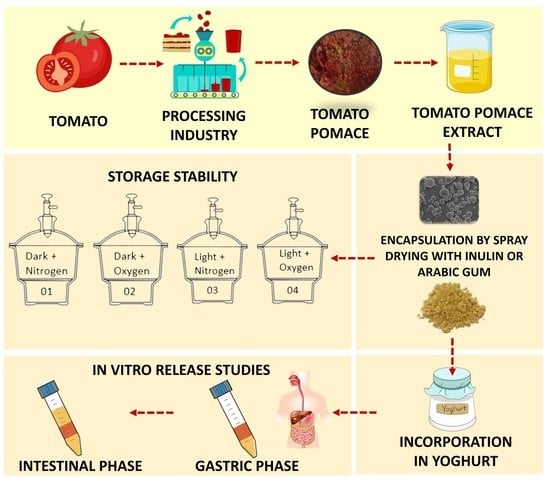
Storage Stability and In Vitro Bioaccessibility of Microencapsulated Tomato (Solanum Lycopersicum L.) Pomace Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tomato Pomace Extract Preparation
2.3. Preparation of Emulsions
2.4. Spray Drying Conditions
2.5. Morphological Characterization of Microparticles
2.6. Analytical Methods
2.6.1. Carotenoids Content in the Tomato Pomace Extract
2.6.2. Antioxidant Activity of the Tomato Pomace Extract
2.6.3. Loading Capacity
2.6.4. Antioxidant Activity of the Encapsulated Material
2.7. Storage Stability of Microencapsulated Tomato Pomace Extract
2.8. In Vitro Digestion Studies
2.8.1. Microencapsulated Tomato Pomace Extract
2.8.2. Yogurt with Incorporated Microparticles
2.8.3. Carotenoid Bioaccessibility
2.9. Viability of Bacteria from the Probiotic Yogurt (Actimel®)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Tomato Pomace Extract
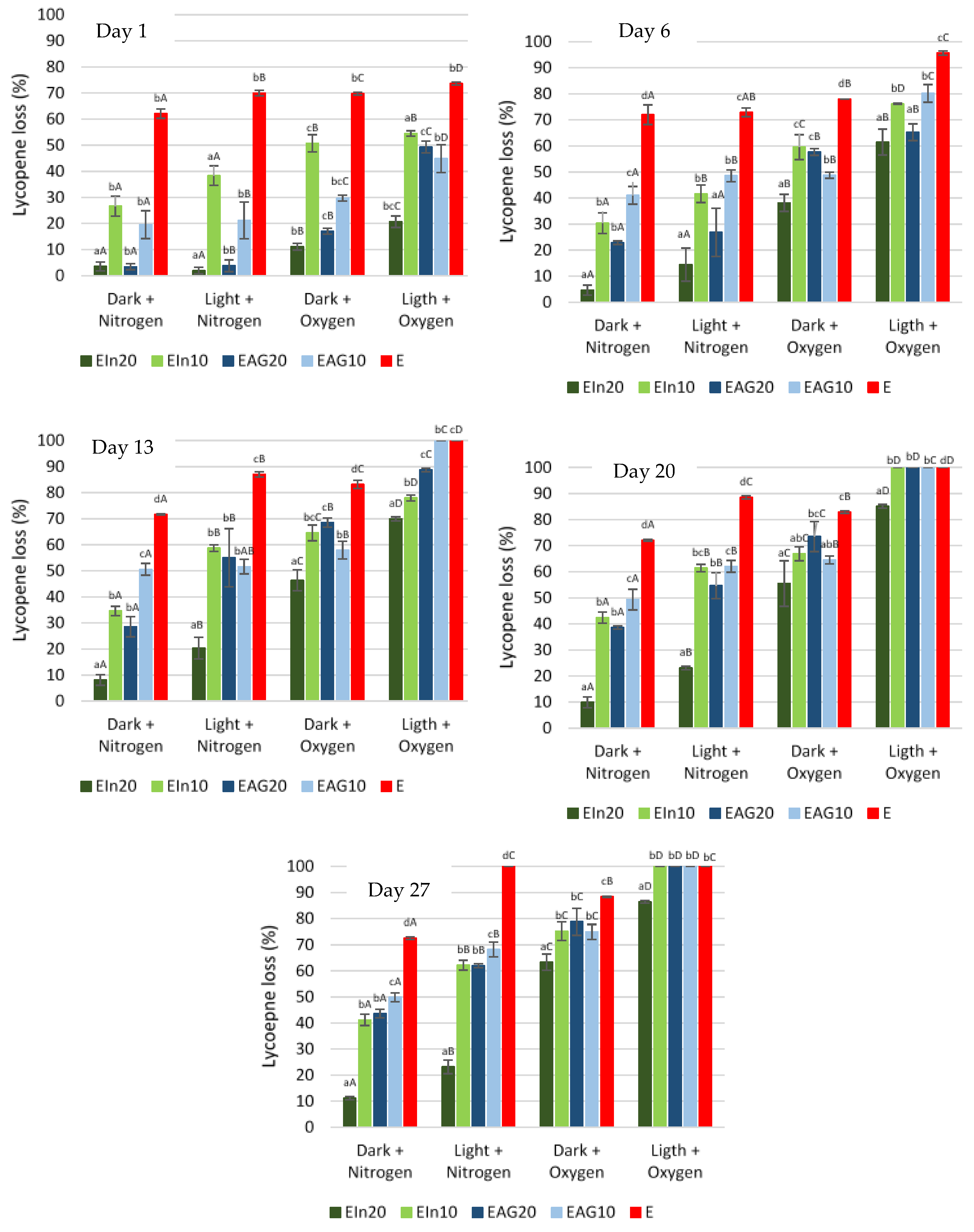
3.2. Storage Stability of Microencapsulated Tomato Pomace Extract
3.2.1. Lycopene Content
3.2.2. Antioxidant Activity
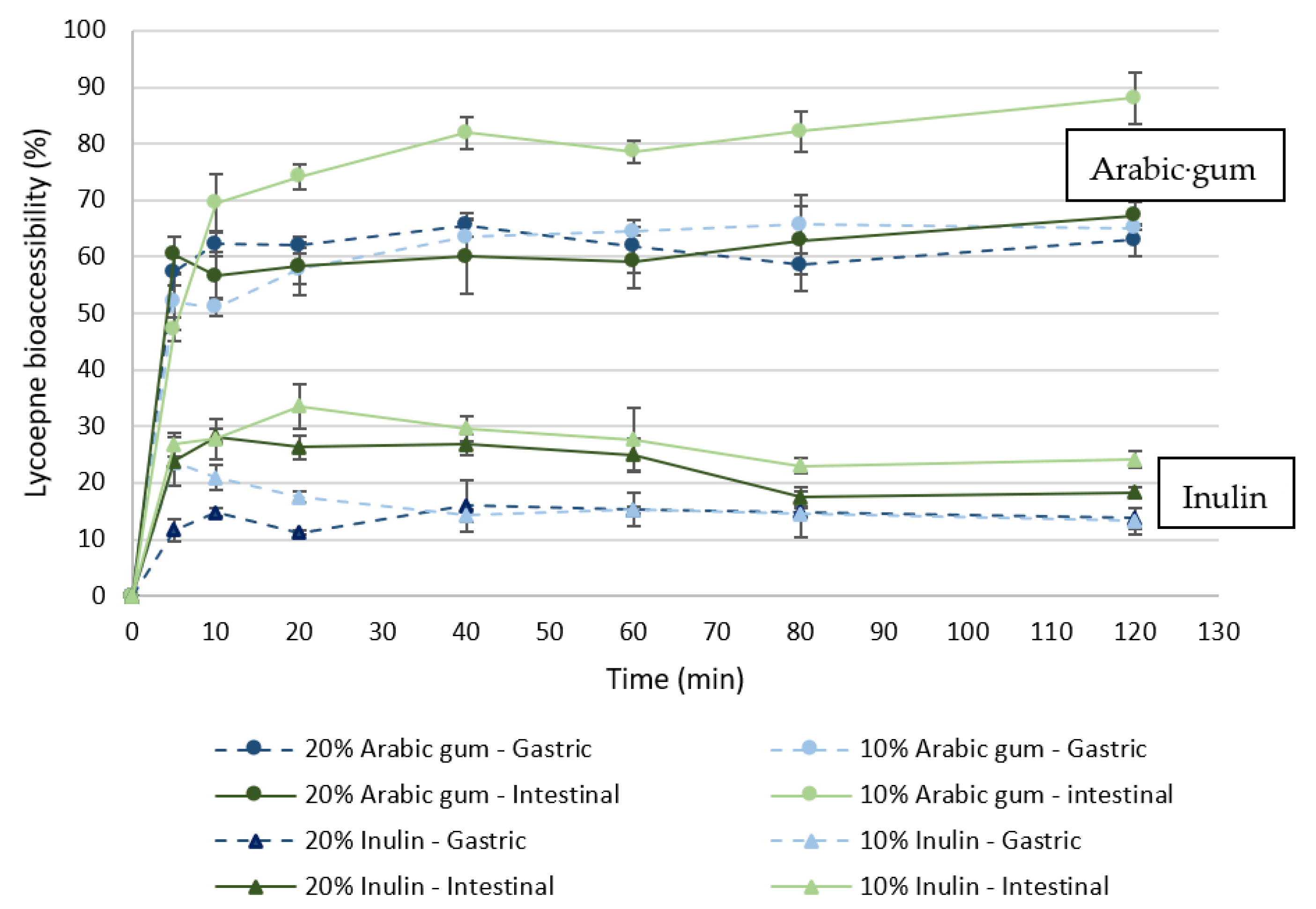
3.3. Carotenoids Bioaccessibility
3.3.1. Microparticles Loaded with Tomato Pomace Extract
3.3.2. Yogurt Enriched with Microparticles
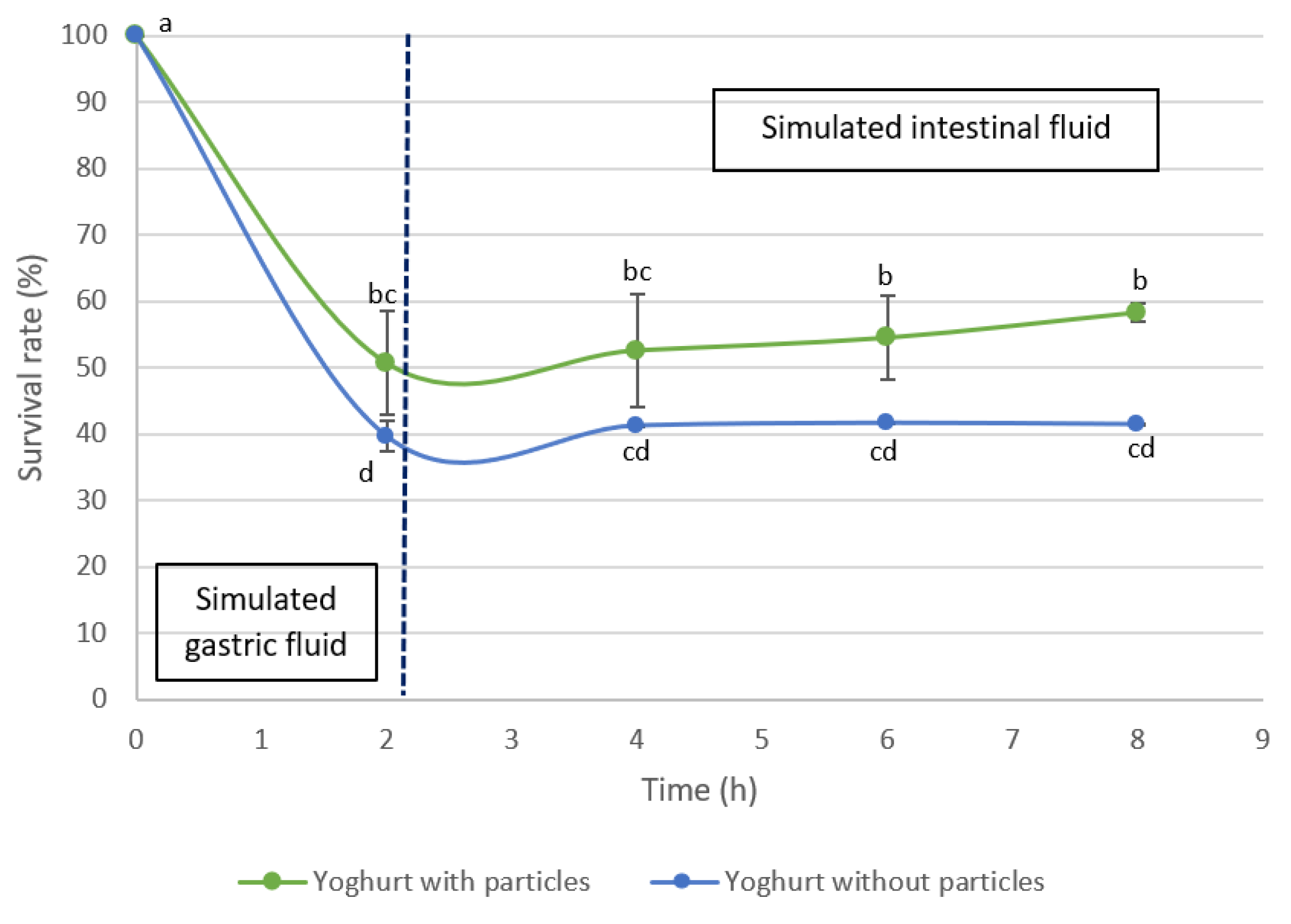
3.4. Viability of Bacteria from Probiotic Yogurt
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fierascu, R.C.; Fierascu, I.; Avramescu, S.M.; Sieniawska, E.; Barba, F.J.; Zhu, Z.; Lorenzo, J.M.; Remize, F. Recovery of Natural Antioxidants from Agro-Industrial Side Streams through Advanced Extraction Techniques. Molecules 2019, 24, 4212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putnik, P.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S.; Jambrak, A.R.; Granato, D.; Montesano, D.; Kovačević, D.B. Novel Food Processing and Extraction Technologies of High-Added Value Compounds from Plant Materials. Foods 2018, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Ojha, K.S.; Aznar, R.; O’Donnell, C.; Tiwari, B.K. Ultrasound Technology for the Extraction of Biologically Active Molecules from Plant, Animal and Marine Sources. TrAC Trends Anal. Chem. 2020, 122, 115663. [Google Scholar] [CrossRef]
