.; Liu, C.; Li, Y.; Ma, Y.; Lyu, F.; et al. Narrative review of the choices of stem cell sources and hydrogels for cartilage tissue engineering. Ann. Transl. Med. 2020, 8, 1598. [Google Scholar] [CrossRef]
- Qi, F.; Deng, Z.; Ma, Y.; Wang, S.; Liu, C.; Lyu, F.; Wang, T.; Zheng, Q. From the perspective of embryonic tendon development: Various cells applied to tendon tissue engineering. Ann. Transl. Med. 2020, 8, 131. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Cai, J.Q.; Lv, F.J.; Xie, H.L.; Yang, Z.M.; Huang, Y.C.; Deng, L. Species variation in the spontaneous calcification of bone marrow-derived mesenchymal stem cells. Cytotherapy 2013, 15, 323–329. [Google Scholar] [CrossRef]
- Lv, F.J.; Tuan, R.S.; Cheung, K.M.; Leung, V.Y. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef]
- Lv, F.; Lu, M.; Cheung, K.M.; Leung, V.Y.; Zhou, G.Q. Intrinsic properties of mesemchymal stem cells from human bone marrow, umbilical cord and umbilical cord blood comparing the different sources of MSC. Curr. Stem Cell Res. Ther. 2012, 7, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, L.; Mayorga, M.; Damaser, M.; Balog, B.; Butler, R.; Penn, M.; Zutshi, M. Mesenchymal stem cells can improve anal pressures after anal sphincter injury. Stem Cell Res. 2013, 10, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, L.; Sopko, N.; Jiang, H.H.; Damaser, M.; Penn, M.; Zutshi, M. Chemokine upregulation in response to anal sphincter and pudendal nerve injury: Potential signals for stem cell homing. Int. J. Colorectal. Dis. 2011, 26, 1577–1581. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ma, X.; Jin, W.; Li, X.; Hu, J.; Jiang, X.; Guo, X. Effects of local injection and intravenous injection of allogeneic bone marrow mesenchymal stem cells on the structure and function of damaged anal sphincter in rats. J. Tissue Eng. Regen. Med. 2020, 14, 989–1000. [Google Scholar] [CrossRef]
- Inoue, Y.; Fujita, F.; Yamaguchi, I.; Kinoe, H.; Kawahara, D.; Sakai, Y.; Kuroki, T.; Eguchi, S. Improvement of Anal Function by Adipose-Derived Stem Cell Sheets. Dig. Surg. 2018, 35, 64–69. [Google Scholar] [CrossRef]
- Jacobs, S.A.; Lane, F.L.; Pham, Q.A.; Nistor, G.; Robles, R.; Chua, C.; Boubion, B.; Osann, K.; Keirstead, H. Safety assessment of myogenic stem cell transplantation and resulting tumor formation. Female Pelvic. Med. Reconstr. Surg. 2013, 19, 362–368. [Google Scholar] [CrossRef]
