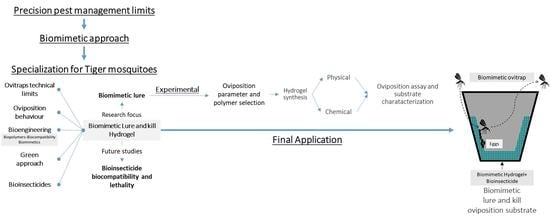
Proof of Concept of Biopolymer Based Hydrogels as Biomimetic Oviposition Substrate to Develop Tiger Mosquitoes (Aedes albopictus) Cost-Effective Lure and Kill Ovitraps
Abstract
1. Introduction
2. Materials and Methods
2.1. Rationale for Oviposition Parameters Selection
2.2. Rationale in Biopolymer and Substrate Composition Selection
2.3. Materials
2.4. Physical Hydrogels Preparation
2.5. Crosslinked (CL) Hydrogels Preparation
2.5.1. Hydroxyethylcellulose Crosslinked Hydrogels, CL-HEC
2.5.2. Sodium Alginate Crosslinked Hydrogel CL-SA2-30
2.6. Preparation of Different Formulations of the Best Performing Hydrogel
2.6.1. Hydrogels with Different Salinity
2.6.2. Hydrogels with Different pH
2.6.3. Hydrogels with Sorbitol
2.6.4. Hydrogels with Different Turbidity
2.7. Oviposition Assays on Ades albopictus
2.7.1. Aedes albopictus Colony
2.7.2. Lab Oviposition Assay Design
2.7.3. Oviposition Assay 1: Proof of Concept, Effects of Hydrogel Type and Polymer Concentration
2.7.4. Oviposition Assay 2: Effects of Salinity, pH, Sorbitol and Turbidity
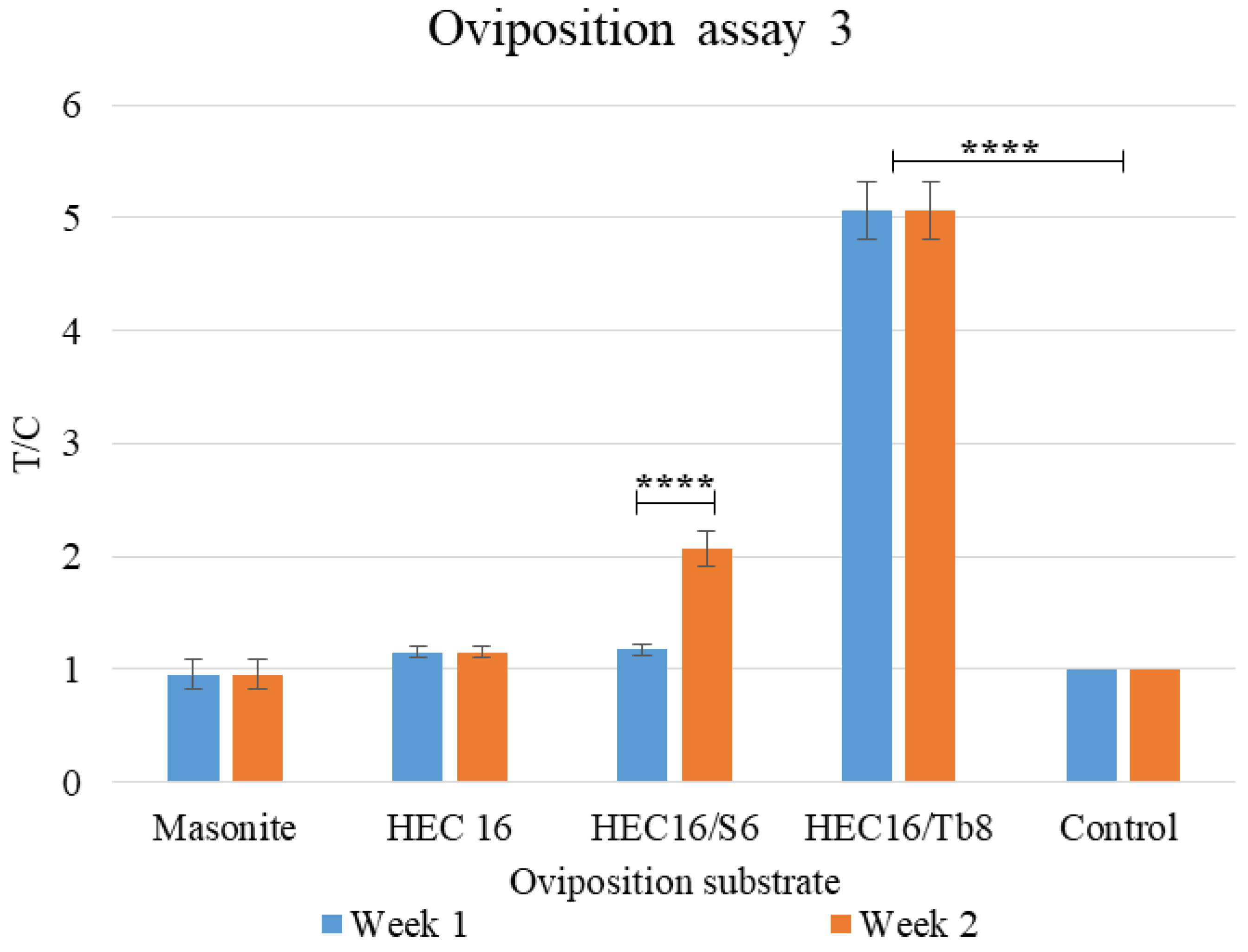
2.7.5. Oviposition Assay 3: Comparison between Best Performing Formulation over 2 Weeks
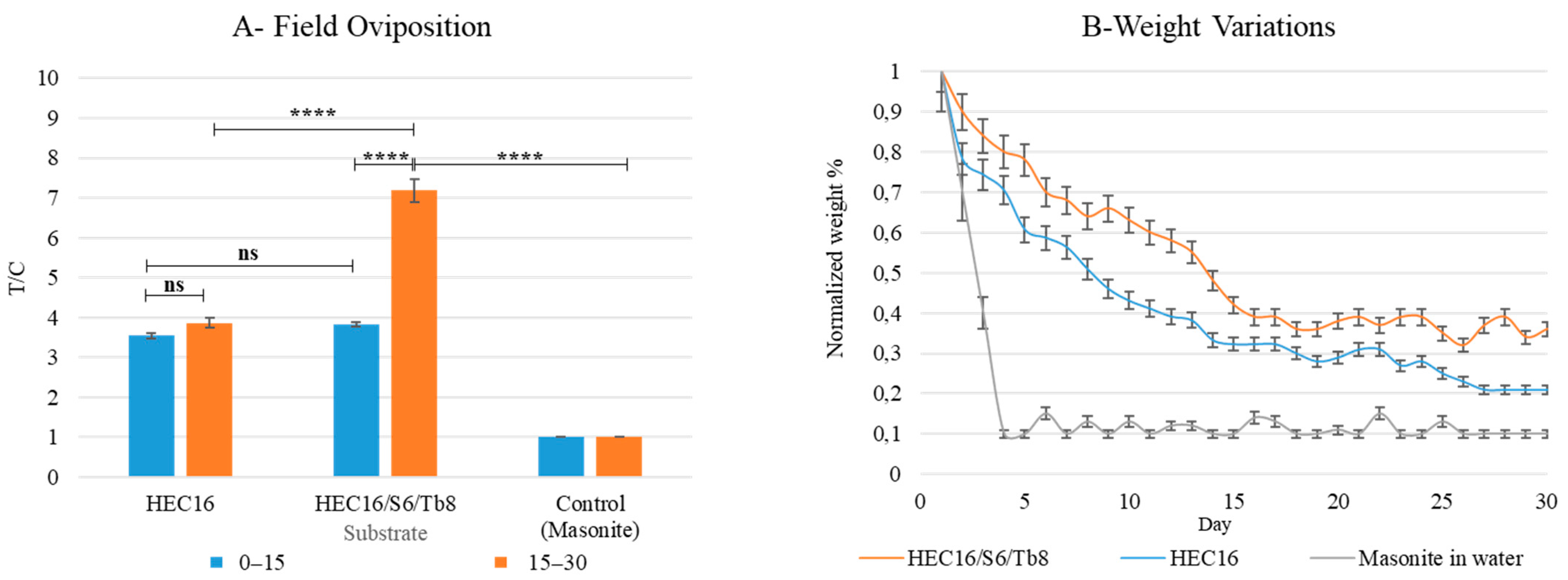
2.7.6. On-Field Oviposition Assay on Best Performing Hydrogel Formulation over 30 Days
2.8. Oviposition Substrates Characterization
2.8.1. Calorimetric Analysis of Free Water Content
2.8.2. Effect of Sorbitol and Polymer Concentration on Water Release at Controlled T and RH
2.8.3. Water Release in Field-like Conditions at Controlled T and RH
2.8.4. Gel Viscosity Measurement
2.8.5. Yield Stress Measurement
2.8.6. Morphological Analysis
2.9. Statistical Analysis
3. Results
3.1. Oviposition Assays on Ades albopictus
3.1.1. Oviposition Assay 1: Proof of Concept, Effects of Hydrogel Type, and Polymer Concentration
3.1.2. Oviposition Assay 2: Effects of Salinity, pH, Sorbitol, and Turbidity on Oviposition
3.1.3. Oviposition Assay 3: Comparison between Best Performing Formulation over Two Weeks
3.1.4. On Field-Oviposition Assay on Best-Performing Hydrogel Formulation over 30 Days
3.2. Substrates Characterization
3.2.1. Calorimetric Analysis of Free Water Content
3.2.2. Effect of Sorbitol and Polymer Concentration on Water Release at Controlled T and RH
3.2.3. Water Release in Field-like Conditions at Controlled T and RH
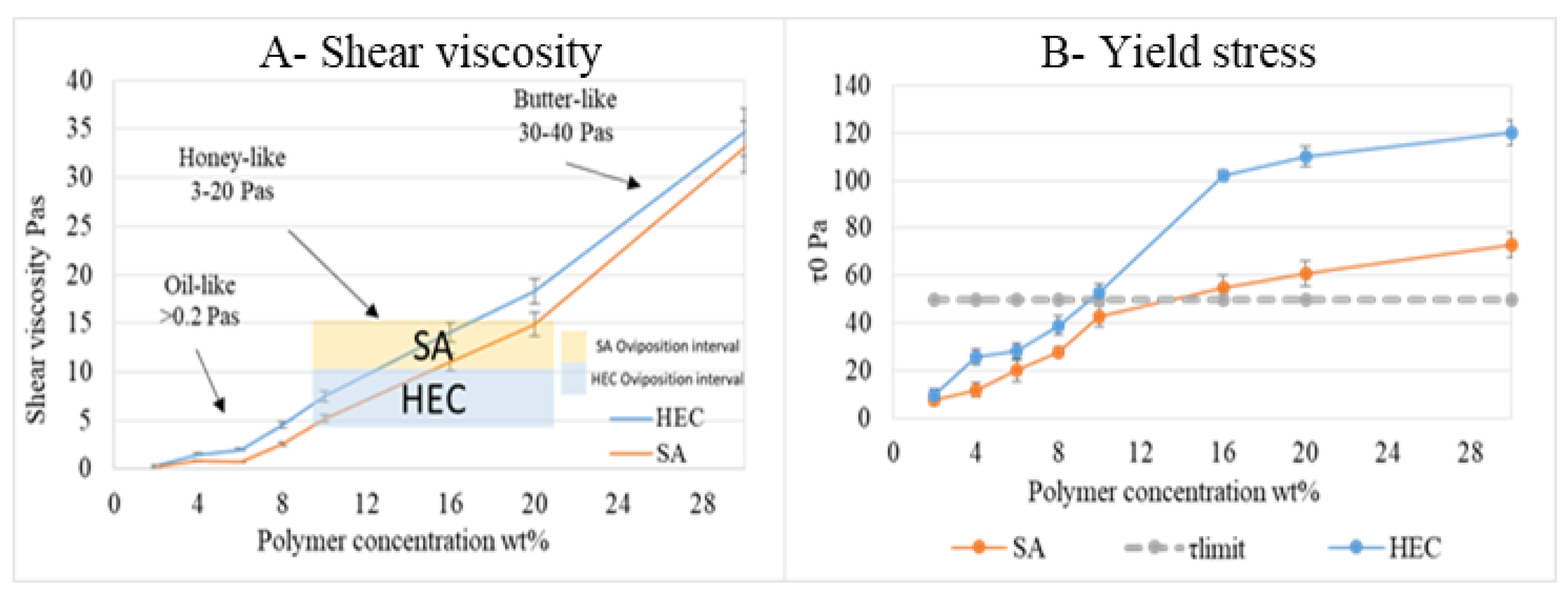