- de Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Fluid Extraction of Carotenoids from Vegetable Waste Matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, A.V.; Rao, L.G. Carotenoids and Human Health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Shi, J.; Maguer, M. Le Lycopene in Tomatoes: Chemical and Physical Properties Affected by Food Processing. Crit. Rev. Food Sci. Nutr. 2000, 40, 1–42. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors Influencing the Chemical Stability of Carotenoids in Foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.; Lourenço, S.; Duarte, D.; Moldão-Martins, M.; Alves, V. Microencapsulation of Tomato (Solanum lycopersicum L.) Pomace Ethanolic Extract by Spray Drying: Optimization of Process Conditions. Appl. Sci. 2019, 9, 612. [Google Scholar] [CrossRef] [Green Version]
- Shu, B.; Yu, W.; Zhao, Y.; Liu, X. Study on Microencapsulation of Lycopene by Spray-Drying. J. Food Eng. 2006, 76, 664–669. [Google Scholar] [CrossRef]
- Provesi, J.G.; Dias, C.O.; Amante, E.R. Changes in Carotenoids during Processing and Storage of Pumpkin Puree. Food Chem. 2011, 128, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Trono, D. Carotenoids in Cereal Food Crops: Composition and Retention throughout Grain Storage and Food Processing. Plants 2019, 8, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etzbach, L.; Meinert, M.; Faber, T.; Klein, C.; Schieber, A.; Weber, F. Effects of Carrier Agents on Powder Properties, Stability of Carotenoids, and Encapsulation Efficiency of Goldenberry (Physalis peruviana L.) Powder Produced by Co-Current Spray Drying. Curr. Res. Food Sci. 2020, 3, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Eun, J.B.; Maruf, A.; Das, P.R.; Nam, S.H. A Review of Encapsulation of Carotenoids Using Spray Drying and Freeze Drying. Crit. Rev. Food Sci. Nutr. 2019, 60, 3547–3572. [Google Scholar] [CrossRef] [PubMed]