- Balaphas, A.; Meyer, J.; Meier, R.; Liot, E.; Buchs, N.; Roche, B.; Toso, C.; Bühler, L.; Gispert, C.; Ris, F. Cell Therapy for Anal Sphincter Incontinence: Where Do We Stand? Cells 2021, 10, 2086. [Google Scholar] [CrossRef]
- Gras, S.; Tolstrup, C.K.; Lose, G. Regenerative medicine provides alternative strategies for the treatment of anal incontinence. Int. Urogynecol. J. 2017, 28, 341–350. [Google Scholar] [CrossRef]
- Trebol, J.; Carabias-Orgaz, A.; Garcia-Arranz, M.; Garcia-Olmo, D. Stem cell therapy for faecal incontinence: Current state and future perspectives. World J. Stem Cells 2018, 10, 82–105. [Google Scholar] [CrossRef]
- Williams, J.K.; Badlani, G.; Dean, A.; Lankford, S.; Poppante, K.; Criswell, T.; Andersson, K.E. Local versus intravenous injections of skeletal muscle precursor cells in nonhuman primates with acute or chronic intrinsic urinary sphincter deficiency. Stem Cell Res. Ther. 2016, 7, 147. [Google Scholar] [CrossRef]
- Das, M.; Mayilsamy, K.; Mohapatra, S.S.; Mohapatra, S. Mesenchymal stem cell therapy for the treatment of traumatic brain injury: Progress and prospects. Rev. Neurosci. 2019, 30, 839–855. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.A.; Tuin, A.J.; Spiekman, M.; Jansma, J.; van der Lei, B.; Harmsen, M.C. Comparison of intraoperative procedures for isolation of clinical grade stromal vascular fraction for regenerative purposes: A systematic review. J. Tissue Eng. Regen. Med. 2018, 12, e261–e274. [Google Scholar] [CrossRef] [PubMed]
- Astori, G.; Vignati, F.; Bardelli, S.; Tubio, M.; Gola, M.; Albertini, V.; Bambi, F.; Scali, G.; Castelli, D.; Rasini, V. “In vitro” and multicolor phenotypic characterization of cell subpopulations identified in fresh human adipose tissue stromal vascular fraction and in the derived mesenchymal stem cells. J. Transl. Med. 2007, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Maffulli, N.; Burgos-Alonso, N. Stromal vascular fraction technologies and clinical applications. Expert Opin. Biol. Ther. 2019, 19, 1289–1305. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Phuong, N.T.T.; Tien, N.L.B.; Tran, D.K.; Minh, L.B.; Thanh, V.V.; Anh, P.G.; Pham, V.H.; Nga, V.T. Adipose Tissue Stem Cells for Therapy: An Update on the Progress of Isolation, Culture, Storage, and Clinical Application. J. Clin. Med. 2019, 8, 917. [Google Scholar] [CrossRef]
