Biomechanics of Transcatheter Aortic Valve Implant
Abstract
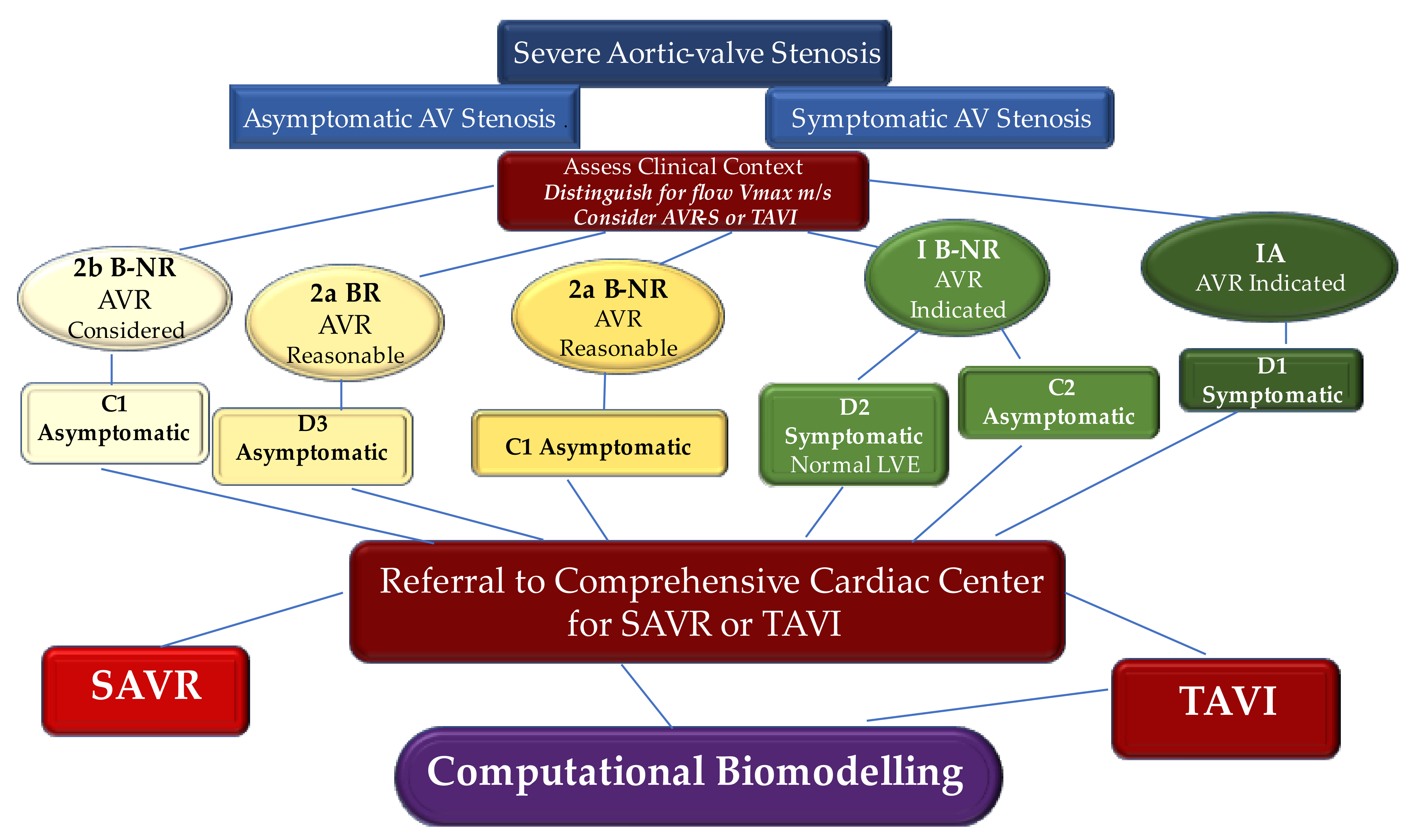
1. Introduction, Search Strategy, and Selection Criteria
2. Engineering to Study the Features of Implanted Transcatheter Heart Valve
2.1. Confluence of Engineering and Medical Sciences
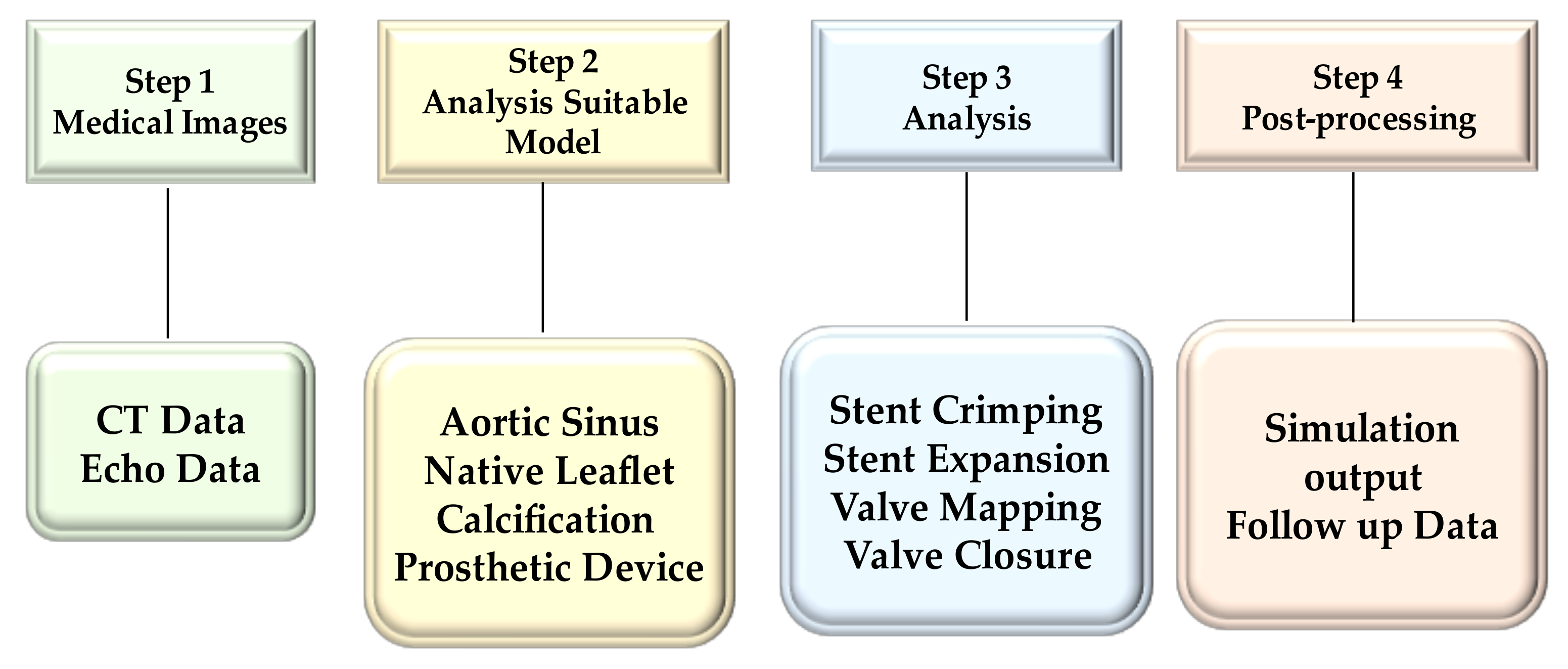
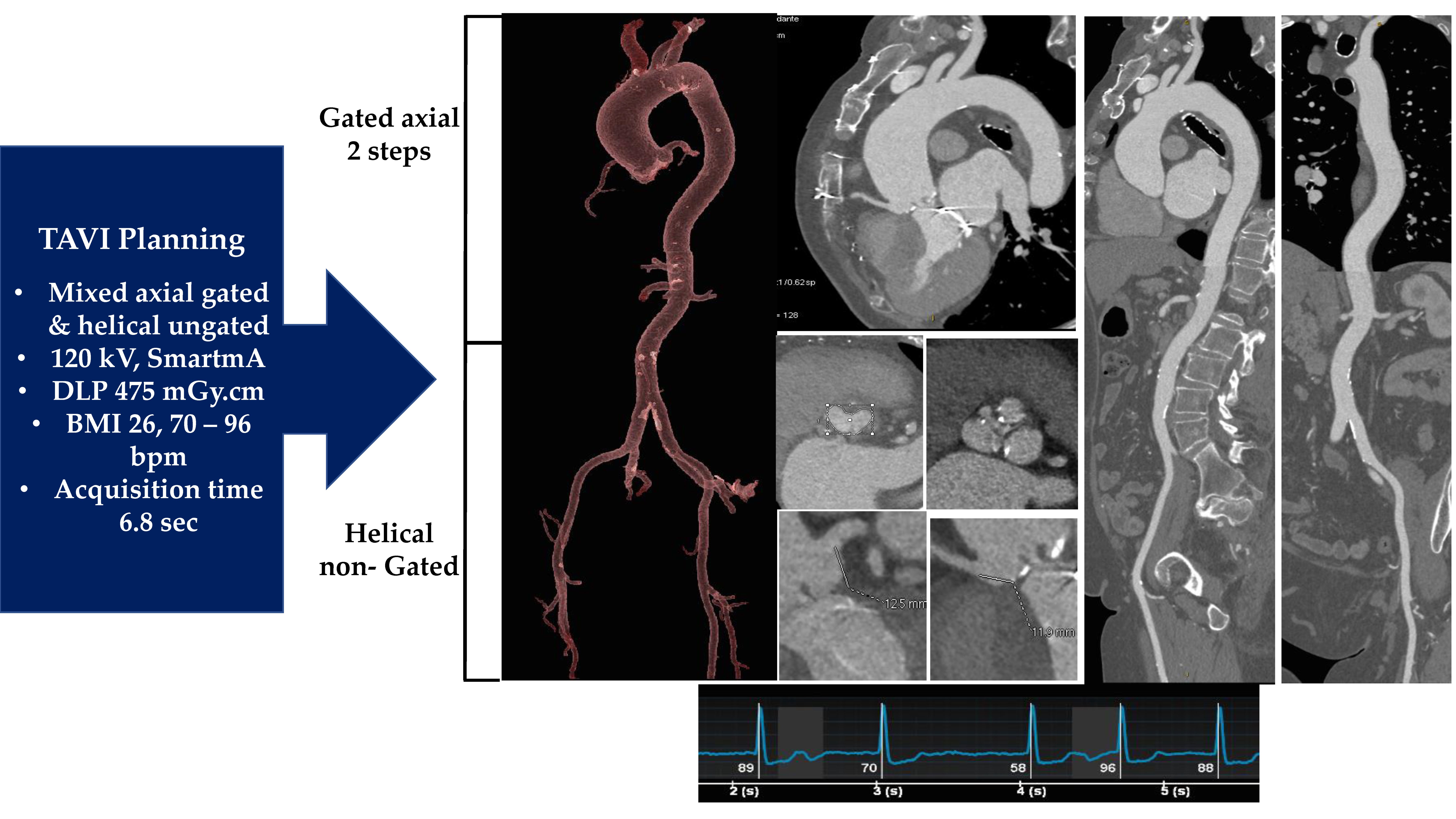
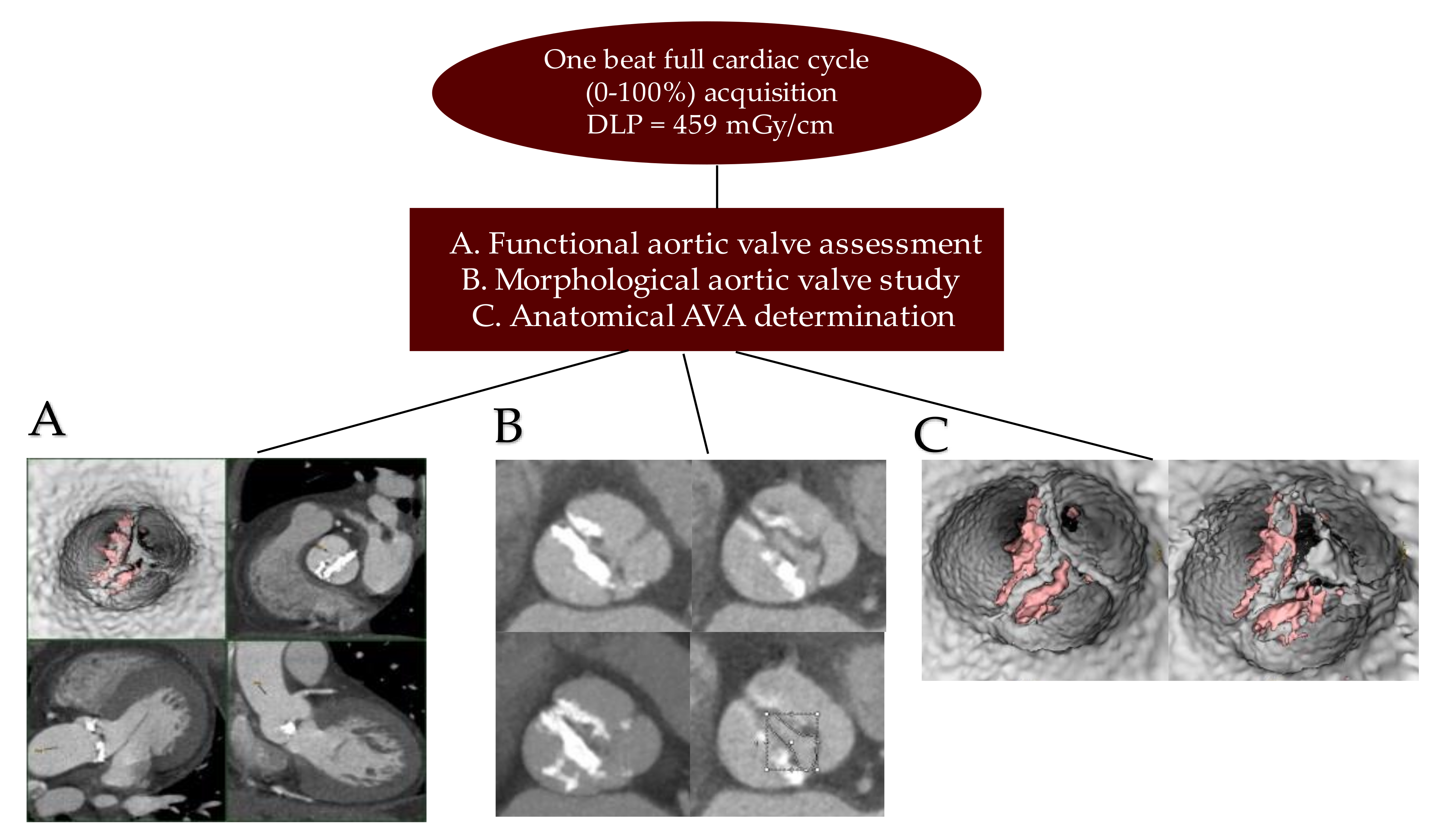
2.2. Medical Image Processing
2.3. Analysis Suitable Model
2.3.1. Native Aortic Valve Model
2.3.2. Prosthesis Model and Material Model
2.3.3. Finite Element Analyses
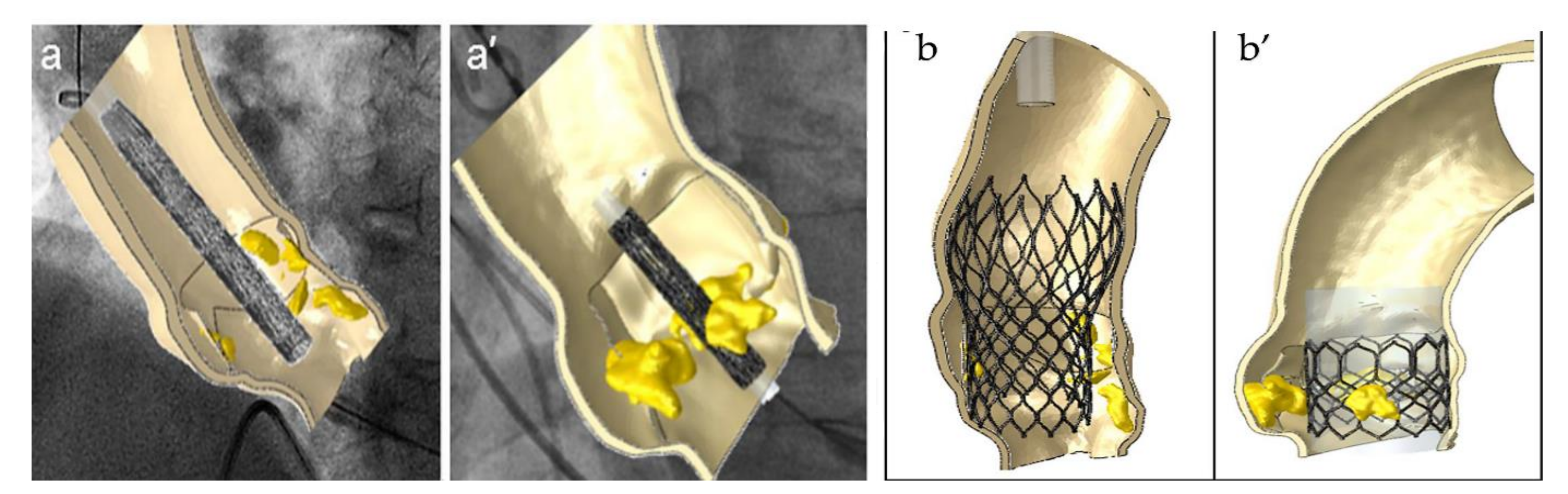
3. Insight on the Use of Biomechanical Evaluation to Predict Paravalvular Aortic Regurgitation
4. Discussion
4.1. Evidence to Deploy Biomechanical Evaluation and to Definitively Accept the Use of Transcatheter Heart Valve Implantation as a New Paradigm Shift
4.2. Biomechanics Computational Modeling to Give Consistency to The Paradigm Shift
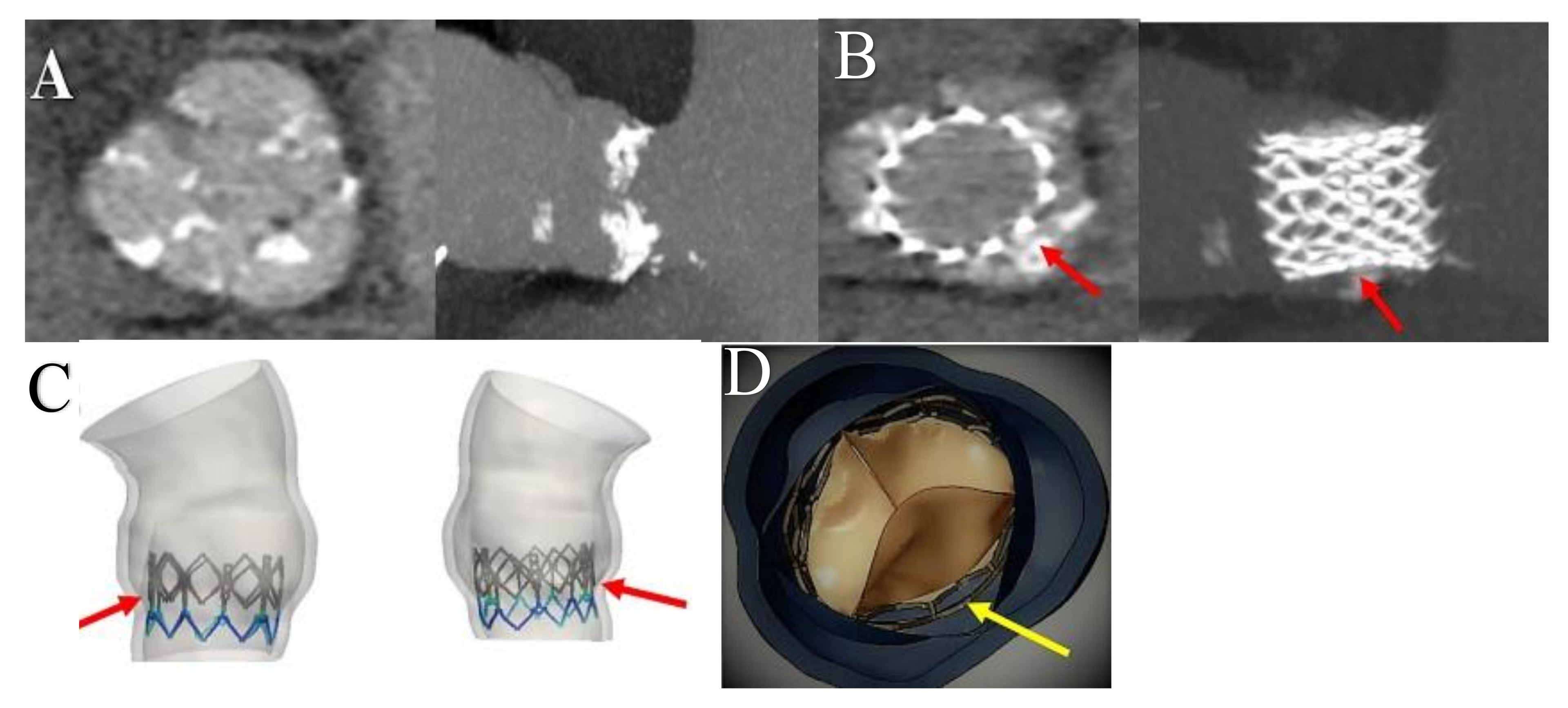
4.2.1. Paravalvular Aortic Regurgitation
4.2.2. Transcatheter Heart Valve Thrombosis
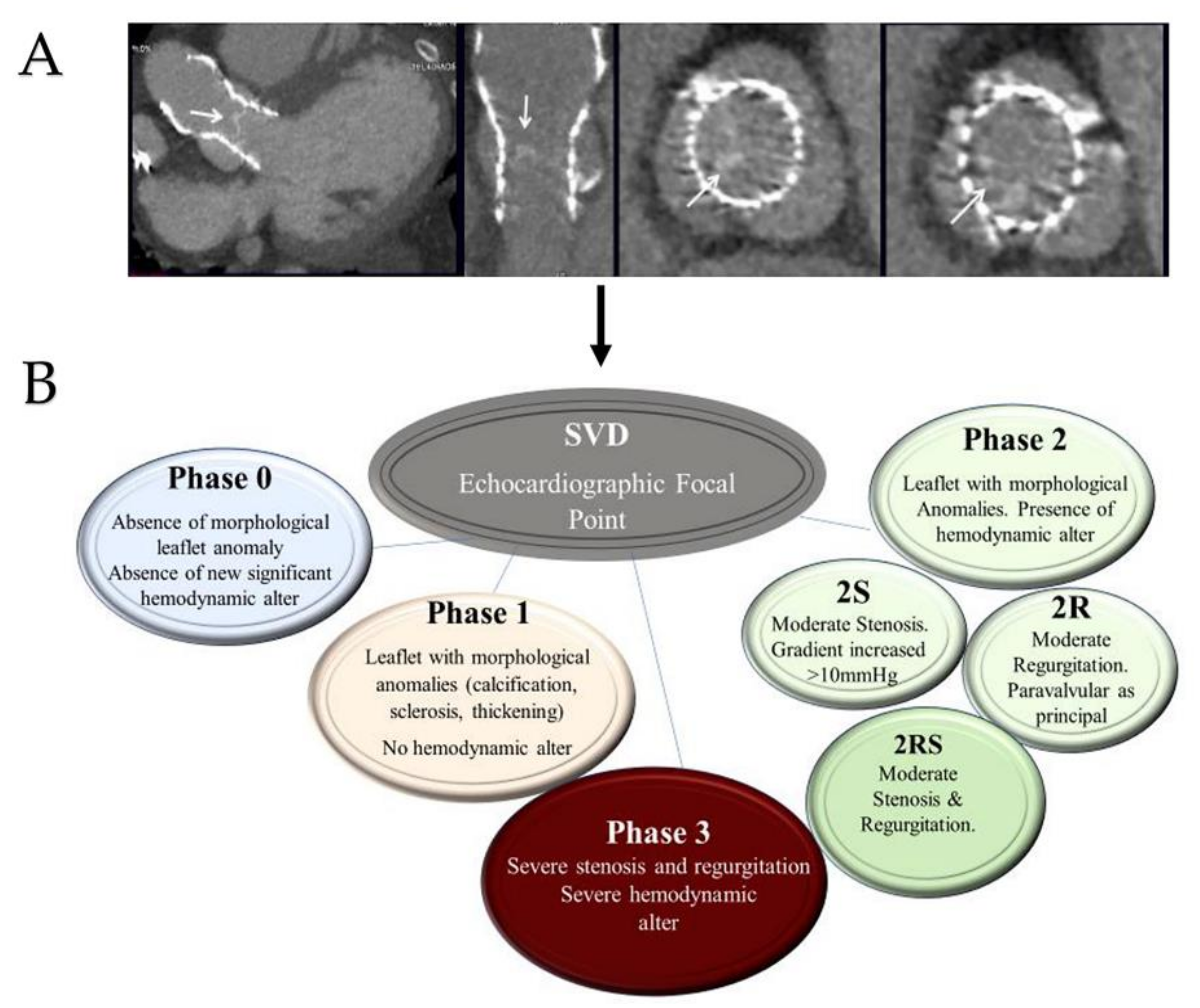
4.2.3. Structural Valve Degeneration
5. Conclusions
6. Limitations
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACC | American College of Cardiology |
| AHA | American Heart Association |
| AR | aortic regurgitation |
| AVS | aortic valve stenosis |
| CT | computed tomography |
| COR | class of recommendation |
| DLP | Dose Length Product |
| ESC | European Society of Cardiology |
| FEA | finite element analysis |
| HF | heart failure |
| LOE | level of evidence |
| LVF | left ventricular function |
| mGy | microgray |
| MDCT | multi-detector row computed tomography |
| PA | pulmonary artery |
| PAVR | paravalvular aortic regurgitation |
| RCT | randomized clinical trial |
| STL | stereolitographic |
| SVD | structural valve degeneration |
| SAVR | surgical aortic valve replacement |
| TAVI | transcatheter aortic valve implantation |
| TVT | transcatheter valve therapy |
| THV | transcatheter heart valve |
| VARC | Valve Academic Research Consortium |
References
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis first human case description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef]
- Webb, J.G.; Altwegg, L.; Boone, R.H. Transcatheter aortic valve implantation: Impact on clinical and valve-related outcomes. Circulation 2009, 119, 3009–3016. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk Patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Fontana, G.P.; Jilaihawi, H. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N. Engl. J. Med. 2012, 366, 1696–1704. [Google Scholar] [CrossRef]
- Kodali, S.K.; Williams, M.R.; Smith, C.R. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N. Engl. J. Med. 2012, 366, 1686–1695. [Google Scholar] [CrossRef]
- Falk, V. Transcatheter aortic valve replacement indications should not be expanded to lower-risk and younger patients. Circulation 2014, 130, 2332–2342. [Google Scholar] [CrossRef][Green Version]
- Adams, D.H.; Popma, J.J.; Reardon, M.J. Transcatheter aortic-valve replacement with a selfexpanding prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Smith, C.R. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1), a randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef]
- Kapadia, S.R.; Leon, M.B.; Makkar, R.R.; PARTNER trial investigators. 5-Year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1), a randomised controlled trial. Lancet 2015, 385, 2485–2491. [Google Scholar] [CrossRef]
- Deeb, G.M.; Reardon, M.J.; Chetcuti, S. 3-Year outcomes in high-risk patients who underwent surgical or transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2016, 67, 2565–2574. [Google Scholar] [CrossRef]
- Siemieniuk, R.A.; Agoritsas, T.; Manja, V. Transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at low and intermediate risk: Systematic review and meta-analysis. BMJ 2016, 354, i5130. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J. Transcatheter or surgical aortic-valve replacement in intermediate risk patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Thourani, V.H.; Kodali, S.; Makkar, R.R. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: A propensity score analysis. Lancet 2016, 387, 2218–2225. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; SURTAVI Investigators. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J. Transcatheter aortic-valve replacement with a selfexpanding valve in low-risk patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Makkar, R.R.; Thourani, V.H.; Mack, M.J.; PARTNER 2 Investigators. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 799–809. [Google Scholar]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Gentile, F. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. ESC/EACTS Scientific Document Group; ESC National Cardiac Societies. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Spadaccio, C.; Fraldi, M.; Sablayrolles, J.L.; Nappi, F.J. TAVI in Lower Risk Patients: Revolution or Nonsense? Keep Calm and Select Patients. J. Am. Coll. Cardiol. 2016, 67, 1380–1381. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Sablayrolles, J.L. Pushing the Limits in Transcatheter Aortic Valve Replacement: High-Volume Center’s Effect, Overconfidence, or Something Else? JACC Cardiovasc. Interv. 2016, 9, 2186–2188. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Sablayrolles, J.L. Delayed prosthesis malposition after transcatheter aortic valve implantation causing coronaries obstruction. Eur. J. Cardiothorac. Surg. 2017, 52, 1227–1228. [Google Scholar] [CrossRef]