- Aizpurua-Olaizola, O.; Navarro, P.; Vallejo, A.; Olivares, M.; Etxebarria, N.; Usobiaga, A. Microencapsulation and Storage Stability of Polyphenols from Vitis Vinifera Grape Wastes. Food Chem. 2016, 190, 614–621. [Google Scholar] [CrossRef]
- Zokti, J.A.; Baharin, B.S.; Mohammed, A.S.; Abas, F. Green Tea Leaves Extract: Microencapsulation, Physicochemical and Storage Stability Study. Molecules 2016, 21, 940. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.; Lozano, M.; Espinosa-Mansilla, A.; González-Gómez, D. In-Vitro Evaluation of the Availability of ϖ-3 and ϖ-6 Fatty Acids and Tocopherols from Microencapsulated Walnut Oil. Food Res. Int. 2012, 48, 316–321. [Google Scholar] [CrossRef]
- Troya, D.; Tupuna-Yerovi, D.S.; Ruales, J. Effects of Wall Materials and Operating Parameters on Physicochemical Properties, Process Efficiency, and Total Carotenoid Content of Microencapsulated Banana Passionfruit Pulp (Passiflora Tripartita Var. Mollissima) by Spray-Drying. Food Bioprocess Technol. 2018, 11, 1828–1839. [Google Scholar] [CrossRef]
- Beirão-da-Costa, S.; Duarte, C.; Bourbon, A.I.; Pinheiro, A.C.; Januário, M.I.N.; Vicente, A.A.; Beirão-da-Costa, M.L.; Delgadillo, I. Inulin Potential for Encapsulation and Controlled Delivery of Oregano Essential Oil. Food Hydrocoll. 2013, 33, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Esmaeilnejad Moghadam, B.; Keivaninahr, F.; Fouladi, M.; Rezaei Mokarram, R.; Nazemi, A. Inulin Addition to Yoghurt: Prebiotic Activity, Health Effects and Sensory Properties. Int. J. Dairy Technol. 2019, 72, 183–198. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Methodologies for Simulation of Gastrointestinal Digestion of Different Controlled Delivery Systems and Further Uptake of Encapsulated Bioactive Compounds. Trends Food Sci. Technol. 2021, 114, 510–520. [Google Scholar] [CrossRef]