3.2.4. Gel Viscosity and Yield Stress Measurement
3.2.5. Morphological Analysis
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Ordinary Two-Way ANOVA | ||||||
|---|---|---|---|---|---|---|
| Alpha | 0.05 | |||||
| Source of Variation | % of total variation | p value | p value summary | |||
| Interaction | 8.009 | <0.0001 | **** | |||
| Polymer concentration | 80.72 | <0.0001 | **** | |||
| Substrate | 6.652 | <0.0001 | **** | |||
| ANOVA table | ||||||
| SS | DF | MS | F (DFn. DFd) | p value | ||
| Interaction | 0.5354 | 7 | 0.07649 | F (7, 32) = 7.933 | p < 0.0001 | |
| Polymer concentration | 5.396 | 7 | 0.7709 | F (7, 32) = 79.96 | p < 0.0001 | |
| Substrate | 0.4447 | 1 | 0.4447 | F (1, 32) = 46.12 | p < 0.0001 | |
| Residual | 0.3085 | 32 | 0.009642 | |||
| Tukey’s Multiple Comparisons Test | ||||||
| Number of families | 2 | |||||
| Number of comparisons per family | 28 | |||||
| Alpha | 0.05 | |||||
| Mean Diff. | 95% CI of diff. | Summary | Adjusted p Value | |||
| HEC | ||||||
| 2% vs. 6% | −0.45 | −0.7097 to −0.1903 | **** | <0.0001 | ||
| 2% vs. 8% | −0.9467 | −1.206 to −0.6870 | **** | <0.0001 | ||
| 2% vs. 10% | −1.06 | −1.320 to −0.8003 | **** | <0.0001 | ||
| 2% vs. 16% | −1.187 | −1.446 to −0.9270 | **** | <0.0001 | ||
| 2% vs. 20% | −0.9733 | −1.233 to −0.7136 | **** | <0.0001 | ||
| 2% vs. 30% | −0.69 | −0.9497 to −0.4303 | **** | <0.0001 | ||
| 2% vs. control | −0.7567 | −1.016 to −0.4970 | **** | <0.0001 | ||
| 6% vs. 8% | −0.4967 | −0.7564 to −0.2370 | **** | <0.0001 | ||
| 6% vs. 10% | −0.61 | −0.8697 to −0.3503 | **** | <0.0001 | ||
| 6% vs. 16% | −0.7367 | −0.9964 to −0.4770 | **** | <0.0001 | ||
| 6% vs. 20% | −0.5233 | −0.7830 to −0.2636 | **** | <0.0001 | ||
| 6% vs. 30% | −0.24 | −0.4997 to 0.01971 | ns | 0.0869 | ||
| 6% vs. control | −0.3067 | −0.5664 to −0.04696 | * | 0.0118 | ||
| 8% vs. 10% | −0.1133 | −0.3730 to 0.1464 | ns | 0.8443 | ||
| 8% vs. 16% | −0.24 | −0.4997 to 0.01971 | ns | 0.0869 | ||
| 8% vs. 20% | −0.02667 | −0.2864 to 0.2330 | ns | >0.9999 | ||
| 8% vs. 30% | 0.2567 | −0.003039 to 0.5164 | ns | 0.0546 | ||
| 8% vs. control | 0.19 | −0.06971 to 0.4497 | ns | 0.2897 | ||
| 10% vs. 16% | −0.1267 | −0.3864 to 0.1330 | ns | 0.7583 | ||
| 10% vs. 20% | 0.08667 | −0.1730 to 0.3464 | ns | 0.9563 | ||
| 10% vs. 30% | 0.37 | 0.1103 to 0.6297 | ** | 0.0014 | ||
| 10% vs. control | 0.3033 | 0.04363 to 0.5630 | * | 0.0131 | ||
| 16% vs. 20% | 0.2133 | −0.04637 to 0.4730 | ns | 0.1719 | ||