- Attias, D.; Nejjari, M.; Nappi, F. How to treat severe symptomatic structural valve deterioration of aortic surgical bioprosthesis: Transcatheter valve-in-valve implantation or redo valve surgery? Eur J Cardiothorac Surg. 2018, 54, 977–985. [Google Scholar] [CrossRef]
- Reardon, M.J.; Adams, D.H.; Kleiman, N.S. 2-year outcomes in patients undergoing surgical or selfexpanding transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2015, 66, 113–121. [Google Scholar] [CrossRef]
- Siontis, G.C.M.; Overtchouk, P.; Cahill, T.J. Transcatheter aortic valve implantation vs. Surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: An updated meta-analysis. Eur. Heart J. 2019, 40, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Didier, R.; Eltchaninoff, H.; Donzeau-Gouge, P. Five-Year Clinical Outcome and Valve Durability After Transcatheter Aortic Valve Replacement in High-Risk Patients. Circulation 2018, 138, 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- Panico, R.A.; Giannini, C.; De Carlo, M. Long-term results and durability of the CoreValve transcatheter aortic bioprosthesis: Outcomes beyond five years. EuroIntervention 2019, 14, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Durand, E.; Sokoloff, A.; Urena-Alcazar, M. Assessment of Long-Term Structural Deterioration of Transcatheter Aortic Bioprosthetic Valves Using the New European Definition. Circ. Cardiovasc. Interv. 2019, 12, e007597. [Google Scholar] [CrossRef]
- Mack, M.; Carroll, J.D.; Thourani, V.; Vemulapalli, S.; Squiers, J.; Manandhar, P. Transcatheter Mitral Valve Therapy in the United States: A Report From the STS-ACC TVT Registry. J. Am. Coll. Cardiol. 2021, 78, 2326–2353. [Google Scholar] [CrossRef]
- Thyregod, H.G.H.; Ihlemann, N.; Jørgensen, T.H. Five-Year Clinical and Echocardiographic Outcomes from the Nordic Aortic Valve Intervention (NOTION) Randomized Clinical Trial in Lower Surgical Risk Patients. Circulation, 2019; Online ahead of print. [Google Scholar] [CrossRef]
- Søndergaard, L.; Ihlemann, N.; Capodanno, D. Durability of Transcatheter and Surgical Bioprosthetic Aortic Valves in Patients at Lower Surgical Risk. J. Am. Coll. Cardiol. 2019, 73, 546–553. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhang, X.W.; Lan, R.F.; Chen, Z.; Wang, L.; Xu, W.; Xu, B. Long-term and Temporal Outcomes of Transcatheter Versus Surgical Aortic-valve Replacement in Severe Aortic Stenosis: A Meta-analysis. Ann Surg. 2021, 273, 459–466. [Google Scholar] [CrossRef]
- Zajarias, A.; Cribier, A.G. Outcomes and safety of percutaneous aortic valve replacement. J. Am. Coll. Cardiol. 2009, 53, 1829–1836. [Google Scholar] [CrossRef]
- Generaux, P.; Head, S.J.; Hahn, R.; Daneault, B.; Kodali, S.; Williams, M.R.; van Mieghem, N. Paravalvular leak after transcatheter aortic valve replacement: The new Achilles’ heel? J. Am. Coll. Cardiol. 2013, 61, 1125–1136. [Google Scholar] [CrossRef]
- Blanke, P.; Siepe, M.; Reinöhl, J.; Zehender, M.; Beyersdorf, F.; Schlensak, C.; Langer, M.; Pache, G. Assessment of aortic annulus dimensions for Edwards SAPIEN Transapical Heart Valve implantation by computed tomography: Calculating average diameter using a virtual ring method. Eur. J. Cardiothorac. Surg. 2010, 38, 750–758. [Google Scholar] [CrossRef][Green Version]
- Détaint, D.; Lepage, L.; Himbert, D.; Brochet, E.; Messika-Zeitoun, D.; Iung, B.; Vahanian, A. Determinants of significant paravalvular regurgitation after transcatheter aortic valve: Implantation impact of device and annulus discongruence. JACC Cardiovasc. Interv. 2009, 2, 821–827. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Head, S.J.; Généreux, P. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. J. Am. Coll. Cardiol. 2012, 60, 1438–1454. [Google Scholar] [CrossRef]
- Nappi, F.; Mazzocchi, L.; Avtaar Singh, S.S. Complementary Role of the Computed Biomodelling through Finite Element Analysis and Computed Tomography for Diagnosis of Transcatheter Heart Valve Thrombosis. Biomed Res Int. 2018, 2018, 1346308. [Google Scholar] [CrossRef]
- Morganti, S.; Conti, M.; Aiello, M. Simulation of transcatheter aortic valve implantation through patient- specific finite element analysis: Two clinical cases. J. Biomech. 2014, 47, 2547–2555. [Google Scholar] [CrossRef]
- Morganti, S.; Brambilla, N.; Petronio, A.S. Prediction of patient-specific post-operative outcomes of TAVI procedure: The impact of the positioning strategy on valve performance. J. Biomech. 2016, 49, 2513–2519. [Google Scholar] [CrossRef]
- Bianchi, M.; Marom, G.; Ghosh, R.P.; Rotman, O.M.; Parikh, P.; Gruberg, L.; Bluestein, D. Patient-specific simulation of transcatheter aortic valve replacement: Impact of deployment options on paravalvular leakage. Biomech. Model Mechanobiol. 2019, 18, 435–451. [Google Scholar] [CrossRef]