- Sumi, M.; Sata, M.; Toya, N.; Yanaga, K.; Ohki, T.; Nagai, R. Transplantation of adipose stromal cells, but not mature adipocytes, augments ischemia-induced angiogenesis. Life Sci. 2007, 80, 559–565. [Google Scholar] [CrossRef]
- Zeyda, M.; Farmer, D.; Todoric, J.; Aszmann, O.; Speiser, M.; Gyori, G.; Zlabinger, G.J.; Stulnig, T.M. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int. J. Obes. 2007, 31, 1420–1428. [Google Scholar] [CrossRef]
- Roato, I.; Belisario, D.C.; Compagno, M.; Verderio, L.; Sighinolfi, A.; Mussano, F.; Genova, T.; Veneziano, F.; Pertici, G.; Perale, G.; et al. Adipose-Derived Stromal Vascular Fraction/Xenohybrid Bone Scaffold: An Alternative Source for Bone Regeneration. Stem Cells Int. 2018, 2018, 4126379. [Google Scholar] [CrossRef]
- Kim, A.; Kim, D.; Song, H.; Kang, W.; Kim, H.; Lim, H.; Cho, D.; Bae, J. Repair of rabbit ulna segmental bone defect using freshly isolated adipose-derived stromal vascular fraction. Cytotherapy 2012, 14, 296–305. [Google Scholar] [CrossRef]
- Kuroshima, S.; Sasaki, M.; Nakajima, K.; Tamaki, S.; Hayano, H.; Sawase, T. Transplantation of Noncultured Stromal Vascular Fraction Cells of Adipose Tissue Ameliorates Osteonecrosis of the Jaw-Like Lesions in Mice. J. Bone Miner. Res. 2018, 33, 154–166. [Google Scholar] [CrossRef]
- Nyberg, E.; Farris, A.; O’Sullivan, A.; Rodriguez, R.; Grayson, W. Comparison of Stromal Vascular Fraction and Passaged Adipose-Derived Stromal/Stem Cells as Point-of-Care Agents for Bone Regeneration. Tissue Eng. Part A 2019, 25, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- El-Habta, R.; Andersson, G.; Kingham, P.J.; Backman, L.J. Anti-apoptotic effect of adipose tissue-derived stromal vascular fraction in denervated rat muscle. Stem Cell Res. Ther. 2021, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.I.; Kishimoto, S.; Kaga, K.; Fuse, M.; Furuta, A.; Yamanishi, T. Autologous and heterotopic transplantation of adipose stromal vascular fraction ameliorates stress urinary incontinence in rats with simulated childbirth trauma. Regen. Ther. 2018, 8, 9–14. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, S.J.; Yoon, H.; Chung, W.S.; Kim, J.Y.; Shin, M.J.; Kang, W.H.; Kim, G.P. Efficacy and Safety of Biodegradable Microparticles in the Regeneration of Injured Rabbit Corpus Cavernosum: Primary Report. World J. Men’s Health 2011, 29, 223–230. [Google Scholar] [CrossRef]
