Improving Vascularization of Biomaterials for Skin and Bone Regeneration by Surface Modification: A Narrative Review on Experimental Research
Abstract
:1. Introduction
2. Methods of Bioactive Modification in Skin and Bone Regeneration
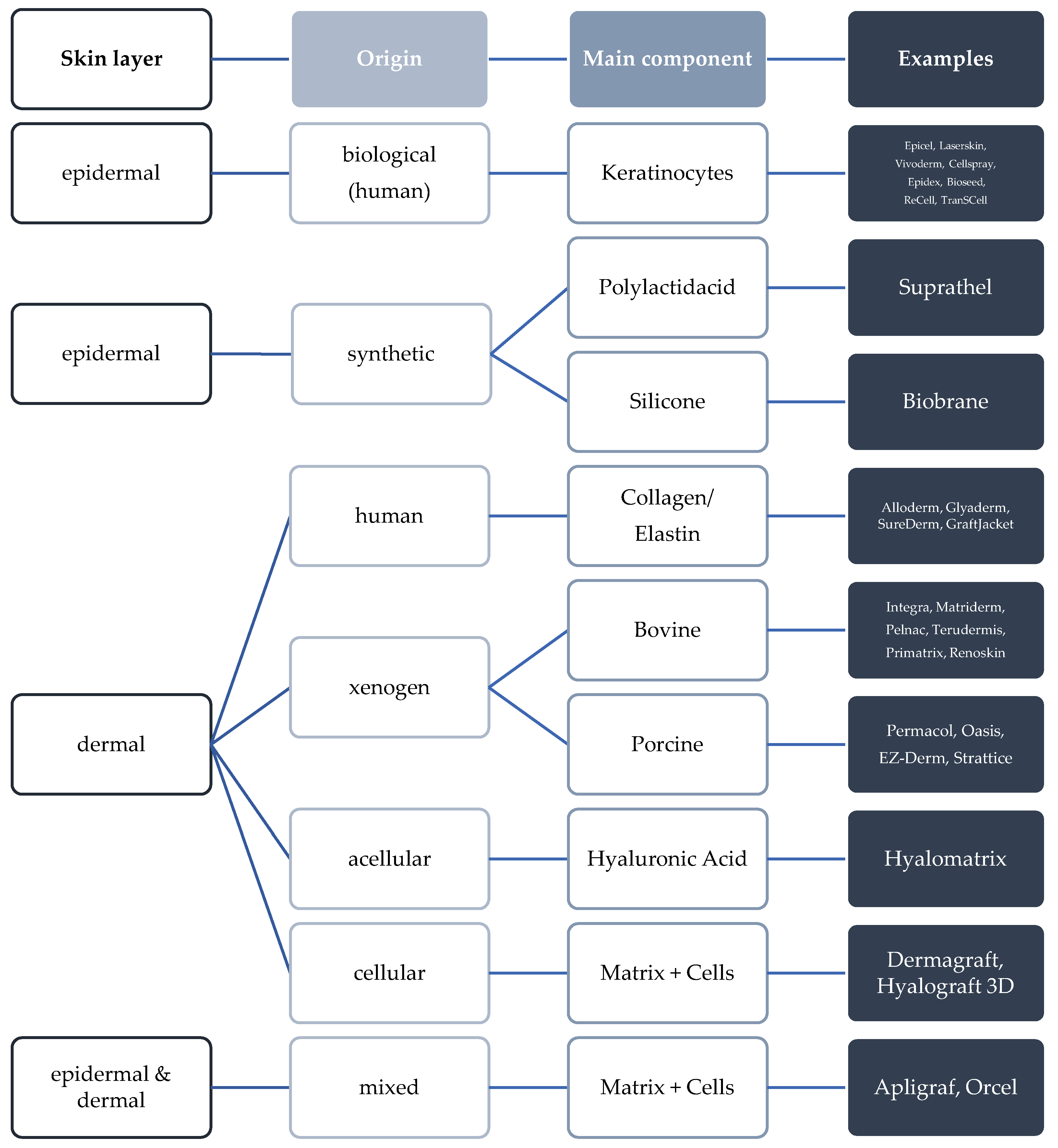
2.1. Challenges in Skin and Bone Tissue Regeneration with Synthetic Biopolymers
2.2. Bioactive Modification for the Improvement of Skin Wound Healing
2.3. Bioactive Modification for the Improvement of Bone Regeneration
2.4. Biomimetic Surface Coating for the Improvement of Skin Regeneration
2.5. Biomimetic Surface Coating for the Improvement of Bone Regeneration
2.6. Physicochemical Surface Changes by Cold Plasma in Skin and Bone Regeneration
2.7. Modification by Changing the Surface Geometry
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harder, Y.; Amon, M.; Laschke, M.; Schramm, R.; Rücker, M.; Wettstein, R.; Bastiaanse, J.; Frick, A.; Machens, H.-G.; Küntscher, M.; et al. An old dream revitalised: Preconditioning strategies to protect surgical flaps from critical ischaemia and ischaemia-reperfusion injury. J. Plast. Reconstr. Aesthetic Surg. 2008, 61, 503–511. [Google Scholar] [CrossRef]
- Laschke, M.; Menger, M. Vascularization in Tissue Engineering: Angiogenesis versus Inosculation. Eur. Surg. Res. 2012, 48, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Holzhüter, G.; Sorg, H.; Wolter, D.; Lenz, S.; Gerber, T.; Vollmar, B. Early matrix change of a nanostructured bone grafting substitute in the rat. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91B, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.; Kuhn, S.; Sorg, H.; Gruene, M.; Schlie, S.; Gaebel, R.; Polchow, B.; Reimers, K.; Stoelting, S.; Ma, N.; et al. Laser Printing of Skin Cells and Human Stem Cells. Tissue Eng. Part C: Methods 2010, 16, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Ring, A.; Tilkorn, D.; Ottomann, C.; Geomelas, M.; Steinstraesser, L.; Langer, S.; Goertz, O. Intravital monitoring of microcirculatory and angiogenic response to lactocapromer terpolymer matrix in a wound model. Int. Wound J. 2011, 8, 112–117. [Google Scholar] [CrossRef]
- Michael, S.; Sorg, H.; Peck, C.-T.; Koch, L.; Deiwick, A.; Chichkov, B.; Vogt, P.M.; Reimers, K. Tissue Engineered Skin Substitutes Created by Laser-Assisted Bioprinting Form Skin-Like Structures in the Dorsal Skin Fold Chamber in Mice. PLoS ONE 2013, 8, e57741. [Google Scholar] [CrossRef]
- Brusselaers, N.; Pirayesh, A.; Hoeksema, H.; Richters, C.D.; Verbelen, J.; Beele, H.; Blot, S.I.; Monstrey, S. Skin Replacement in Burn Wounds. J. Trauma Inj. Infect. Crit. Care 2010, 68, 490–501. [Google Scholar] [CrossRef]
- Smeets, D.R.; Hanken, H.; Jung, O.; Rothamel, D.; Handschel, J.; Al-Dam, A.; Blessmann, M.; Heiland, M.; Kolk, A. Knochenersatzmaterialien. Der MKG-Chirurg 2014, 7, 53–67. [Google Scholar] [CrossRef]
- Fernandez De Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [Green Version]
- Bordel, R.; Laschke, M.W.; Menger, M.D.; Vollmar, B. Inhibition of p53 during physiological angiogenesis in the hamster ovary does not affect extent of new vessel formation but delays vessel maturation. Cell Tissue Res. 2005, 320, 427–435. [Google Scholar] [CrossRef]