- Gomes, G.V.L.; Sola, M.R.; Marostegan, L.F.P.; Jange, C.G.; Cazado, C.P.S.; Pinheiro, A.C.; Vicente, A.A.; Pinho, S.C. Physico-Chemical Stability and in Vitro Digestibility of Beta-Carotene-Loaded Lipid Nanoparticles of Cupuacu Butter (Theobroma Grandiflorum) Produced by the Phase Inversion Temperature (PIT) Method. J. Food Eng. 2017, 192, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Pettinato, M.; Casazza, A.A.; Perego, P. A Comprehensive Optimization of Ultrasound-Assisted Extraction for Lycopene Recovery from Tomato Waste and Encapsulation by Spray Drying. Processes 2022, 10, 308. [Google Scholar] [CrossRef]
- Souza, A.L.R.; Hidalgo-Chávez, D.W.; Pontes, S.M.; Gomes, F.S.; Cabral, L.M.C.; Tonon, R.V. Microencapsulation by Spray Drying of a Lycopene-Rich Tomato Concentrate: Characterization and Stability. LWT 2018, 91, 286–292. [Google Scholar] [CrossRef]
- Álvarez-Henao, M.V.; Saavedra, N.; Medina, S.; Jiménez Cartagena, C.; Alzate, L.M.; Londoño-Londoño, J. Microencapsulation of Lutein by Spray-Drying: Characterization and Stability Analyses to Promote Its Use as a Functional Ingredient. Food Chem. 2018, 256, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Çam, M.; Içyer, N.C.; Erdoǧan, F. Pomegranate Peel Phenolics: Microencapsulation, Storage Stability and Potential Ingredient for Functional Food Development. LWT Food Sci. Technol. 2014, 55, 117–123. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Dumoulin, E.D.; Richard, H.M.J.; Noleau, I.; Lebert, A.M. Flavor Encapsulation by Spray Drying: Application to Citral and Linalyl Acetate. J. Food Sci. 1992, 57, 217–221. [Google Scholar] [CrossRef]
- Rocha, G.A.; Fávaro-Trindade, C.S.; Grosso, C.R.F. Microencapsulation of Lycopene by Spray Drying: Characterization, Stability and Application of Microcapsules. Food Bioprod. Process. 2012, 90, 37–42. [Google Scholar] [CrossRef]