- Wang, Q.; Sirois, E.; Sun, W. Patient-specific modeling of biomechanical interaction in transcatheter aortic valve deployment. J. Biomech. 2012, 45, 1965–1971. [Google Scholar] [CrossRef]
- De Jaegere, P. Patient-specific computer modeling to predict aortic regurgitation after transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 2016, 9, 508–512. [Google Scholar] [CrossRef]
- Capelli, C. Patient-specific simulations of transcatheter aortic valve stent implantation. Med. Biol. Eng. Comput. 2012, 50, 183–192. [Google Scholar] [CrossRef]
- Bosmans, B.; Famaey, N.; Verhoelst, E.; Bosmans, J.; Vander Sloten, J. A validated methodology for patient specific computational modeling of self-expandable transcatheter aortic valve implantation. J. Biomech. 2016, 49, 2824–2830. [Google Scholar] [CrossRef]
- Bianchi, M.; Marom, G.; Ghosh, R.P.; Fernandez, H.A.; Taylor, J.R., Jr.; Slepian, M.J.; Bluestein, D. Effect of balloon-expandable transcatheter aortic valve replacement positioning: A patient-specific numerical model. Artif Organs. 2016, 40, E292–E304. [Google Scholar] [CrossRef]
- Bosi, G.M.; Capelli, C.; Hong Cheang, M.; Delahunty, N.; Mullen, M.; Taylor, A.M.; Schievano, S. Population-specific material properties of the implantation site for transcatheter aortic valve replacement finite element simulations. J. Biomech. 2018, 71, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Dowling, C.; Firoozi, S.; Brecker, S.J. First-in-Human Experience with Patient-Specific Computer Simulation of TAVR in Bicuspid Aortic Valve Morphology. JACC Cardiovasc. Interv. 2020, 13, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Mazzocchi, L.; Spadaccio, C.; Attias, D.; Timofeva, I.; Macron, L.; Iervolino, A.; Morganti, S.; Auricchio, F. CoreValve vs. Sapien 3 Transcatheter Aortic Valve Replacement: A Finite Element Analysis Study. Bioengineering 2021, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Mazzocchi, L.; Timofeva, I.; Macron, L.; De Cecco, C.N.; Morganti, S.; Auricchio, F.; Nappi, F. Bioengineering Case Study to Evaluate Complications of Adverse Anatomy of Aortic Root in Transcatheter Aortic Valve Replacement: Combining Biomechanical Modelling with CT imaging. Bioengineering 2020, 7, 121. [Google Scholar] [CrossRef]
- Nappi, F.; Mazzocchi, L.; Timofeva, I.; Macron, L.; Morganti, S.; Avtaar Singh, S.S.; Attias, D.; Congedo, A.; Auricchio, F. A Finite Element Analysis Study from 3D CT to Predict Transcatheter Heart Valve Thrombosis. Diagnostics 2020, 10, 183. [Google Scholar] [CrossRef]
- Bonhoeffer, P.; Boudjemline, Y.; Saliba, Z. Transcatheter implantation of a bovine valve in pulmonary position: A lamb study. Circulation 2000, 102, 813–816. [Google Scholar] [CrossRef]
- Nappi, F.; Nenna, A.; Larobina, D.; Carotenuto, A.R.; Jarraya, M.; Spadaccio, C.; Fraldi, M.; Chello, M.; Acar, C.; Carrel, T. Simulating the ideal geometrical and biomechanical parameters of the pulmonary autograft to prevent failure in the Ross operation. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 269–276. [Google Scholar] [CrossRef]
- Nappi, F.; Nenna, A.; Lemmo, F.; Chello, M.; Chachques, J.C.; Acar, C.; Larobina, D. Finite Element Analysis Investigate Pulmonary Autograft Root and Leaflet Stresses to Understand Late Durability of Ross Operation. Biomimetics 2020, 5, 37. [Google Scholar] [CrossRef]
- Xuan, Y.; Krishnan, K.; Ye, J.; Dvir, D.; Guccione, J.M.; Ge, L. Stent and leaflet stresses in a 26-mm first-generation balloon-expandable transcatheter aortic valve. J. Thorac. Cardiovasc. Surg. 2017, 153, 1065–1073. [Google Scholar] [CrossRef]
- Li, K.; Sun, W. Simulated thin pericardial bioprosthetic valve leaflet deformation under static pressure-only loading conditions: Implications for percutaneous valves. Ann. Biomed. Eng. 2010, 38, 2690–2701. [Google Scholar] [CrossRef]
- Nappi, F.; Carotenuto, A.R.; Cutolo, A. Compliance mismatch and compressive wall stresses drive anomalous remodelling of pulmonary trunks reinforced with Dacron grafts. J. Mech. Behav. Biomed. Mater. 2016, 63, 287–302. [Google Scholar] [CrossRef]
- Nappi, F.; Fraldi, M.; Spadaccio, C. Biomechanics drive histological wall remodeling of neoaortic root: A mathematical model to study the expression levels of ki 67, metalloprotease, and apoptosis transition. J. Biomed. Mater. Res. A. 2016, 104, 2785–2793. [Google Scholar] [CrossRef]
- Nappi, F.; Attias, D.; Avtaar Singh, S.S. Finite element analysis applied to the transcatheter mitral valve therapy: Studying the present, imagining the future. J. Thorac. Cardiovasc. Surg. 2019, 157, e149–e151. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Al-Attar, N.; Acar, C. The Ross procedure at the crossroads: Lessons from biology: Is Dr Ross’s dream concluded? Int. J. Cardiol. 2015, 178, 37–39. [Google Scholar] [CrossRef]
- Nataf, P.; Guettier, C.; Bourbon, A.; Nappi, F.; Lima, L.; Dorent, R.; Pavie, A.; Gandjbakhch, I. Influence of arterial allograft preparation techniques on chronic vascular rejection: A histological study. Transplant Proc. 1996, 28, 2890–2892. [Google Scholar]