- Amirsadeghi, A.; Jafari, A.; Eggermont, L.J.; Hashemi, S.S.; Bencherif, S.A.; Khorram, M. Vascularization strategies for skin tissue engineering. Biomater. Sci. 2020, 8, 4073–4094. [Google Scholar] [CrossRef]
- Ring, A.; Langer, S.; Homann, H.H.; Kuhnen, C.; Schmitz, I.; Steinau, H.U.; Drücke, D. Analysis of neovascularization of PEGT/PBT-copolymer dermis substitutes in balb/c-mice. Burns 2006, 32, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Heilborn, J.D.; Nilsson, M.F.; Kratz, G.; Weber, G.; Sorensen, O.; Borregaard, N.; Stahle-Backdahl, M. The Cathelicidin Anti-Microbial Peptide LL-37 is Involved in Re-Epithelialization of Human Skin Wounds and is Lacking in Chronic Ulcer Epithelium. J. Investig. Dermatol. 2003, 120, 379–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koczulla, R.; von Degenfeld, G.; Kupatt, C.; Krötz, F.; Zahler, S.; Gloe, T.; Issbrücker, K.; Unterberger, P.; Zaiou, M.; Lebherz, C. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J. Clin. Investig. 2003, 111, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Steinstraesser, L.; Ring, A.; Bals, R.; Steinau, H.U.; Langer, S. The human host defense peptide LL37/hCAP accelerates angiogenesis in PEGT/PBT biopolymers. Ann. Plast. Surg. 2006, 56, 93–98. [Google Scholar] [CrossRef]
- Steinstraesser, L.; Koehler, T.; Jacobsen, F.; Daigeler, A.; Goertz, O.; Langer, S.; Kesting, M.; Steinau, H.; Eriksson, E.; Hirsch, T. Host Defense Peptides in Wound Healing. Mol. Med. 2008, 14, 528–537. [Google Scholar] [CrossRef]
- Burton, M.F.; Steel, P.G. The chemistry and biology of LL-37. Nat. Prod. Rep. 2009, 26, 1572–1584. [Google Scholar] [CrossRef]
- Ramos, R.; Silva, J.P.; Rodrigues, A.C.; Costa, R.; Guardão, L.; Schmitt, F.; Soares, R.; Vilanova, M.; Domingues, L.; Gama, M. Wound healing activity of the human antimicrobial peptide LL37. Peptides 2011, 32, 1469–1476. [Google Scholar] [CrossRef] [Green Version]
- Chereddy, K.K.; Her, C.-H.; Comune, M.; Moia, C.; Lopes, A.; Porporato, P.E.; Vanacker, J.; Lam, M.C.; Steinstraesser, L.; Sonveaux, P.; et al. PLGA nanoparticles loaded with host defense peptide LL37 promote wound healing. J. Control. Release 2014, 194, 138–147. [Google Scholar] [CrossRef]
- Fabisiak, A.; Murawska, N.; Fichna, J. LL-37: Cathelicidin-related antimicrobial peptide with pleiotropic activity. Pharmacol. Rep. 2016, 68, 802–808. [Google Scholar] [CrossRef]
- Hertog, A.L.D.; Van Marle, J.; Van Veen, H.A.; Hof, W.V.; Bolscher, J.G.M.; Veerman, E.C.I.; Amerongen, A.V.N. Candidacidal effects of two antimicrobial peptides: Histatin 5 causes small membrane defects, but LL-37 causes massive disruption of the cell membrane. Biochem. J. 2005, 388, 689–695. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Garcia, B.; Lee, P.H.; Yamasaki, K.; Gallo, R.L. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J. Investig. Dermatol. 2005, 125, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Walter-Jallow, L.; Broliden, K.; Agerberth, B.; Söderlund, J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr. HIV Res. 2007, 5, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Watson, K.M.; Buckheit, R.W., Jr. Anti-Human Immunodeficiency Virus Type 1 Activities of Antimicrobial Peptides Derived from Human and Bovine Cathelicidins. Antimicrob. Agents Chemother. 2008, 52, 3438–3440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.W.; Rehm, B.H.A.; Hancock, R.E.W. Human Host Defense Peptide LL-37 Prevents Bacterial Biofilm Formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef] [Green Version]