| 16% vs. 30% | 0.4967 | 0.2370 to 0.7564 | **** | <0.0001 | ||
| 16% vs. control | 0.43 | 0.1703 to 0.6897 | *** | 0.0002 | ||
| 20% vs. 30% | 0.2833 | 0.02363 to 0.5430 | * | 0.0247 | ||
| 20% vs. control | 0.2167 | −0.04304 to 0.4764 | ns | 0.1586 | ||
| 30% vs. control | −0.06667 | −0.3264 to 0.1930 | ns | 0.9898 | ||
| SA | ||||||
| 2% vs. 6% | −0.4133 | −0.6730 to −0.1536 | *** | 0.0003 | ||
| 2% vs. 8% | −0.52 | −0.7797 to −0.2603 | **** | <0.0001 | ||
| 2% vs. 10% | −0.8533 | −1.113 to −0.5936 | **** | <0.0001 | ||
| 2% vs. 16% | −0.9833 | −1.243 to −0.7236 | **** | <0.0001 | ||
| 2% vs. 20% | −1.053 | −1.313 to −0.7936 | **** | <0.0001 | ||
| 2% vs. 30% | −0.97 | −1.230 to −0.7103 | **** | <0.0001 | ||
| 2% vs. control | −0.9033 | −1.163 to −0.6436 | **** | <0.0001 | ||
| 6% vs. 8% | −0.1067 | −0.3664 to 0.1530 | ns | 0.8803 | ||
| 6% vs. 10% | −0.44 | −0.6997 to −0.1803 | *** | 0.0001 | ||
| 6% vs. 16% | −0.57 | −0.8297 to −0.3103 | **** | <0.0001 | ||
| 6% vs. 20% | −0.64 | −0.8997 to −0.3803 | **** | <0.0001 | ||
| 6% vs. 30% | −0.5567 | −0.8164 to −0.2970 | **** | <0.0001 | ||
| 6% vs. control | −0.49 | −0.7497 to −0.2303 | **** | <0.0001 | ||
| 8% vs. 10% | −0.3333 | −0.5930 to −0.07363 | ** | 0.0049 | ||
| 8% vs. 16% | −0.4633 | −0.7230 to −0.2036 | **** | <0.0001 | ||
| 8% vs. 20% | −0.5333 | −0.7930 to −0.2736 | **** | <0.0001 | ||
| 8% vs. 30% | −0.45 | −0.7097 to −0.1903 | **** | <0.0001 | ||
| 8% vs. control | −0.3833 | −0.6430 to −0.1236 | *** | 0.0009 | ||
| 10% vs. 16% | −0.13 | −0.3897 to 0.1297 | ns | 0.7343 | ||
| 10% vs. 20% | −0.2 | −0.4597 to 0.05971 | ns | 0.2339 | ||
| 10% vs. 30% | −0.1167 | −0.3764 to 0.1430 | ns | 0.8245 | ||
| 10% vs. control | −0.05 | −0.3097 to 0.2097 | ns | 0.9983 | ||
| 16% vs. 20% | −0.07 | −0.3297 to 0.1897 | ns | 0.9865 | ||
| 16% vs. 30% | 0.01333 | −0.2464 to 0.2730 | ns | >0.9999 | ||
| 16% vs. control | 0.08 | −0.1797 to 0.3397 | ns | 0.9714 | ||
| 20% vs. 30% | 0.08333 | −0.1764 to 0.3430 | ns | 0.9644 | ||
| 20% vs. control | 0.15 | −0.1097 to 0.4097 | ns | 0.5793 | ||
| 30% vs. control | 0.06667 | −0.1930 to 0.3264 | ns | 0.9898 | ||
| Ordinary Two-Way ANOVA | |||||
|---|---|---|---|---|---|
| Alpha | 0.05 | ||||
| Source of Variation | % of total variation | p value | p value summary | ||
| Interaction | 8.009 | <0.0001 | **** | ||
| Row Factor | 80.72 | <0.0001 | **** | ||
| Column Factor | 6.652 | <0.0001 | **** | ||
| ANOVA table | |||||