- Matioli, G.; Rodriguez-Amaya, D.B. Lycopene Encapsulated with Gum Arabic and Maltodextrin: Stability Study. Braz. J. Food Technol 2002, 5, 197–203. [Google Scholar]
- Pelissari, J.R.; Souza, V.B.; Pigoso, A.A.; Tulini, F.L.; Thomazini, M.; Rodrigues, C.E.C.; Urbano, A.; Favaro-Trindade, C.S. Production of Solid Lipid Microparticles Loaded with Lycopene by Spray Chilling: Structural Characteristics of Particles and Lycopene Stability. Food Bioprod. Process. 2016, 98, 86–94. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Food Carotenoids: Chemistry, Biology and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 1118733304. [Google Scholar]
- Gomes, F.S.; Cabral, L.M.C.; Couri, S.; Costa, P.A.; Campos, M.B.D. Lycopene Content and Antioxidant Capacity of Watermelon Powder. Acta Hortic. 2014, 1040, 105–110. [Google Scholar] [CrossRef]
- Ramakrishnan, Y.; Adzahan, N.M.; Yusof, Y.A.; Muhammad, K. Effect of Wall Materials on the Spray Drying Efficiency, Powder Properties and Stability of Bioactive Compounds in Tamarillo Juice Microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar] [CrossRef]
- Yeo, Y.; Park, K. Control of Encapsulation Efficiency and Initial Burst in Polymeric Microparticle Systems. Arch. Pharmacal Res. 2004, 27, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourenço, S.C.; Torres, C.A.V.; Nunes, D.; Duarte, P.; Freitas, F.; Reis, M.A.M.; Fortunato, E.; Moldão-Martins, M.; da Costa, L.B.; Alves, V.D. Using a Bacterial Fucose-Rich Polysaccharide as Encapsulation Material of Bioactive Compounds. Int. J. Biol. Macromol. 2017, 104, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.P.; Kong, F. In Vitro Release Kinetics of Microencapsulated Materials and the Effect of the Food Matrix. Annu. Rev. Food Sci. Technol. 2017, 8, 237–259. [Google Scholar] [CrossRef]