- Nappi, F.; Carotenuto, A.R.; Avtaar Singh, S.S.; Mihos, C.; Fraldi, M. Euler’s Elastica-Based Biomechanics of the Papillary Muscle Approximation in Ischemic Mitral Valve Regurgitation: A Simple 2D Analytical Model. Materials 2019, 12, 1518. [Google Scholar] [CrossRef]
- Rama, A.; Nappi, F.; Praschker, B.G.; Gandjbakhch, I. Papillary muscle approximation for ischemic mitral valve regurgitation. J. Card Surg. 2008, 23, 733–735. [Google Scholar] [CrossRef]
- Spadaccio, C.; Nappi, F.; De Marco, F. Implantation of a Poly-L-Lactide GCSF-Functionalized Scaffold in a Model of Chronic Myocardial Infarction. J. Cardiovasc. Transl. Res. 2017, 10, 47–65. [Google Scholar] [CrossRef]
- Spadaccio, C.; Nappi, F.; De Marco, F. Preliminary In Vivo Evaluation of a Hybrid Armored Vascular Graft Combining Electrospinning and Additive Manufacturing Techniques. Drug Target Insights 2016, 10 (Suppl. S1), 1–7. [Google Scholar] [CrossRef]
- Nappi, F.; Carotenuto, A.R.; Di Vito, D.; Spadaccio, C.; Acar, C.; Fraldi, M. Stress-shielding, growth and remodeling of pulmonary artery reinforced with copolymer scaffold and transposed into aortic position. Biomech. Model Mechanobiol. 2016, 15, 1141–1157. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Prakoso, A.T.; Basri, H.; van der Heide, E. Computational Contact Pressure Prediction of CoCrMo, SS 316L and Ti6Al4V Femoral Head against UHMWPE Acetabular Cup under Gait Cycle. J. Funct. Biomater. 2022, 13, 64. [Google Scholar] [CrossRef]
- Smuts, A.N.; Blaine, D.C.; Scheffer, C.; Weich, H.; Doubell, A.F.; Dellimore, K.H. Application of finite element analysis to the design of tissue leaflets for a percutaneous aortic valve. J. Mech. Behav. Biomed. Mater. 2011, 4, 85–98. [Google Scholar] [CrossRef]
- Sun, W.; Li, K.; Sirois, E. Simulated elliptical bioprosthetic valve deformation: Implications for asymmetric transcatheter valve deployment. J. Biomech. 2010, 43, 3085–3090. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, F.; Conti, M.; Morganti, S.; Reali, A. Simulation of transcatheter aortic valve implantation: A patient-specific finite element approach. Comput. Methods Biomech. Biomed. Engin. 2014, 17, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Sun, W. Comparison of transcatheter aortic valve and surgical bioprosthetic valve durability: A fatigue simulation study. J. Biomech. 2015, 48, 3026–3034. [Google Scholar] [CrossRef] [PubMed]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Akbar, I.; Basri, H.; van der Heide, E.; Jamari, J. Tresca Stress Simulation of Metal-on-Metal Total Hip Arthroplasty during Normal Walking Activity. Materials 2021, 14, 7554. [Google Scholar] [CrossRef]
- Raby, J.; Newton, J.D.; Dawkins, S.; Lewis, A.J.M. Cardiovascular magnetic resonance facilitates entirely contrast-free transcatheter aortic valve implantation: Case report. Eur. Heart J. Case Rep. 2021, 5, ytab378. [Google Scholar] [CrossRef]
- Bittner, D.O.; Arnold, M.; Klinghammer, L.; Schuhbaeck, A.; Hell, M.M.; Muschiol, G.; Gauss, S.; Lell, M.; Uder, M.; Hoffmann, U. Contrast volume reduction using third generation dual source computed tomography for the evaluation of patients prior to transcatheter aortic valve implantation. Eur. Radiol. 2016, 26, 4497–4504. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Antiga, L.; Piccinelli, M.; Botti, L.; Ene Iordache, B.; Remuzzi, A.; Steinman, D.A. An image-based modeling framework for patient-specific computational hemodynamics. Med. Biol. Eng. Comput. 2008, 46, 1097–1112. [Google Scholar] [CrossRef]
- Piccinelli, M.; Veneziani, A.; Steinman, D.A.; Remuzzi, A.; Antiga, L. A framework for geometric analysis of vascular structures: Application to cerebral aneurysms. IEEE Trans. Med. Imaging 2009, 28, 1141–1155. [Google Scholar] [CrossRef]
- Marchandise, E.; Geuzaine, C.; Remacle, J.F. Cardiovascular and lung mesh generation based on centerlines. Int. J. Numer. Method Biomed. Eng. 2013, 29, 665–682. [Google Scholar] [CrossRef]
- Dillard, S.I.; Mousel, J.A.; Shrestha, L.; Raghavan, M.L.; Vigmostad, S.C. From medical images to flow computations without user-generated meshes. Int. J. Numer. Method Biomed. Eng. 2014, 30, 1057–1083. [Google Scholar] [CrossRef]
- Xiong, F.L.; Goetz, W.A.; Chong, C.K.; Chua, Y.L.; Pfeifer, S.; Wintermantel, E.; Yeo, J.H. Finite element investigation of stentless pericardial aortic valves: Relevance of leaflet geometry. Ann Biomed Eng. 2010, 38, 1908–1918. [Google Scholar] [CrossRef]
- Stradins, P.; Lacis, R.; Ozolanta, I.; Purina, B.; Ose, V.; Feldmane, L.; Kasyanov, V. Comparison of biomechanical and structural properties between human aortic and pulmonary valve. Eur. J. Cardiothorac Surg. 2004, 26, 634–639. [Google Scholar] [CrossRef]
- Gnyaneshwar, R.; Kumar, R.K.; Balakrishnan, K.R. Dynamic analysis of the aortic valve using a finite element model. Ann. Thorac. Surg. 2002, 73, 1122–1129. [Google Scholar] [CrossRef]
- Selvadurai, A.P.S. Deflections of a rubber membrane. J. Mech. Phys. Solids 2006, 54, 1093–1119. [Google Scholar] [CrossRef]
- Yeoh, O.H. Some forms of the strain energy function for rubber. Rubber Chem. Technol. 1993, 66, 754–771. [Google Scholar] [CrossRef]