| SS | DF | MS | F (DFn. DFd) | p value | |
| Interaction | 0.5354 | 7 | 0.07649 | F (7, 32) = 7.933 | p < 0.0001 |
| Row Factor | 5.396 | 7 | 0.7709 | F (7, 32) = 79.96 | p < 0.0001 |
| Column Factor | 0.4447 | 1 | 0.4447 | F (1, 32) = 46.12 | p < 0.0001 |
| Residual | 0.3085 | 32 | 0.009642 | ||
| Bonferroni’s multiple comparisons test | |||||
| Number of families | 1 | ||||
| Number of comparisons per family | 8 | ||||
| Alpha | 0.05 | ||||
| Mean Diff. | 95% CI of diff. | Significant? | Summary | Adjusted p Value | |
| HEC-SA | |||||
| 2% | 0.1467 | −0.08802 to 0.3813 | No | ns | 0.6134 |
| 6% | 0.1833 | −0.05135 to 0.4180 | No | ns | 0.2318 |
| 8% | 0.5733 | 0.3387 to 0.8080 | Yes | **** | <0.0001 |
| 10% | 0.3533 | 0.1187 to 0.5880 | Yes | *** | 0.0009 |
| 16% | 0.3500 | 0.1153 to 0.5847 | Yes | *** | 0.0010 |
| 20% | 0.06667 | −0.1680 to 0.3013 | No | ns | >0.9999 |
| 30% | −0.1333 | −0.3680 to 0.1013 | No | ns | 0.8485 |
| Control | 0.000 | −0.2347 to 0.2347 | No | ns | >0.9999 |
| Ordinary Two-Way ANOVA | |||||
|---|---|---|---|---|---|
| Alpha | 0.05 | ||||
| Source of Variation | % of total variation | p value | p value summary | ||
| Interaction | 0.5089 | 0.2648 | ns | ||
| Concentration | 97.71 | <0.0001 | **** | ||
| Substrate | 0.04771 | 0.3557 | ns | ||
| ANOVA table | |||||
| SS | DF | MS | F (DFn. DFd) | p value | |
| Interaction | 0.02569 | 7 | 0.00367 | F (7, 32) = 1.339 | p = 0.2648 |
| Polymer Concentration | 4.932 | 7 | 0.7046 | F (7, 32) = 257.0 | p < 0.0001 |
| Substrate | 0.002408 | 1 | 0.002408 | F (1, 32) = 0.8784 | p = 0.3557 |
| Residual | 0.08773 | 32 | 0.002742 | ||
| Tukey’s multiple comparisons test | |||||
| Number of families | 2 | ||||
| Number of comparisons per family | 28 | ||||
| Alpha | 0.05 | ||||
| Mean Diff. | 95% CI of diff. | Summary | Adjusted p Value | ||
| CL-HEC | |||||
| 2% vs. 6% | −0.03333 | −0.1718 to 0.1052 | ns | 0.9931 | |
| 2% vs. 8% | 0 | −0.1385 to 0.1385 | ns | >0.9999 | |
| 2% vs. 10% | −0.03333 | −0.1718 to 0.1052 | ns | 0.9931 | |
| 2% vs. 16% | −0.03333 | −0.1718 to 0.1052 | ns | 0.9931 | |
| 2% vs. 20% | −0.03333 | −0.1718 to 0.1052 | ns | 0.9931 | |
| 2% vs. 30% | −0.03333 | −0.1718 to 0.1052 | ns | 0.9931 | |
| 2% vs. control | −1 | −1.138 to −0.8615 | **** | <0.0001 | |
| 6% vs. 8% | 0.03333 | −0.1052 to 0.1718 | ns | 0.9931 | |
| 6% vs. 10% | −2.8 × 10−17 | −0.1385 to 0.1385 | ns | >0.9999 | |
| 6% vs. 16% | −5.6 × 10−17 | −0.1385 to 0.1385 | ns | >0.9999 | |