- Jain, A.; Thakur, D.; Ghoshal, G.; Katare, O.P.; Shivhare, U.S. Characterization of Microcapsulated β-Carotene Formed by Complex Coacervation Using Casein and Gum Tragacanth. Int. J. Biol. Macromol. 2016, 87, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Sittipummongkol, K.; Pechyen, C. RETRACTED: Production, Characterization and Controlled Release Studies of Biodegradable Polymer Microcapsules Incorporating Neem Seed Oil by Spray Drying. Food Packag. Shelf Life 2018, 18, 131–139. [Google Scholar] [CrossRef]
- Moreno, T.; Cocero, M.J.; Rodríguez-Rojo, S. Storage Stability and Simulated Gastrointestinal Release of Spray Dried Grape Marc Phenolics. Food Bioprod. Process. 2018, 112, 96–107. [Google Scholar] [CrossRef]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; da Rosa, C.G.; da Silva, M.M. Elaboration of Microparticles of Carotenoids from Natural and Synthetic Sources for Applications in Food. Food Chem. 2016, 202, 324–333. [Google Scholar] [CrossRef]
- Kalyani Nair, K.; Kharb, S.; Thompkinson, D.K. Inulin Dietary Fiber with Functional and Health Attributes—A Review. Food Rev. Int. 2010, 26, 189–203. [Google Scholar] [CrossRef]
- Montenegro, M.A.; Boiero, M.L.; Valle, L.; Borsarelli, C.D. Gum Arabic: More Than an Edible Emulsifier. In Products and Applications of Biopolymers; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.Y.; Chung, T.S.; Ng, N.P. Morphology, Drug Distribution, and in Vitro Release Profiles of Biodegradable Polymeric Microspheres Containing Protein Fabricated by Double-Emulsion Solvent Extraction/Evaporation Method. Biomaterials 2001, 22, 231–241. [Google Scholar] [CrossRef]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; Crizel-Cardozo, M.M.; Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of Palm Oil by Complex Coacervation for Application in Food Systems. Food Chem. 2017, 220, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Flamm, G.; Glinsmann, W.; Kritchevsky, D.; Prosky, L.; Roberfroid, M. Inulin and Oligofructose as Dietary Fiber: A Review of the