- Auricchio, F.; Ferrara, A.; Morganti, S. Comparison and critical analysis of invariant-based models with respect to their ability in fitting human aortic valve data. Ann. Solid Struct. Mech. 2012, 4, 1–14. [Google Scholar] [CrossRef]
- Hanlon, J.G.; Suggit, R.W.; Love, J.W. Pre-use intraoperative testing of autologous tissue for valvular surgery: A proof-of-concept study. J. Heart Valve Dis. 1999, 8, 614–623; [Google Scholar]
- Lee, J.M.; Haberer, S.A.; Boughner, D.R. The bovine pericardial xenograft: I. Effect of fixation in aldehydes without constraint on the tensile viscoelastic properties of bovine pericardium. J. Biomed. Mater Res. 1989, 23, 457–475. [Google Scholar] [CrossRef]
- Trowbridge, E.A.; Black, M.M.; Daniel, C.L. The mechanical response of glutaraldehyde fixed bovine pericardium to uniaxial load. J. Mater. Sci. 2011, 20, 114–140. [Google Scholar] [CrossRef]
- Willson, A.B.; Rodés-Cabau, J.; Wood, D.A.; Leipsic, J.; Cheung, A.; Toggweiler, S.; Binder, R.K.; Freeman, M. Transcatheter aortic valve replacement with the St. Jude Medical Portico valve: First-in-human experience. J. Am. Coll. Cardiol. 2012, 60, 581–586. [Google Scholar] [CrossRef]
- Möllmann, H.; Diemert, P.; Grube, E.; Baldus, S.; Kempfert, J.; Abizaid, A. Symetis ACURATE TF™ aortic bioprosthesis. EuroIntervention 2013, 9, S107–S110. [Google Scholar] [CrossRef]
- Meredith, I.T.; Hood, K.L.; Haratani, N.; Allocco, D.J.; Dawkins, K.D. Boston Scientific Lotus valve. EuroIntervention 2012, 8, Q70–Q74. [Google Scholar] [CrossRef]
- Feldman, T.E.; Reardon, M.J.; Rajagopal, V. Effect of Mechanically Expanded vs Self-Expanding Transcatheter Aortic Valve Replacement on Mortality and Major Adverse Clinical Events in High-Risk Patients Wit50,52h Aortic Stenosis: The REPRISE III Randomized Clinical Trial. JAMA 2018, 319, 27–37. [Google Scholar] [CrossRef]
- Wiggers, C.J. Circulatory Dynamics: Physiological Studies. JAMA 1952, 150, 1357. [Google Scholar]
- Delgado, V.; Ng, A.C.; van de Veire, N.R.; van der Kley, F.; Schuijf, J.D.; Tops, L.F. Transcatheter aortic valve implantation: Role of multi-detector row computed tomography to evaluate prosthesis positioning and deployment in relation to valve function. Eur. Heart J. 2010, 31, 1114–1123. [Google Scholar] [CrossRef]
- Santos, N.; de Agustín, J.A.; Almería, C.; Gonçalves, A.; Marcos-Alberca, P.; Fernández-Golfín, C. Prosthesis/annulus discongruence assessed by three-dimensional transoesophageal echocardiography: A predictor of significant paravalvular aortic regurgitation after transcatheter aortic valve implantation. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 931–937. [Google Scholar] [CrossRef]
- Pontone, G.; Andreini, D.; Bartorelli, A.L.; Bertella, E.; Cortinovis, S.; Mushtaq, S.; Annoni, A. Aortic annulus area assessment by multidetector computed tomography for predicting paravalvular regurgitation in patients undergoing balloon-expandable transcatheter aortic valve implantation: A comparison with transthoracic and transoesophageal echocardiography. Am. Heart J. 2012, 164, 576–584. [Google Scholar]
- Katsanos, S.; Ewe, S.H.; Debonnaire, P.; van der Kley, F.; de Weger, A.; Palmen, M.; Scholte, A.J. Multidetector row computed tomography parameters associated with paravalvular regurgitation after transcatheter aortic valve implantation. Am. J. Cardiol. 2013, 112, 1800–1806. [Google Scholar] [CrossRef]
- Madukauwa-David, I.D.; Midha, P.A.; Sharma, R.; McLain, K.; Mitra, R.; Crawford, K.; Yoon, S.H. Characterization of aortic root geometry in transcatheter aortic valve replacement patients. Catheter. Cardiovasc. Interv. 2019, 93, 134–140. [Google Scholar] [CrossRef]
- Feuchtner, G.; Plank, F.; Bartel, T.; Mueller, S.; Leipsic, J.; Schachner, T.; Müller, L. Prediction of paravalvular regurgitation after transcatheter aortic valve implantation by computed tomography: Value of aortic valve and annular calcification. Ann. Thorac. Surg. 2013, 96, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Eker, A.; Sozzi, F.B.; Civaia, F.; Bourlon, F. Aortic annulus rupture during transcatheter aortic valve implantation: Safe aortic root replacement. Eur. J. Cardiothorac. Surg. 2012, 41, 1205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Q.; Kodali, S.; Primiano, C.; Sun, W. Simulations of transcatheter aortic valve implantation: Implications for aortic root rupture. Biomech. Model Mechanobiol. 2015, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, F.; Conti, M.; Morganti, S.; Totaro, P. A computational tool to support pre-operative planning of stentless aortic valve implant. Med. Eng. Phys. 2011, 33, 1183–1192. [Google Scholar] [CrossRef]
- Auricchio, F.; Conti, M.; Ferrara, A.; Morganti, S.; Reali, A. Patient-specific simulation of a stentless aortic valve implant: The impact of fibres on leaflet performance. Comput. Methods Biomech. Biomed. Eng. 2012, 17, 740–749. [Google Scholar] [CrossRef]
- Morlacchi, S.; Colleoni, S.G.; Cárdenes, R.; Chiastra, C.; Diez, J.L.; Larrabide, I.; Migliavacca, F. Patient-specific simulations of stenting procedures in coronary bifurcations: Two clinical cases. Med. Eng. Phys. 2013, 35, 1272–1281. [Google Scholar] [CrossRef]