- Mohammadi, R.; Sanaei, N.; Ahsan, S.; Rostami, H.; Abbasipour-Dalivand, S.; Amini, K. Repair of nerve defect with chitosan graft supplemented by uncultured characterized stromal vascular fraction in streptozotocin induced diabetic rats. Int. J. Surg. 2013, 12, 33–40. [Google Scholar] [CrossRef][Green Version]
- Tada, K.; Nakada, M.; Matsuta, M.; Murai, A.; Hayashi, K.; Tsuchiya, H. Enhanced nerve autograft using stromal vascular fraction. Eur. J. Orthop. Surg. Traumatol. 2020, 31, 183–188. [Google Scholar] [CrossRef]
- Shimizu, M.; Matsumine, H.; Osaki, H.; Ueta, Y.; Tsunoda, S.; Kamei, W.; Hashimoto, K.; Niimi, Y.; Watanabe, Y.; Miyata, M.; et al. Adipose-derived stem cells and the stromal vascular fraction in polyglycolic acid-collagen nerve conduits promote rat facial nerve regeneration. Wound Repair Regen. 2018, 26, 446–455. [Google Scholar] [CrossRef]
- Karatan, B.; Aksam, E.; Erden, E.; Demirseren, M.E. Effects of adipose derived stromal vascular fraction on diabetic neuropathy: An experimental study. J. Plast. Surg. Hand. Surg. 2019, 53, 335–340. [Google Scholar] [CrossRef]
- Dromard, C.; Barreau, C.; Andre, M.; Berger-Muller, S.; Casteilla, L.; Planat-Benard, V. Mouse adipose tissue stromal cells give rise to skeletal and cardiomyogenic cell sub-populations. Front Cell Dev. Biol. 2014, 2, 42. [Google Scholar] [CrossRef]
- Premaratne, G.U.; Ma, L.P.; Fujita, M.; Lin, X.; Bollano, E.; Fu, M. Stromal vascular fraction transplantation as an alternative therapy for ischemic heart failure: Anti-inflammatory role. J. Cardiothorac. Surg. 2011, 6, 43. [Google Scholar] [CrossRef]
- Wang, W.Z. Microcirculatory Response In Vivo on Local Intraarterial Infusion of Autogenic Adipose-derived Stem Cells or Stromal Vascular Fraction. Plast. Reconstr. Surg. Glob. Open 2016, 4, e1067. [Google Scholar] [CrossRef] [PubMed]