Evidence. Crit. Rev. Food Sci. Nutr. 2001, 41, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Nag, A.; Han, K.S.; Singh, H. Microencapsulation of Probiotic Bacteria Using PH-Induced Gelation of Sodium Caseinate and Gellan Gum. Int. Dairy J. 2011, 21, 247–253. [Google Scholar] [CrossRef]
- dos Santos, D.X.; Casazza, A.A.; Aliakbarian, B.; Bedani, R.; Saad, S.M.I.; Perego, P. Improved Probiotic Survival to in Vitro Gastrointestinal Stress in a Mousse Containing Lactobacillus Acidophilus La-5 Microencapsulated with Inulin by Spray Drying. LWT 2019, 99, 404–410. [Google Scholar] [CrossRef]



| Gastric Fluid | Intestinal Fluid |
|---|---|
| 4.8 g/L NaCl | 5.0 g/L NaCl |
| 2.2 g/L KCl | 0.6 g/L KCl |
| 0.22 g/L CaCl2 | 0.25 g/L CaCl2 |
| 1.5 g/L NaHCO3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrêa-Filho, L.C.; Santos, D.I.; Brito, L.; Moldão-Martins, M.; Alves, V.D. Storage Stability and In Vitro Bioaccessibility of Microencapsulated Tomato (Solanum Lycopersicum L.) Pomace Extract. Bioengineering 2022, 9, 311. https://doi.org/10.3390/bioengineering9070311
Corrêa-Filho LC, Santos DI, Brito L, Moldão-Martins M, Alves VD. Storage Stability and In Vitro Bioaccessibility of Microencapsulated Tomato (Solanum Lycopersicum L.) Pomace Extract. Bioengineering. 2022; 9(7):311. https://doi.org/10.3390/bioengineering9070311
Chicago/Turabian StyleCorrêa-Filho, Luiz C., Diana I. Santos, Luísa Brito, Margarida Moldão-Martins, and Vítor D. Alves. 2022. "Storage Stability and In Vitro Bioaccessibility of Microencapsulated Tomato (Solanum Lycopersicum L.) Pomace Extract" Bioengineering 9, no. 7: 311. https://doi.org/10.3390/bioengineering9070311
APA StyleCorrêa-Filho, L. C., Santos, D. I., Brito, L., Moldão-Martins, M., & Alves, V. D. (2022). Storage Stability and In Vitro Bioaccessibility of Microencapsulated Tomato (Solanum Lycopersicum L.) Pomace Extract. Bioengineering, 9(7), 311. https://doi.org/10.3390/bioengineering9070311