- Chennupati, S.K.; Chiu, A.G.; Tamashiro, E.; Banks, C.A.; Cohen, M.B.; Bleier, B.S.; Kofonow, J.M.; Tam, E.; Cohen, N.A. Effects of an LL-37-Derived Antimicrobial Peptide in an Animal Model of Biofilm Pseudomonas Sinusitis. Am. J. Rhinol. Allergy 2009, 23, 46–51. [Google Scholar] [CrossRef]
- Steinstraesser, L.; Lam, M.C.; Jacobsen, F.; Porporato, P.E.; Chereddy, K.K.; Becerikli, M.; Stricker, I.; Hancock, R.E.; Lehnhardt, M.; Sonveaux, P.; et al. Skin electroporation of a plasmid encoding hCAP-18/LL-37 host defense peptide promotes wound healing. Mol. Ther. 2014, 22, 734–742. [Google Scholar] [CrossRef] [Green Version]
- Porporato, P.E.; Payen, V.L.; De Saedeleer, C.J.; Préat, V.; Thissen, J.-P.; Feron, O.; Sonveaux, P. Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis 2012, 15, 581–592. [Google Scholar] [CrossRef]
- Yang, X.; Guo, J.-L.; Han, J.; Si, R.-J.; Liu, P.-P.; Zhang, Z.-R.; Wang, A.-M.; Zhang, J. Chitosan hydrogel encapsulated with LL-37 peptide promotes deep tissue injury healing in a mouse model. Mil. Med Res. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Zhang, Z.; Shively, J.E. Generation of Novel Bone Forming Cells (Monoosteophils) from the Cathelicidin-Derived Peptide LL-37 Treated Monocytes. PLOS ONE 2010, 5, e13985. [Google Scholar] [CrossRef]
- Zhang, Z.; Shively, J.E. Acceleration of Bone Repair in NOD/SCID Mice by Human Monoosteophils, Novel LL-37-Activated Monocytes. PLoS ONE 2013, 8, e67649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supanchart, C.; Thawanaphong, S.; Makeudom, A.; Bolscher, J.G.; Nazmi, K.; Kornak, U.; Krisanaprakornkit, S. The antimicrobial peptide, LL-37, inhibits in vitro osteoclastogenesis. J. Dent. Res. 2012, 91, 1071–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Mu, C.; Shen, X.; Yuan, Z.; Liu, J.; Chen, W.; Lin, C.; Tao, B.; Liu, B.; Cai, K. Peptide LL-37 coating on micro-structured titanium implants to facilitate bone formation in vivo via mesenchymal stem cell recruitment. Acta Biomater. 2018, 80, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Fan, Y.; Li, X. The use of bioactive peptides to modify materials for bone tissue repair. Regen. Biomater. 2017, 4, 191–206. [Google Scholar] [CrossRef] [Green Version]
- Blaydon, S.M.; Shepler, T.R.; Neuhaus, R.W.; White, W.L.; Shore, J.W. Reply re: “The Porous Polyethylene (Medpor) Spherical Orbital Implant: A Retrospective Study of 136 Cases”. Ophthalmic Plast. Reconstr. Surg. 2004, 20, 403–404. [Google Scholar] [CrossRef]
- Reddy, V.J.; Radhakrishnan, S.; Ravichandran, R.; Mukherjee, S.; Balamurugan, R.; Sundarrajan, S.; Ramakrishna, S. Nanofibrous structured biomimetic strategies for skin tissue regeneration. Wound Repair Regen. 2012, 21, 1–16. [Google Scholar] [CrossRef]