- Grover, A.; Gorman, K.; Dall, T.M.; Jonas, R.; Lytle, B.; Shemin, R. Shortage of Cardiothoracic Surgeons Is Likely by 2020. Circulation 2009, 120, 488–494. [Google Scholar] [CrossRef]
- Kuhn, T.S. The Structure of Scientific Revolutions. Am. Hist. Rev. 1963, 68, 700–701. [Google Scholar]
- Holmes, D.R., Jr.; Firth, B.G.; Wood, D.L. Paradigm shifts in cardiovascular medicine. J. Am. Coll. Cardiol. 2004, 43, 507–512. [Google Scholar] [CrossRef]
- Sacks, C.A.; Jarcho, J.A.; Curfman, G.D. Paradigm shifts in heart-failure therapy—A timeline. New Engl. J. Med. 2014, 371, 989–991. [Google Scholar] [CrossRef]
- Kanwar, A.; Thaden, J.J.; Nkomo, V.T. Management of patients with aortic valve stenosis. Mayo Clin. Proc. 2018, 93, 488–508. [Google Scholar] [CrossRef]
- Pilgrim, T.; Windecker, S. Transcatheter aortic valve replacement: Lessons gained from extreme-risk patients. J. Am. Coll. Cardiol. 2015, 66, 1335–1338. [Google Scholar] [CrossRef]
- Bagur, R.; Rodés-Cabau, J.; Gurvitch, R.; Dumont, É.; Velianou, J.L.; Manazzoni, J.; Toggweiler, S. Need for permanent pacemaker as a complication of transcatheter aortic valve implantation and surgical aortic valve replacement in elderly patients with severe aortic stenosis and similar baseline electrocardiographic findings. JACC Cardiovasc. Interv. 2012, 5, 540–551. [Google Scholar] [CrossRef]
- Van der Boon, R.M.; Nuis, R.J.; Van Mieghem, N.M.; Jordaens, L.; Rodés-Cabau, J.; van Domburg, R.T.; Serruys, P.W. New conduction abnormalities after TAVI—frequency and causes. Nat. Rev. Cardiol. Nat. Rev. Cardiol. 2012, 9, 454–463. [Google Scholar] [CrossRef]
- Ribeiro, H.B. Coronary obstruction following transcatheter aortic valve implantation: A systematic review. JACC Cardiovasc. Interv. 2013, 6, 452–461. [Google Scholar] [CrossRef]
- Scotten, L.N.; Siegel, R. Thrombogenic potential of transcatheter aortic valve implantation with trivial paravalvular leakage. Ann. Transl. Med. 2014, 2, 43. [Google Scholar] [CrossRef]
- Maisano, F.; Taramasso, M.; Nietlispach, F. Prognostic influence of paravalvular leak following TAVI: Is aortic regurgitation an active incremental risk factor or just a mere indicator? Eur. Heart J. 2015, 36, 413–415. [Google Scholar] [CrossRef]
- Gilbert, O.N. Comparison of paravalvular aortic leak characteristics in the Medtronic CoreValve versus Edwards Sapien Valve: Paravalvular aortic leak characteristics. Catheter. Cardiovasc. Interv. 2018, 92, 972–980. [Google Scholar] [CrossRef]
- Still, S.; Szerlip, M.; Mack, M. TAVR Vs. SAVR in intermediate-risk patients: What influences our choice of therapy. Curr. Cardiol. Rep. 2018, 20, 82. [Google Scholar] [CrossRef]
- Pibarot, P.; Hahn, R.T.; Weissman, N.J.; Monaghan, M.J. Assessment of paravalvular regurgitation following TAVR: A proposal of unifying grading scheme. JACC Cardiovasc. Imag. 2015, 8, 340–360. [Google Scholar] [CrossRef]
- Hatoum, H.; Yousefi, A.; Lilly, S.; Maureira, P.; Crestanello, J.; Dasi, L.P. An in-vitro evaluation of turbulence after transcatheter aortic valve implantation. J. Thorac. Cardiovasc. Surg. 2018, 1, 1. [Google Scholar] [CrossRef]
- Abdelghani, M.; Soliman, O.I.I.; Schultz, C.; Vahanian, A.; Serruys, P.W. Adjudicating paravalvular leaks of transcatheter aortic valves: A critical appraisal. Eur. Heart J. 2016, 37, 2627–2644. [Google Scholar] [CrossRef]
- Eggebrecht, H.; Doss, M.; Schmermund, A.; Nowak, B.; Krissel, J.; Voigtländer, T. Interventional options for severe aortic regurgitation after transcatheter aortic valve implantation: Balloons, snares, valve-in-valve. Clin. Res. Cardiol. 2012, 101, 503–507. [Google Scholar] [CrossRef]
- Dvir, D. Multicenter evaluation of Edwards SAPIEN positioning during transcatheter aortic valve implantation with correlates for device movement during final deployment. JACC Cardiovasc. Interv. 2012, 5, 563–570. [Google Scholar] [CrossRef][Green Version]
- Nombela-Franco, L. Predictive factors, efficacy, and safety of balloon post-dilation after transcatheter aortic valve implantation with a balloon-expandable valve. JACC Cardiovasc. Interv. 2012, 5, 499–512. [Google Scholar] [CrossRef]
- Takagi, K. Predictors of moderate-to-severe paravalvular aortic regurgitation immediately after CoreValve implantation and the impact of postdilatation. Catheter. Cardiovasc. Interv. 2011, 78, 432–443. [Google Scholar] [CrossRef] [PubMed]
- McGee, O.M.; Gunning, P.S.; McNamara, A.; McNamara, L.M. The impact of implantation depth of the Lotus™ valve on mechanical stress in close proximity to the bundle of His. Biomech Model Mechanobiol. 2018, 1, 1. [Google Scholar] [CrossRef]
- Sturla, F. Impact of different aortic valve calcification patterns on the outcome of Transcatheter Aortic Valve Implantation: A finite element study. J. Biomech. 2016, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C. Patient-specific image-based computer simulation for the prediction of valve morphology and calcium displacement after TAVI with the Medtronic CoreValve and the Edwards SAPIEN valve. EuroIntervention 2016, 11, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Rong-Hui, L.; Sheng-Ping, Z.; Li-Zhen, W.; Yu-Bo, F. Effect of stent designs on the paravalvular regurgitation of transcatheter aortic valve implantation. Int. J. Comput. Methods 2018, 16, 1842007. [Google Scholar]