| 6% vs. 20% | 0 | −0.1385 to 0.1385 | ns | >0.9999 | |
| 6% vs. 30% | 8.33 × 10−17 | −0.1385 to 0.1385 | ns | >0.9999 | |
| 6% vs. control | −0.9667 | −1.105 to −0.8282 | **** | <0.0001 | |
| 8% vs. 10% | −0.03333 | −0.1718 to 0.1052 | ns | 0.9931 | |
| 8% vs. 16% | −0.03333 | −0.1718 to 0.1052 | ns | 0.9931 | |
| 8% vs. 20% | −0.03333 | −0.1718 to 0.1052 | ns | 0.9931 | |
| 8% vs. 30% | −0.03333 | −0.1718 to 0.1052 | ns | 0.9931 | |
| 8% vs. control | −1 | −1.138 to −0.8615 | **** | <0.0001 | |
| 10% vs. 16% | −2.8 × 10−17 | −0.1385 to 0.1385 | ns | >0.9999 | |
| 10% vs. 20% | 2.78 × 10−17 | −0.1385 to 0.1385 | ns | >0.9999 | |
| 10% vs. 30% | 1.11 × 10−16 | −0.1385 to 0.1385 | ns | >0.9999 | |
| 10% vs. control | −0.9667 | −1.105 to −0.8282 | **** | <0.0001 | |
| 16% vs. 20% | 5.55 × 10−17 | −0.1385 to 0.1385 | ns | >0.9999 | |
| 16% vs. 30% | 1.39 × 10−16 | −0.1385 to 0.1385 | ns | >0.9999 | |
| 16% vs. control | −0.9667 | −1.105 to −0.8282 | **** | <0.0001 | |
| 20% vs. 30% | 8.33 × 10−17 | −0.1385 to 0.1385 | ns | >0.9999 | |
| 20% vs. control | −0.9667 | −1.105 to −0.8282 | **** | <0.0001 | |
| 30% vs. control | −0.9667 | −1.105 to −0.8282 | **** | <0.0001 | |
| CL-SA | |||||
| 2% vs. 6% | −0.03667 | −0.1752 to 0.1018 | ns | 0.9878 | |
| 2% vs. 8% | −0.02 | −0.1585 to 0.1185 | ns | 0.9997 | |
| 2% vs. 10% | 0.06333 | −0.07515 to 0.2018 | ns | 0.8113 | |
| 2% vs. 16% | 0.03 | −0.1085 to 0.1685 | ns | 0.9963 | |
| 2% vs. 20% | 0.06333 | −0.07515 to 0.2018 | ns | 0.8113 | |
| 2% vs. 30% | 0.06333 | −0.07515 to 0.2018 | ns | 0.8113 | |
| 2% vs. control | −0.9367 | −1.075 to −0.7982 | **** | <0.0001 | |
| 6% vs. 8% | 0.01667 | −0.1218 to 0.1552 | ns | >0.9999 | |
| 6% vs. 10% | 0.1 | −0.03849 to 0.2385 | ns | 0.3047 | |
| 6% vs. 16% | 0.06667 | −0.07182 to 0.2052 | ns | 0.7698 | |
| 6% vs. 20% | 0.1 | −0.03849 to 0.2385 | ns | 0.3047 | |
| 6% vs. 30% | 0.1 | −0.03849 to 0.2385 | ns | 0.3047 | |
| 6% vs. control | −0.9 | −1.038 to −0.7615 | **** | <0.0001 | |
| 8% vs. 10% | 0.08333 | −0.05515 to 0.2218 | ns | 0.5295 | |
| 8% vs. 16% | 0.05 | −0.08849 to 0.1885 | ns | 0.9348 | |
| 8% vs. 20% | 0.08333 | −0.05515 to 0.2218 | ns | 0.5295 | |
| 8% vs. 30% | 0.08333 | −0.05515 to 0.2218 | ns | 0.5295 | |
| 8% vs. control | −0.9167 | −1.055 to −0.7782 | **** | <0.0001 | |
| 10% vs. 16% | −0.03333 | −0.1718 to 0.1052 | ns | 0.9931 | |
| 10% vs. 20% | 0 | −0.1385 to 0.1385 | ns | >0.9999 | |