- Yu, Q.; Han, Y.; Tian, T.; Zhou, Q.; Yi, Z.; Chang, J.; Wu, C. Chinese sesame stick-inspired nano-fibrous scaffolds for tumor therapy and skin tissue reconstruction. Biomaterials 2018, 194, 25–35. [Google Scholar] [CrossRef]
- McLuckie, M.; Schmidt, C.A.; Oosthuysen, A.; Sanchez-Macedo, N.; Merker, H.; Bezuidenhout, D.; Hoerstrup, S.P.; Lindenblatt, N. High heparin content surface-modified polyurethane discs promote rapid and stable angiogenesis in full thickness skin defects through VEGF immobilization. J. Biomed. Mater. Res. Part A 2017, 105, 2543–2550. [Google Scholar] [CrossRef]
- Aguirre, A.; González, A.; Navarro, M.; Castaño Linares, Ó.; Planell Estany, J.A.; Engel, E. Control of microenvironmental cues with a smart biomaterial composite promotes endothelial progenitor cell angiogenesis. Eur. Cells Mater. 2012, 24, 90–106. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Chen, S.; Yi, J.; Zhang, H.; Ameer, G.A. A Cooperative Copper Metal-Organic Framework-Hydrogel System Improves Wound Healing in Diabetes. Adv. Funct. Mater. 2017, 27, 1604872. [Google Scholar] [CrossRef]
- Ishihara, J.; Ishihara, A.; Fukunaga, K.; Sasaki, K.; White, M.J.V.; Briquez, P.S.; Hubbell, J.A. Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Q.; Lim, T.; Wei, X.-J.; Wang, Q.-Y.; Xu, J.-C.; Shen, L.-Y.; Zhu, Z.-Z.; Zhang, C.-Q. A free-standing multilayer film as a novel delivery carrier of platelet lysates for potential wound-dressing applications. Biomaterials 2020, 255, 120138. [Google Scholar] [CrossRef] [PubMed]
- Ring, A.; Steinstraesser, L.; Muhr, G.; Steinau, H.-U.; Hauser, J.; Langer, S. Improved Neovascularization of PEGT/PBT Copolymer Matrices in Response to Surface Modification by Biomimetic Coating. Eur. Surg. Res. 2007, 39, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Munaron, L. Intracellular calcium, endothelial cells and angiogenesis. Recent Pat. Anti-Cancer Drug Discov. 2006, 1, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Munaron, L.; Fiorio Pla, A. Endothelial calcium machinery and angiogenesis: Understanding physiology to interfere with pathology. Curr. Med. Chem. 2009, 16, 4691–4703. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Kang, Y.J. Role of copper in angiogenesis and its medicinal implications. Curr. Med. Chem. 2009, 16, 1304–1314. [Google Scholar] [CrossRef]
- Milan, P.B.; Khamseh, S.; Zarintaj, P.; Ramezanzadeh, B.; Badawi, M.; Morisset, S.; Vahabi, H.; Saeb, M.R.; Mozafari, M. Copper-enriched diamond-like carbon coatings promote regeneration at the bone–implant interface. Heliyon 2020, 6, e03798. [Google Scholar] [CrossRef]
- Bai, L.; Chen, P.; Zhao, Y.; Hang, R.; Yao, X.; Tang, B.; Liu, C.; Xiao, Y.; Hang, R. A micro/nano-biomimetic coating on titanium orchestrates osteo/angio-genesis and osteoimmunomodulation for advanced osseointegration. Biomaterials 2021, 278, 121162. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, J.; Crevier, M.-C.; Pelletier, J.; Philip, N.; Saoudi, B. Plasma sterilization. Methods and mechanisms. Pure Appl. Chem. 2002, 74, 349–358. [Google Scholar] [CrossRef]