- Mao, W.; Wang, Q.; Kodali, S.; Sun, W. Numerical parametric study of paravalvular leak following a transcatheter aortic valve deployment into a patient-specific aortic root. J. Biomech. Eng. 2018, 140, 101007. [Google Scholar] [CrossRef]
- Vahidkhah, K.; Azadani, A.N. Supra-annular Valve-in-Valve implantation reduces blood stasis on the transcatheter aortic valve leaflets. J. Biomech. 2017, 58, 114–122. [Google Scholar] [CrossRef]
- Latib, A.; Naganuma, T.; Abdel-Wahab, M. Treatment and clinical outcomes of transcatheter heart valve thrombosis. Circ. Cardiovasc. Interv. 2015, 8, e001779. [Google Scholar] [CrossRef]
- Stortecky, S.; Windecker, S. Stroke: An infrequent but devastating complication in cardiovascular interventions. Circulation 2012, 126, 2921–2924. [Google Scholar] [CrossRef]
- Leetmaa, T.; Hansson, N.C.; Leipsic, J. Early aortic transcatheter heart valve thrombosis: Diagnostic value of contrast-enhanced multidetector computed tomography. Circ. Cardiovasc. Interv. 2015, 8, e001596. [Google Scholar] [CrossRef]
- Hansson, N.C.; Grove, E.L.; Andersen, H.R. Transcatheter aortic heart valve thrombosis: Incidence, predisposing factors, and clinical implications. J. Am. Coll. Cardiol. 2016, 68, 2059–2069. [Google Scholar] [CrossRef]
- Wolberg, A.S.; Aleman, M.M.; Leiderman, K. Procoagulant activity in hemostasis and thrombosis: Virchow’s triad revisited. Anesth. Analg. 2012, 114, 275–285. [Google Scholar] [CrossRef]
- Turbill, P.; Beugeling, T.; Poot, A.A. Proteins involved in the Vroman effect during exposure of human blood plasma to glass and polyethylene. Biomaterials 1996, 17, 1279–1287. [Google Scholar] [CrossRef]
- Noble, S.; Asgar, A.; Cartier, R. Anatomopathological analysis after CoreValve Revalving system implantation. EuroIntervention 2009, 5, 78–85. [Google Scholar] [CrossRef]
- Makkar, R.R.; Fontana, G.; Jilaihawi, H. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N. Engl. J. Med. 2015, 373, 2015–2024. [Google Scholar] [CrossRef]
- Chakravarty, T.; Søndergaard, L.; Friedman, J.; RESOLVE; SAVORY Investigators. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: An observational study. Lancet 2017, 389, 2383–2392. [Google Scholar] [CrossRef]
- Pache, G.; Schoechlin, S.; Blanke, P. Early hypo-attenuated leaflet thickening in balloon-expandable transcatheter aortic heart valves. Eur. Heart J. 2016, 37, 2263–2271. [Google Scholar] [CrossRef]
- Vollema, E.M.; Kong, W.K.F.; Katsanos, S. Transcatheter aortic valve thrombosis: The relation between hypo-attenuated leaflet thickening, abnormal valve haemodynamics, and stroke. Eur. Heart J. 2017, 38, 1207–1217. [Google Scholar] [CrossRef]
- Nührenberg, T.G.; Hromek, J.; Kille, A. Impact of On-Clopidogrel Platelet Reactivity on Incidence of Hypoattenuated Leaflet Thickening After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 12–18. [Google Scholar] [CrossRef]
- De Backer, O.; Dangas, G.D.; Jilaihawi, H. GALILEO-4D Investigators. Reduced Leaflet Motion after Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 130–139. [Google Scholar] [CrossRef]
- Khalique, O.K.; Hahn, R.T.; Gada, H. Quantity and location of aortic valve complex calcification predicts severity and location of paravalvular regurgitation and frequency of post-dilation after balloon-expandable transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 2014, 7, 885–894. [Google Scholar] [CrossRef]
- Couture, E.L.; Lepage, S.; Masson, J.-B.; Daneault, B. Very late transcatheter heart valve thrombosis. World J. Cardiol. 2017, 9, 196–199. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J. Recommendations for the imaging assessment of prosthetic heart valves: A report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 589–590. [Google Scholar]
- Capodanno, D.; Petronio, A.S.; Prendergast, B. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: A consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. J. Cardiothorac. Surg. 2017, 52, 408–417. [Google Scholar]
- Zilberszac, R.; Gabriel, H.; Schemper, M. Outcome of combined stenotic and regurgitant aortic valve disease. J. Am. Coll. Cardiol. 2013, 61, 1489–1495. [Google Scholar] [CrossRef]
- Masters, R.G.; Walley, V.M.; Pipe, A.L. Long-term experience with the Ionescu-Shiley pericardial valve. Ann. Thorac. Surg. 1995, 60 (Suppl. S2), S288–S291. [Google Scholar] [CrossRef]
- Puvimanasinghe, J.P.; Steyerberg, E.W.; Takkenberg, J.J. Prognosis after aortic valve replacement with a bioprosthesis: Predictions based on meta-analysis and microsimulation. Circulation 2001, 103, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Furnary, A.P.; Li, H.F. Bioprosthetic aortic valve durability: A meta-regression of published studies. Ann. Thorac. Surg. 2017, 104, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, F.; Guyatt, G.H.; O’Brien, K. Prognosis after surgical replacement with a bioprosthetic aortic valve in patients with severe symptomatic aortic stenosis: Systematic review of observational studies. BMJ 2016, 354, i5065. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Nenna, A.; Petitti, T.; Spadaccio, C.; Gambardella, I.; Lusini, M.; Chello, M.; Acar, C. Long-term outcome of cryopreserved allograft for aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2018, 156, 1357–1365.e6. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, S.; Tesar, P.J.; Pearse, B. Long-term clinical outcomes after aortic valve replacement using cryopreserved aortic allograft. J. Thorac. Cardiovasc. Surg. 2014, 148, 65–72.e2. [Google Scholar] [CrossRef] [PubMed]