| 10% vs. 30% | 1.39 × 10−16 | −0.1385 to 0.1385 | ns | >0.9999 | |
| 10% vs. control | −1 | −1.138 to −0.8615 | **** | <0.0001 | |
| 16% vs. 20% | 0.03333 | −0.1052 to 0.1718 | ns | 0.9931 | |
| 16% vs. 30% | 0.03333 | −0.1052 to 0.1718 | ns | 0.9931 | |
| 16% vs. control | −0.9667 | −1.105 to −0.8282 | **** | <0.0001 | |
| 20% vs. 30% | 1.39 × 10−16 | −0.1385 to 0.1385 | ns | >0.9999 | |
| 20% vs. control | −1 | −1.138 to −0.8615 | **** | <0.0001 | |
| 30% vs. control | −1 | −1.138 to −0.8615 | **** | <0.0001 | |
| Pearson r | ||
|---|---|---|
| r | 0.9053 | 0.9357 |
| 95% confidence interval | 0.1144 to 0.9938 | 0.5160 to 0.9931 |
| R squared | 0.8196 | 0.8756 |
| pvalue | ||
| p(two-tailed) | 0.0345 | 0.0061 |
| pvalue summary | * | ** |
| Significant? (alpha = 0.05) | Yes | Yes |
| Number of XY Pairs | 5 | 6 |
| ANOVA Summary | |||||
|---|---|---|---|---|---|
| F | 132.1 | ||||
| p value | <0.0001 | ||||
| p value summary | **** | ||||
| Significant diff. among means (p < 0.05)? | Yes | ||||
| R square | 0.9822 | ||||
| ANOVA table | |||||
| SS | DF | MS | F (DFn. DFd) | p value | |
| Treatment (between columns) | 4.631 | 5 | 0.9262 | F (5, 12) = 132.1 | p < 0.0001 |
| Residual (within columns) | 0.08413 | 12 | 0.007011 | ||
| Total | 4.715 | 17 | |||
| Number of families | 1 | ||||
| Number of comparisons per family | 15 | ||||
| Alpha | 0.05 | ||||
| Tukey’s multiple comparisons test | |||||
| Mean Diff. | 95% CI of diff. | Summary | Adjusted p Value | ||
| <1% (HEC16) vs. 2% | 0.3228 | 0.09314 to 0.5524 | ** | 0.0051 | |
| <1% (HEC16) vs. 3% | 0.5509 | 0.3212 to 0.7805 | **** | <0.0001 | |
| <1% (HEC16) vs. 4% | 1.319 | 1.090 to 1.549 | **** | <0.0001 | |
| <1% (HEC16) vs. 5% | 1.319 | 1.090 to 1.549 | **** | <0.0001 | |
| <1% (HEC16) vs. Control | 0.3191 | 0.08951 to 0.5488 | ** | 0.0056 | |
| 2% vs. 3% | 0.2281 | −0.001558 to 0.4577 | ns | 0.0519 | |
| 2% vs. 4% | 0.9964 | 0.7667 to 1.226 | **** | <0.0001 | |
| 2% vs. 5% | 0.9964 | 0.7667 to 1.226 | **** | <0.0001 | |
| 2% vs. Control | −0.003630 | −0.2333 to 0.2260 | ns | >0.9999 | |
| 3% vs. 4% | 0.7683 | 0.5387 to 0.9979 | **** | <0.0001 | |
| 3% vs. 5% | 0.7683 | 0.5387 to 0.9979 | **** | <0.0001 | |
| 3% vs. Control | −0.2317 | −0.4613 to −0.002072 | * | 0.0475 | |
| 4% vs. 5% | 0.000 | −0.2296 to 0.2296 | ns | >0.9999 | |
| 4% vs. Control | −1.000 | −1.230 to −0.7704 | **** | <0.0001 | |
| 5% vs. Control | −1.000 | −1.230 to −0.7704 | **** | <0.0001 | |
| ANOVA Summary | |||||