- Laroussi, M.; Tendero, C.; Lu, X.; Alla, S.; Hynes, W.L. Inactivation of Bacteria by the Plasma Pencil. Plasma Process. Polym. 2006, 3, 470–473. [Google Scholar] [CrossRef]
- Brun, P.; Bernabè, G.; Marchiori, C.; Scarpa, M.; Zuin, M.; Cavazzana, R.; Zaniol, B.; Martines, E. Antibacterial efficacy and mechanisms of action of low power atmospheric pressure cold plasma: Membrane permeability, biofilm penetration and antimicrobial sensitization. J. Appl. Microbiol. 2018, 125, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Ermolaeva, S.A.; Varfolomeev, A.F.; Chernukha, M.Y.; Yurov, D.S.; Vasiliev, M.M.; Kaminskaya, A.A.; Moisenovich, M.M.; Romanova, J.M.; Murashev, A.N.; Selezneva, I.I.; et al. Bactericidal effects of non-thermal argon plasma in vitro, in biofilms and in the animal model of infected wounds. J. Med Microbiol. 2011, 60, 75–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohd Nasir, N.; Lee, B.K.; Yap, S.S.; Thong, K.L.; Yap, S.L. Cold plasma inactivation of chronic wound bacteria. Arch. Biochem. Biophys. 2016, 605, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.-F.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.K.; et al. Cold Atmospheric Plasma (CAP) Changes Gene Expression of Key Molecules of the Wound Healing Machinery and Improves Wound Healing In Vitro and In Vivo. PLOS ONE 2013, 8, e79325. [Google Scholar] [CrossRef] [Green Version]
- Arndt, S.; Landthaler, M.; Zimmermann, J.L.; Unger, P.; Wacker, E.; Shimizu, T.; Li, Y.-F.; Morfill, G.E.; Bosserhoff, A.-K.; Karrer, S. Effects of Cold Atmospheric Plasma (CAP) on ß-Defensins, Inflammatory Cytokines, and Apoptosis-Related Molecules in Keratinocytes In Vitro and In Vivo. PLoS ONE 2015, 10, e0120041. [Google Scholar] [CrossRef] [Green Version]
- Arndt, S.; Unger, P.; Berneburg, M.; Bosserhoff, A.-K.; Karrer, S. Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. J. Dermatol. Sci. 2018, 89, 181–190. [Google Scholar] [CrossRef]
- Xu, G.; Shi, X.; Cai, J.; Chen, S.; Li, P.; Yao, C.; Chang, Z.; Zhang, G. Dual effects of atmospheric pressure plasma jet on skin wound healing of mice. Wound Repair Regen. 2015, 23, 878–884. [Google Scholar] [CrossRef]
- Arjunan, K.P.; Friedman, G.; Fridman, A.; Clyne, A.M. Non-thermal dielectric barrier discharge plasma induces angiogenesis through reactive oxygen species. J. R. Soc. Interface 2011, 9, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Ring, A.; Langer, S.; Schaffran, A.; Stricker, I.; Awakowicz, P.; Steinau, H.-U.; Hauser, J. Enhanced neovascularization of dermis substitutes via low-pressure plasma-mediated surface activation. Burns 2010, 36, 1222–1227. [Google Scholar] [CrossRef]
- Roach, P.; Farrar, D.; Perry, C.C. Interpretation of Protein Adsorption: Surface-Induced Conformational Changes. J. Am. Chem. Soc. 2005, 127, 8168–8173. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of Biomaterial–Cell Interactions by Adsorbed Proteins: A Review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Jesmer, A.H.; Wylie, R.G. Controlling Experimental Parameters to Improve Characterization of Biomaterial Fouling. Front. Chem. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.F.; Palgrave, R.G.; Seifalian, A.M.; Butler, P.E.; Kalaskar, D.M. Enhancing tissue integration and angiogenesis of a novel nanocomposite polymer using plasma surface polymerisation, an in vitro and in vivo study. Biomater. Sci. 2015, 4, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.; Palgrave, R.; Baldovino-Medrano, V.G.; Butler, P.E.; Kalaskar, D.M. Argon plasma improves the tissue integration and angiogenesis of subcutaneous implants by modifying surface chemistry and topography. Int. J. Nanomed. 2018, 13, 6123–6141. [Google Scholar] [CrossRef] [Green Version]
- Griffin, M.F.; Naderi, N.; Kalaskar, D.M.; Seifalian, A.; Butler, P.E. Argon plasma surface modification promotes the therapeutic angiogenesis and tissue formation of tissue-engineered scaffolds in vivo by adipose-derived stem cells. Stem Cell Res. Ther. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hauser, J.; Koeller, M.; Bensch, S.; Halfmann, H.; Awakowicz, P.; Steinau, H.-U.; Esenwein, S. Plasma mediated collagen-I-coating of metal implant materials to improve biocompatibility. J. Biomed. Mater. Res. Part A 2010, 94A, 19–26. [Google Scholar] [CrossRef]
- Hauser, J.; Ring, A.; Schaffran, A.; Henrich, L.; Esenwein, S.; Steinau, H.; Stricker, I.; Langer, S. In vivo Analysis of Tissue Response to Plasma-Treated Collagen-I-Coated Titanium Alloys. Eur. Surg. Res. 2009, 43, 262–268. [Google Scholar] [CrossRef]
- Mercado-Pagán, E.; Stahl, A.M.; Shanjani, Y.; Yang, Y. Vascularization in Bone Tissue Engineering Constructs. Ann. Biomed. Eng. 2015, 43, 718–729. [Google Scholar] [CrossRef] [Green Version]
- Marrella, A.; Lee, T.Y.; Lee, D.H.; Karuthedom, S.; Syla, D.; Chawla, A.; Khademhosseini, A.; Jang, H.L. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater. Today 2017, 21, 362–376. [Google Scholar] [CrossRef]
- Bienert, M. Angiogenesis in Bone Tissue Engineering. J. Stem Cell Res. Med. 2019, 4, 1–2. [Google Scholar] [CrossRef]
- Ring, M.A.; Goertz, O.; Al-Benna, S.; Ottomann, C.; Langer, S.; Steinstraesser, L.; Schmitz, I.; Tilkorn, D. Accelerated Angiogenic Induction and Vascular Integration in a Novel Synthetic Scaffolding Matrix for Tissue Replacement. Int. J. Artif. Organs 2010, 33, 877–884. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Ishikawa, K.; Takechi, M.; Toh, T.; Yuasa, T.; Nagayama, M.; Suzuki, K. Histological and compositional evaluations of three types of calcium phosphate cements when implanted in subcutaneous tissue immediately after mixing. J. Biomed. Mater. Res. 1999, 48, 36–42. [Google Scholar] [CrossRef]
- Schildhauer, T.; Bauer, T.; Josten, C.; Muhr, G. Open Reduction and Augmentation of Internal Fixation with an Injectable Skeletal Cement for the Treatment of Complex Calcaneal Fractures. J. Orthop. Trauma 2000, 14, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.B.; Weinzweig, J.; Kirschner, R.E.; Bartlett, S.P. Applications of a New Carbonated Calcium Phosphate Bone Cement: Early Experience in Pediatric and Adult Craniofacial Reconstruction. Plast. Reconstr. Surg. 2002, 109, 1789–1796. [Google Scholar] [CrossRef]