- Trébol, J.; Georgiev-Hristov, T.; Vega-Clemente, L.; Garcia-Gomez, I.; Carabias-Orgaz, A.; Garcia-Arranz, M.; Garcia-Olmo, D. Rat model of anal sphincter injury and two approaches for stem cell administration. World J. Stem Cells 2018, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zutshi, M.; Salcedo, L.B.; Zaszczurynski, P.J.; Hull, T.L.; Butler, R.S.; Damaser, M.S. Effects of sphincterotomy and pudendal nerve transection on the anal sphincter in a rat model. Dis. Colon. Rectum. 2009, 52, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, L.; Penn, M.; Damaser, M.; Balog, B.; Zutshi, M. Functional outcome after anal sphincter injury and treatment with mesenchymal stem cells. Stem Cells Transl. Med. 2014, 3, 760–767. [Google Scholar] [CrossRef]
- Sun, L.; Xie, Z.; Kuang, M.; Penn, M.; Damaser, M.S.; Zutshi, M. Regenerating the Anal Sphincter: Cytokines, Stem Cells, or Both? Dis. Colon Rectum 2017, 60, 416–425. [Google Scholar] [CrossRef]
- Kang, S.B.; Lee, H.N.; Lee, J.Y.; Park, J.S.; Lee, H.S.; Lee, J.Y. Sphincter contractility after muscle-derived stem cells autograft into the cryoinjured anal sphincters of rats. Dis. Colon Rectum 2008, 51, 1367–1373. [Google Scholar] [CrossRef][Green Version]
- Salcedo, L.; Damaser, M.; Butler, R.; Jiang, H.H.; Hull, T.; Zutshi, M. Long-Term Effects on Pressure and Electromyography in a Rat Model of Anal Sphincter Injury. Dis. Colon Rectum 2010, 53, 1209–1217. [Google Scholar] [CrossRef]
- Kang, S.; Lee, H.; Lim, J.; Oh, S.; Kim, S.; Hong, S.; Jang, J.; Cho, J.; Lee, S.; Lee, J. Injection of porous polycaprolactone beads containing autologous myoblasts in a dog model of fecal incontinence. J. Korean Surg. Soc. 2013, 84, 216. [Google Scholar] [CrossRef]
- Kim, H.S.; Mandakhbayar, N.; Kim, H.W.; Leong, K.W.; Yoo, H.S. Protein-reactive nanofibrils decorated with cartilage-derived decellularized extracellular matrix for osteochondral defects. Biomaterials 2021, 269, 120214. [Google Scholar] [CrossRef]
- Semon, J.A.; Zhang, X.; Pandey, A.C.; Alandete, S.M.; Maness, C.; Zhang, S.J.; Scruggs, B.A.; Strong, A.L.; Sharkey, S.A.; Beuttler, M.M.; et al. Administration of murine stromal vascular fraction ameliorates chronic experimental autoimmune encephalomyelitis. Stem Cells Transl. Med. 2013, 2, 789–796. [Google Scholar] [CrossRef]
- Cohen, S.R.; Hewett, S.; Ross, L.; Delaunay, F.; Goodacre, A.; Ramos, C.; Leong, T.; Saad, A. Regenerative Cells For Facial Surgery: Biofilling and Biocontouring. Aesthet. Surg. J. 2017, 37, S16–S32. [Google Scholar] [CrossRef] [PubMed]