|---|---|---|---|---|---|
| F | 66.89 | ||||
| p value | <0.0001 | ||||
| p value summary | **** | ||||
| Significant diff. among means (p < 0.05)? | Yes | ||||
| R square | 0.9663 | ||||
| ANOVA table | |||||
| SS | DF | MS | F (DFn. DFd) | p value | |
| Treatment (between columns) | 6.890 | 6 | 1.148 | F (6, 14) = 66.89 | p < 0.0001 |
| Residual (within columns) | 0.2403 | 14 | 0.01717 | ||
| Total | 7.130 | 20 | |||
| Tukey’s multiple comparisons test | |||||
| Number of families | 1 | ||||
| Number of comparisons per family | 21 | ||||
| Alpha | 0.05 | ||||
| Mean Diff. | 95% CI of diff. | Summary | Adjusted p Value | ||
| <4.5 vs. 5.5–4.5 | −0.8333 | −1.199 to −0.4680 | **** | <0.0001 | |
| <4.5 vs. 6.5–5.5 | −1.186 | −1.551 to −0.8205 | **** | <0.0001 | |
| <4.5 vs. 6.5–7.5 (HEC16) | −1.417 | −1.782 to −1.051 | **** | <0.0001 | |
| <4.5 vs. 8–9 | 0.000 | −0.3653 to 0.3653 | ns | >0.9999 | |
| <4.5 vs. 10 | 0.000 | −0.3653 to 0.3653 | ns | >0.9999 | |
| <4.5 vs. Control | −1.000 | −1.365 to −0.6347 | **** | <0.0001 | |
| 5.5–4.5 vs. 6.5–5.5 | −0.3525 | −0.7178 to 0.01281 | ns | 0.0618 | |
| 5.5–4.5 vs. 6.5–7.5 (HEC16) | −0.5833 | −0.9486 to −0.2180 | ** | 0.0013 | |
| 5.5–4.5 vs. 8–9 | 0.8333 | 0.4680 to 1.199 | **** | <0.0001 | |
| 5.5–4.5 vs. 10 | 0.8333 | 0.4680 to 1.199 | **** | <0.0001 | |
| 5.5–4.5 vs. Control | −0.1667 | −0.5320 to 0.1986 | ns | 0.7086 | |
| 6.5–5.5 vs. 6.5–7.5 (HEC16) | −0.2309 | −0.5961 to 0.1344 | ns | 0.3737 | |
| 6.5–5.5 vs. 8–9 | 1.186 | 0.8205 to 1.551 | **** | <0.0001 | |
| 6.5–5.5 vs. 10 | 1.186 | 0.8205 to 1.551 | **** | <0.0001 | |
| 6.5–5.5 vs. Control | 0.1858 | −0.1795 to 0.5511 | ns | 0.6052 | |
| 6.5–7.5 (HEC16) vs. 8–9 | 1.417 | 1.051 to 1.782 | **** | <0.0001 | |
| 6.5–7.5 (HEC16) vs. 10 | 1.417 | 1.051 to 1.782 | **** | <0.0001 | |
| 6.5–7.5 (HEC16) vs. Control | 0.4167 | 0.05137 to 0.7820 | * | 0.0210 | |
| 8–9 vs. 10 | 0.000 | −0.3653 to 0.3653 | ns | >0.9999 | |
| 8–9 vs. Control | −1.000 | −1.365 to −0.6347 | **** | <0.0001 | |
| 10 vs. Control | −1.000 | −1.365 to −0.6347 | **** | <0.0001 | |
| ANOVA Summary | |||||
|---|---|---|---|---|---|
| F | 16.33 | ||||
| p value | <0.0001 | ||||
| p value summary | **** | ||||
| Significant diff. among means (p < 0.05)? | Yes | ||||
| R square | 0.8750 | ||||
| ANOVA table | |||||
| SS | DF | MS | F (DFn. DFd) | p value | |
| Treatment (between columns) | 0.3405 | 6 | 0.05675 | F (6, 14) = 16.33 | p < 0.0001 |