- Elsner, A.; Jubel, A.; Prokop, A.; Koebke, J.; Rehm, K.E.; Andermahr, J. Augmentation of Intraarticular Calcaneal Fractures with Injectable Calcium Phosphate Cement: Densitometry, Histology, and Functional Outcome of 18 Patients. J. Foot Ankle Surg. 2005, 44, 390–395. [Google Scholar] [CrossRef]
- Gómez, E.; Martín, M.; Arias, J.; Carceller, F. Clinical applications of Norian SRS (calcium phosphate cement) in craniofacial reconstruction in children: Our experience at Hospital La Paz since 2001. J. Oral Maxillofac. Surg. 2005, 63, 8–14. [Google Scholar] [CrossRef]
- Roetman, B.; Ring, A.; Langer, S.; Schildhauer, T.A.; Muhr, G.; Köller, M. Microvascular response to calcium phosphate bone substitutes: An intravital microscopy analysis. Langenbeck’s Arch. Surg. 2010, 395, 1147–1155. [Google Scholar] [CrossRef]
- Ring, A.; Tilkorn, D.J.; Goertz, O.; Langer, S.; Schaffran, A.; Awakowicz, P.; Hauser, J. Surface modification by glow discharge gasplasma treatment improves vascularization of allogenic bone implants. J. Orthop. Res. 2011, 29, 1237–1244. [Google Scholar] [CrossRef]

| Autologous | Xenogen | Allogen | Alloplastic | Phycogenic | |
|---|---|---|---|---|---|
| biological | synthetic | Hydroxyapatite from 100% inorganic calcium phosphate, of which 95% is present as apatite; source material is calcareous encrusting marine algae | |||
| spongious | Bovine | Living donor | Hydroxyapatite | Ca3(PO4)2 cements | |
| cortico-spongious | Porcine | Cadaver donor | Platelet rich plasma | Hydroxyapatite | |
| vascular | Equine | CaSo4 | β-tricalciumphosphate | ||
| Corals | Bioactive glasses | ||||
| Polymer-based substitute materials | |||||
| Metals | |||||
| Material | Component | Ca/P Index | Porosity (%) | |
|---|---|---|---|---|
| Cement | Calcibon ® | α-TCP, CaHPO4, CaCO3, pHA | 1.57 | 8 |
| Biobon ® | α-TCP, DCPD | 1.45 | 50–60 | |
| Norian SRS ® | α-TCP, CaCO3, MCPM | 1.67 | ||
| Granules | Algipore ® | Coraline HA | 1.8–2.15 | 75–80 |
| BioOss ® | Bovine HA | 2.03 | 59.7 | |
| ChronOs ® | β-TCP | 1.5 | 60 | |
| Endobon ® | Bovine HA | 1.67 | 45–85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorg, H.; Tilkorn, D.J.; Hauser, J.; Ring, A. Improving Vascularization of Biomaterials for Skin and Bone Regeneration by Surface Modification: A Narrative Review on Experimental Research. Bioengineering 2022, 9, 298. https://doi.org/10.3390/bioengineering9070298
Sorg H, Tilkorn DJ, Hauser J, Ring A. Improving Vascularization of Biomaterials for Skin and Bone Regeneration by Surface Modification: A Narrative Review on Experimental Research. Bioengineering. 2022; 9(7):298. https://doi.org/10.3390/bioengineering9070298
Chicago/Turabian StyleSorg, Heiko, Daniel J. Tilkorn, Jörg Hauser, and Andrej Ring. 2022. "Improving Vascularization of Biomaterials for Skin and Bone Regeneration by Surface Modification: A Narrative Review on Experimental Research" Bioengineering 9, no. 7: 298. https://doi.org/10.3390/bioengineering9070298
APA StyleSorg, H., Tilkorn, D. J., Hauser, J., & Ring, A. (2022). Improving Vascularization of Biomaterials for Skin and Bone Regeneration by Surface Modification: A Narrative Review on Experimental Research. Bioengineering, 9(7), 298. https://doi.org/10.3390/bioengineering9070298