- Boyer, O.; Bridoux, V.; Giverne, C.; Bisson, A.; Koning, E.; Leroi, A.M.; Chambon, P.; Dehayes, J.; Le Corre, S.; Jacquot, S. Autologous Myoblasts for the Treatment of Fecal Incontinence: Results of a Phase 2 Randomized Placebo-controlled Study (MIAS). Ann. Surg. 2018, 267, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Frudinger, A.; Kolle, D.; Schwaiger, W.; Pfeifer, J.; Paede, J.; Halligan, S. Muscle-derived cell injection to treat anal incontinence due to obstetric trauma: Pilot study with 1 year follow-up. Gut 2010, 59, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Frudinger, A.; Pfeifer, J.; Paede, J.; Kolovetsiou-Kreiner, V.; Marksteiner, R.; Halligan, S. Autologous skeletal-muscle-derived cell injection for anal incontinence due to obstetric trauma: A 5-year follow-up of an initial study of 10 patients. Colorectal. Dis. 2015, 17, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Frudinger, A.; Marksteiner, R.; Pfeifer, J.; Margreiter, E.; Paede, J.; Thurner, M. Skeletal muscle-derived cell implantation for the treatment of sphincter-related faecal incontinence. Stem Cell Res. Ther. 2018, 9, 233. [Google Scholar] [CrossRef]
- Park, E.J.; Kang, J.; Baik, S.H. Treatment of faecal incontinence using allogeneic-adipose-derived mesenchymal stem cells: A study protocol for a pilot randomised controlled trial. BMJ Open 2016, 6, e10450. [Google Scholar] [CrossRef]
- de la Portilla, F.; Guerrero, J.L.; Maestre, M.V.; Leyva, L.; Mera, S.; Garcia-Olmo, D.; Rodriguez, A.; Mata, R.; Lora, F. Treatment of faecal incontinence with autologous expanded mesenchymal stem cells: Results of a pilot study. Colorectal. Dis. 2021, 23, 698–709. [Google Scholar] [CrossRef]
- Plair, A.; Bennington, J.; Williams, J.K.; Parker-Autry, C.; Matthews, C.A.; Badlani, G. Regenerative medicine for anal incontinence: A review of regenerative therapies beyond cells. Int. Urogynecol. J. 2021, 32, 2337–2347. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.E. D.I.4 Satellite cells and skeletal muscle regeneration. Neuromuscul. Disord. 2011, 21, 640. [Google Scholar] [CrossRef]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar]