- Arabkhani, B.; Bekkers, J.A.; Andrinopoulou, E.R. Allografts in aortic position: Insights from a 27-year, single-center prospective study. J. Thorac. Cardiovasc. Surg. 2016, 152, 1572–1579.e3. [Google Scholar] [CrossRef]
- David, T.E.; Feindel, C.M.; Bos, J. Aortic valve replacement with Toronto SPV bioprosthesis: Optimal patient survival but suboptimal valve durability. J. Thorac. Cardiovasc. Surg. 2008, 135, 19–24. [Google Scholar] [CrossRef]
- Schaefer, A.; Dickow, J.; Schoen, G. Stentless vs stented bioprosthesis for aortic valve replacement: A case matched comparison of long-term follow-up and subgroup analysis of patients with native valve endocarditis. PLoS ONE 2018, 13, e0191171. [Google Scholar] [CrossRef]
- Nishida, T.; Tominaga, R. A look at recent improvements in the durability of tissue valves. Gen. Thorac. Cardiovasc. Surg. 2013, 61, 182–190. [Google Scholar] [CrossRef]
- Garrido-Olivares, L.; Maganti, M.; Armstrong, S.; David, T. Aortic valve replacement with Hancock II bioprosthesis with and without replacement of the ascending aorta. Ann. Thorac. Surg. 2011, 92, 541–547. [Google Scholar] [CrossRef]
- David, T.E.; Armstrong, S.; Maganti, M. Hancock II bioprosthesis for aortic valve replacement: The gold standard of bioprosthetic valves durability? Ann. Thorac. Surg. 2010, 90, 775–781. [Google Scholar] [CrossRef]
- Glaser, N.; Franco-Cereceda, A.; Sartipy, U. Late survival after aortic valve replacement with the perimount versus the mosaic bioprosthesis. Ann. Thorac. Surg. 2014, 97, 1314–1320. [Google Scholar] [CrossRef]
- Bourguignon, T.; Bouquiaux-Stablo, A.L.; Candolfi, P. Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann. Thorac. Surg. 2015, 99, 831–837. [Google Scholar] [CrossRef]
- Johnston, D.R.; Soltesz, E.G.; Vakil, N. Long-term durability of bioprosthetic aortic valves: Implications from 12,569 implants. Ann. Thorac. Surg. 2015, 99, 1239–1247. [Google Scholar] [CrossRef]
- Senage, T.; Le Tourneau, T.; Foucher, Y. Early structural valve deterioration of Mitroflow aortic bioprosthesis: Mode, incidence, and impact on outcome in a large cohort of patients. Circulation 2014, 130, 2012–2020. [Google Scholar] [CrossRef]
- Goldman, S.; Cheung, A.; Bavaria, J.E. Midterm, multicenter clinical and hemodynamic results for the Trifecta aortic pericardial valve. J. Thorac. Cardiovasc. Surg. 2017, 153, 561–569.e2. [Google Scholar] [CrossRef]
- Kalra, A.; Rehman, H.; Ramchandani, M. Early Trifecta valve failure: Report of a cluster of cases from a tertiary care referral center. J. Thorac. Cardiovasc. Surg. 2017, 154, 1235–1240. [Google Scholar] [CrossRef]
- Fischlein, T.; Meuris, B.; Hakim-Meibodi, K. The sutureless aortic valve at 1 year: A large multicenter cohort study. J. Thorac. Cardiovasc. Surg. 2016, 151, 1617–1626.e4. [Google Scholar] [CrossRef]
- Kocher, A.A.; Laufer, G.; Haverich, A. One-year outcomes of the Surgical Treatment of Aortic Stenosis With a Next Generation Surgical Aortic Valve (TRITON) trial: A prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. J. Thorac. Cardiovasc. Surg. 2013, 145, 110–115. [Google Scholar] [CrossRef]
- Durand, E.; Tron, C.; Eltchaninoff, H. Emergency Transcatheter Aortic Valve Implantation for Acute and Early Failure of Sutureless Perceval Aortic Valve. Can. J. Cardiol. 2015, 31, 1204.e13–1204.e15. [Google Scholar] [CrossRef]
- Tseng, E.; Wisneski, A.; Azadani, A.; Ge, L. Engineering perspective on transcatheter aortic valve implantation. Interv. Cardiol. 2013, 5, 53–70. [Google Scholar] [CrossRef]
- Sun, W.; Abad, A.; Sacks, M.S. Simulated bioprosthetic heart valve deformation under quasi-static loading. J. Biomech. Eng. 2005, 127, 905–914. [Google Scholar] [CrossRef]
- Alavi, S.H.; Groves, E.M.; Kheradvar, A. The effects of transcatheter valve crimping on pericardial leaflets. Ann. Thorac. Surg. 2014, 97, 1260–1266. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nappi, F.; Avtaar Singh, S.S.; Nappi, P.; Fiore, A. Biomechanics of Transcatheter Aortic Valve Implant. Bioengineering 2022, 9, 299. https://doi.org/10.3390/bioengineering9070299
Nappi F, Avtaar Singh SS, Nappi P, Fiore A. Biomechanics of Transcatheter Aortic Valve Implant. Bioengineering. 2022; 9(7):299. https://doi.org/10.3390/bioengineering9070299
Chicago/Turabian StyleNappi, Francesco, Sanjeet Singh Avtaar Singh, Pierluigi Nappi, and Antonio Fiore. 2022. "Biomechanics of Transcatheter Aortic Valve Implant" Bioengineering 9, no. 7: 299. https://doi.org/10.3390/bioengineering9070299
APA StyleNappi, F., Avtaar Singh, S. S., Nappi, P., & Fiore, A. (2022). Biomechanics of Transcatheter Aortic Valve Implant. Bioengineering, 9(7), 299. https://doi.org/10.3390/bioengineering9070299