- Rustad, K.C.; Gurtner, G.C. Mesenchymal Stem Cells Home to Sites of Injury and Inflammation. Adv Wound Care 2012, 1, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhang, Y.; Li, X.; Fu, Q. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. CMLS 2020, 77, 2771–2794. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.Y.; Aladin, D.M.; Lv, F.; Tam, V.; Sun, Y.; Lau, R.Y.; Hung, S.C.; Ngan, A.H.; Tang, B.; Lim, C.T. Mesenchymal stem cells reduce intervertebral disc fibrosis and facilitate repair. Stem Cells 2014, 32, 2164–2177. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Fan, X.L.; Zhang, Z.; Ma, C.Y.; Fu, Q.L. Mesenchymal stem cells for inflammatory airway disorders: Promises and challenges. Biosci. Rep. 2019, 39, BSR20182160. [Google Scholar] [CrossRef]
- Huang, Y.C.; Li, Z.; Li, J.; Lyu, F.J. Interaction between Stem Cells and the Microenvironment for Musculoskeletal Repair. Stem Cells Int. 2020, 2020, 7587428. [Google Scholar] [CrossRef]
- Lyu, F.J. Impact of Microenvironmental Changes during Degeneration on Intervertebral Disc Progenitor Cells: A Comparison with Mesenchymal Stem Cells. Bioengineering 2022, 9, 148. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef]
- Kuriyan, A.E.; Albini, T.A.; Townsend, J.H.; Rodriguez, M.; Pandya, H.K.; Leonard, R.E.; Parrott, M.B.; Rosenfeld, P.J.; Flynn, H.W.; Goldberg, J.L. Vision Loss after Intravitreal Injection of Autologous “Stem Cells” for AMD. N. Engl. J. Med. 2017, 376, 1047–1053. [Google Scholar] [CrossRef]



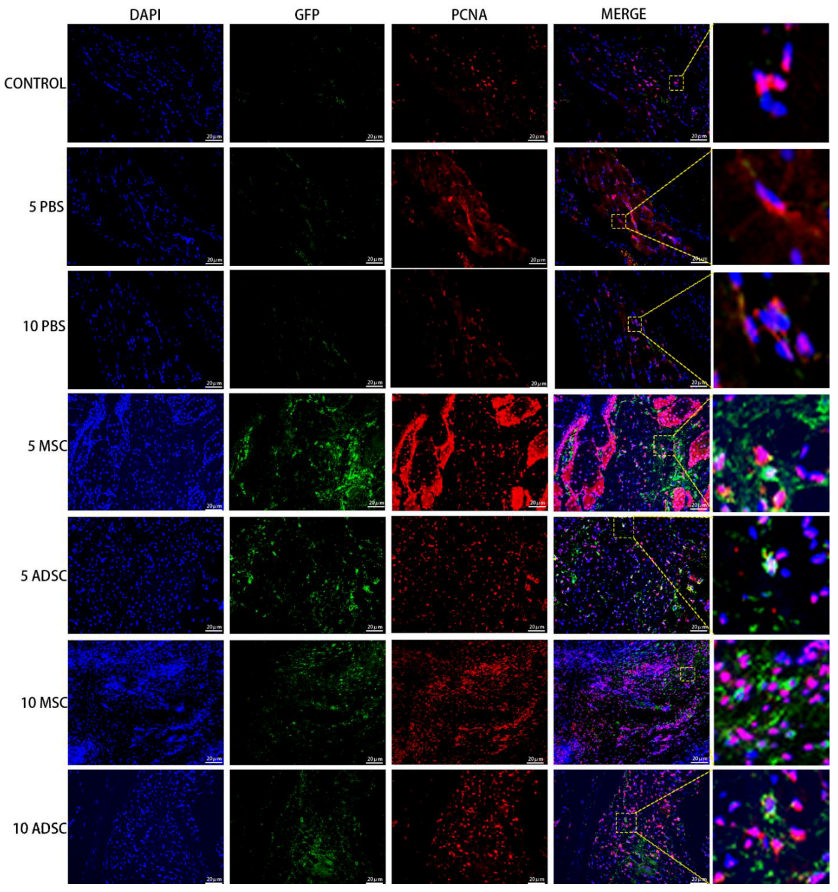
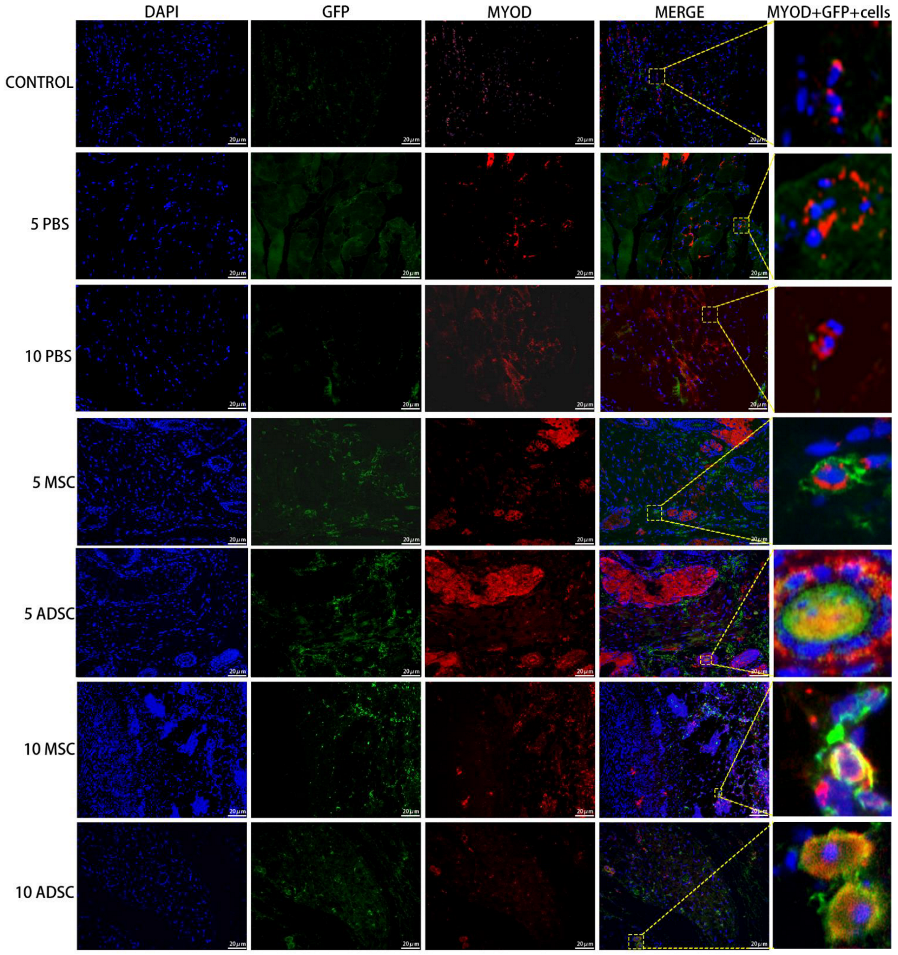
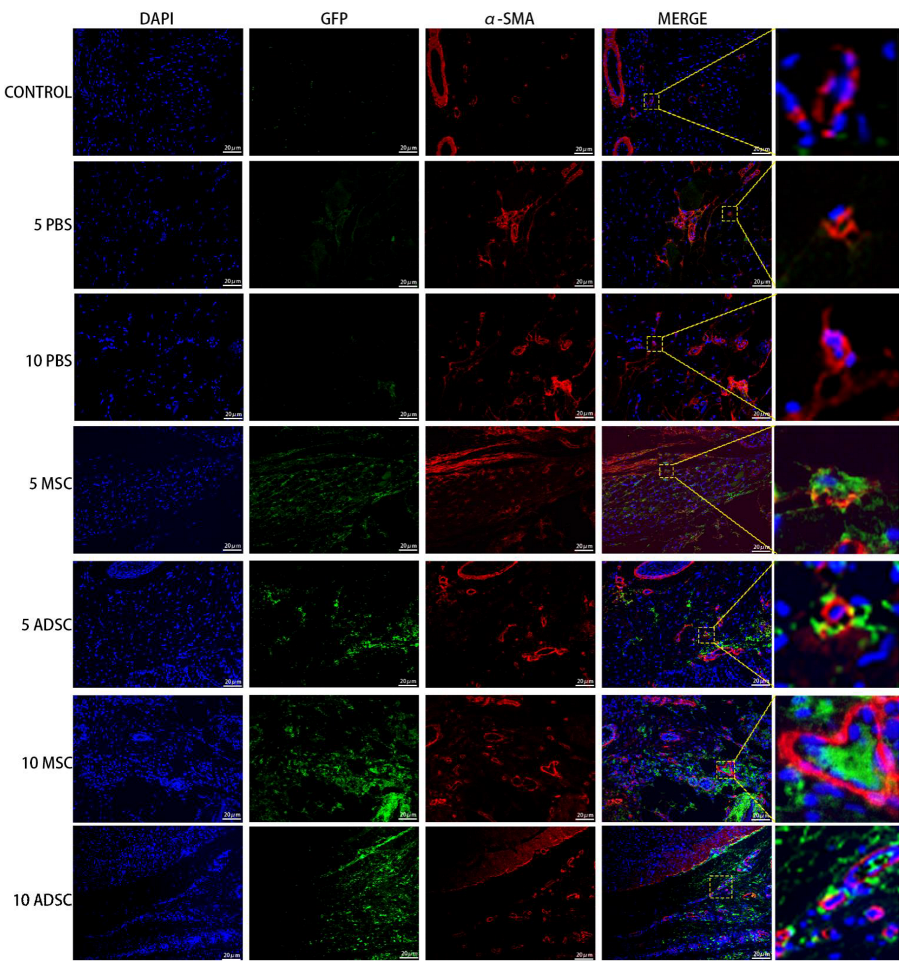
| Variable | Control | PBS | MSC | ADSC | CSVF |
|---|---|---|---|---|---|
| Baseline pressures (cmH2O) Pre-excision | 7.47 ± 0.57 | 6.90 ± 0.44 | 7.18 ± 0.77 | 7.06 ± 1.30 | 7.42 ± 0.76 |
| DAY(0) | 6.88 ± 0.45 | 4.10 ± 0.88 * | 4.21 ± 0.33 * | 4.47 ± 1.00 * | 4.24 ± 1.12 * |
| DAY(5) | 7.22 ± 0.91 | 4.70 ± 1.25 * | 4.07 ± 1.61 ** | 4.14 ± 1.28 ** | 5.29 ± 0.72 |
| DAY(10) | 7.15 ± 0.96 | 4.23 ± 1.65 * | 8.43 ± 1.61 &&& | 7.4 ± 1.70 && | 7.89 ± 2.10 && |
| Peak pressures (cmH2O) Pre-excision | 14.94 ± 1.49 | 15.30 ± 1.31 | 15.95 ± 0.55 | 15.31 ± 1.72 | 16.33 ± 2.10 |
| DAY(0) | 15.06 ± 0.37 | 9.06 ± 1.27 *** | 8.05 ± 1.17 **** | 9.00 ± 1.17 *** | 9.95 ± 2.21 *** |
| DAY(5) | 15.61 ± 0.25 | 8.94 ± 0.75 **** | 9.98 ± 0.79 *** | 9.49 ± 1.07 **** | 11.4 ± 2.08 ** |
| DAY(10) | 16.08 ± 1.22 | 9.64 ± 0.17 **** | 11.45 ± 1.56 ** | 11.71 ± 1.59 ** | 12.73 ± 2.45 * |
| Variable | Control | PBS | MSC | ADSC | CSVF |
|---|---|---|---|---|---|
| Peak EMG(μv) Pre-excision | 134.65 ± 9.10 | 132.55 ± 6.55 | 142.40 ± 9.59 | 136.96 ± 4.53 | 137.00 ± 7.17 |
| DAY(0) | 137.52 ± 6.93 | 84.89 ± 9.42 **** | 88.03 ± 7.07 **** | 84.33 ± 2.28 **** | 78.00 ± 3.58 **** |
| DAY(5) | 142.44 ± 6.15 | 86.56 ± 9.46 **** | 93.53 ± 8.24 **** | 100.87 ± 3.99 ****& | 104.02 ± 6.34 ****& |
| DAY(10) | 140.09 ± 2.12 | 95.16 ± 9.81 **** | 128.87 ± 9.60 &&&& | 130.64 ± 3.64 &&&& | 124.69 ± 1.99 *&&&& |
| EMG frequency(Hz) Pre-excision | 27.33 ± 1.24 | 28.33 ± 2.86 | 28.00 ± 2.94 | 27.66 ± 2.62 | 26.00 ± 2.73 |
| DAY(0) | 26.00 ± 0.81 | 15.33 ± 2.05 **** | 14.33 ± 1.69 **** | 14.33 ± 3.39 **** | 14.66 ± 0.47 **** |
| DAY(5) | 28.00 ± 2.94 | 17.25 ± 1.08 **** | 19.25 ± 1.47 **** | 19.00 ± 2.16 **** | 20.66 ± 1.24 *** |
| DAY(10) | 26.00 ± 0.81 | 18.25 ± 1.08 *** | 25.75 ± 1.47 &&&& | 25.66 ± 1.69 &&& | 22.25 ± 2.04 *& |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; He, Z.; Li, S.; Wu, Z.; Tan, J.; Yang, W.; Li, G.; Pan, X.; Liu, Y.; Lyu, F.-J.; et al. The Effect of Mesenchymal Stem Cells, Adipose Tissue Derived Stem Cells, and Cellular Stromal Vascular Fraction on the Repair of Acute Anal Sphincter Injury in Rats. Bioengineering 2022, 9, 318. https://doi.org/10.3390/bioengineering9070318
Chen W, He Z, Li S, Wu Z, Tan J, Yang W, Li G, Pan X, Liu Y, Lyu F-J, et al. The Effect of Mesenchymal Stem Cells, Adipose Tissue Derived Stem Cells, and Cellular Stromal Vascular Fraction on the Repair of Acute Anal Sphincter Injury in Rats. Bioengineering. 2022; 9(7):318. https://doi.org/10.3390/bioengineering9070318
Chicago/Turabian StyleChen, Wenbin, Zijian He, Shuyu Li, Zixin Wu, Jin Tan, Weifeng Yang, Guanwei Li, Xiaoting Pan, Yuying Liu, Feng-Juan Lyu, and et al. 2022. "The Effect of Mesenchymal Stem Cells, Adipose Tissue Derived Stem Cells, and Cellular Stromal Vascular Fraction on the Repair of Acute Anal Sphincter Injury in Rats" Bioengineering 9, no. 7: 318. https://doi.org/10.3390/bioengineering9070318
APA StyleChen, W., He, Z., Li, S., Wu, Z., Tan, J., Yang, W., Li, G., Pan, X., Liu, Y., Lyu, F.-J., & Li, W. (2022). The Effect of Mesenchymal Stem Cells, Adipose Tissue Derived Stem Cells, and Cellular Stromal Vascular Fraction on the Repair of Acute Anal Sphincter Injury in Rats. Bioengineering, 9(7), 318. https://doi.org/10.3390/bioengineering9070318