| Residual (within columns) | 0.04867 | 14 | 0.003476 | ||
| Total | 0.3892 | 20 | |||
| Tukey’s multiple comparisons test | |||||
| Number of families | 1 | ||||
| Number of comparisons per family | 21 | ||||
| Alpha | 0.05 | ||||
| Mean Diff. | 95% CI of diff. | Summary | Adjusted p Value | ||
| S10 vs. S8 | 0.01667 | −0.1477 to 0.1810 | ns | 0.9998 | |
| S10 vs. S6 | −0.2167 | −0.3810 to −0.05229 | ** | 0.0070 | |
| S10 vs. S4 | −0.1533 | −0.3177 to 0.01104 | ns | 0.0749 | |
| S10 vs. S2 | −0.1733 | −0.3377 to −0.008955 | * | 0.0358 | |
| S10 vs. S0 (HEC16) | −0.1900 | −0.3544 to −0.02562 | * | 0.0191 | |
| S10 vs. Control | 0.1500 | −0.01438 to 0.3144 | ns | 0.0845 | |
| S8 vs. S6 | −0.2333 | −0.3977 to −0.06896 | ** | 0.0037 | |
| S8 vs. S4 | −0.1700 | −0.3344 to −0.005622 | * | 0.0406 | |
| S8 vs. S2 | −0.1900 | −0.3544 to −0.02562 | * | 0.0191 | |
| S8 vs. S0 (HEC16) | −0.2067 | −0.3710 to −0.04229 | * | 0.0102 | |
| S8 vs. Control | 0.1333 | −0.03104 to 0.2977 | ns | 0.1510 | |
| S6 vs. S4 | 0.06333 | −0.1010 to 0.2277 | ns | 0.8341 | |
| S6 vs. S2 | 0.04333 | −0.1210 to 0.2077 | ns | 0.9665 | |
| S6 vs. S0 (HEC16) | 0.02667 | −0.1377 to 0.1910 | ns | 0.9972 | |
| S6 vs. Control | 0.3667 | 0.2023 to 0.5310 | **** | <0.0001 | |
| S4 vs. S2 | −0.02000 | −0.1844 to 0.1444 | ns | 0.9994 | |
| S4 vs. S0 (HEC16) | −0.03667 | −0.2010 to 0.1277 | ns | 0.9852 | |
| S4 vs. Control | 0.3033 | 0.1390 to 0.4677 | *** | 0.0003 | |
| S2 vs. S0 (HEC16) | −0.01667 | −0.1810 to 0.1477 | ns | 0.9998 | |
| S2 vs. Control | 0.3233 | 0.1590 to 0.4877 | *** | 0.0002 | |
| S0 (HEC16) vs. Control | 0.3400 | 0.1756 to 0.5044 | **** | <0.0001 | |
| ANOVA Summary | |||||
|---|---|---|---|---|---|
| F | 80.01 | ||||
| p value | <0.0001 | ||||
| p value summary | **** | ||||
| Significant diff. among means (p < 0.05)? | Yes | ||||
| R square | 0.9717 | ||||
| ANOVA table | |||||
| SS | DF | MS | F (DFn. DFd) | p value | |
| Treatment (between columns) | 13.22 | 6 | 2.204 | F (6, 14) = 80.01 | p < 0.0001 |
| Residual (within columns) | 0.3856 | 14 | 0.02754 | ||
| Total | 13.61 | 20 | |||
| Tukey’s multiple comparisons test | |||||
| Number of families | 1 | ||||
| Number of comparisons per family | 21 | ||||
| Alpha | 0.05 | ||||
| Mean Diff. | 95% CI of diff. | Summary | Adjusted p Value | ||
| Tb10 vs. Tb8 | −0.1333 | −0.5960 to 0.3294 | ns | 0.9496 | |
| Tb10 vs. Tb6 | 0.9333 | 0.4706 to 1.396 | *** | 0.0001 | |
| Tb10 vs. Tb4 | 1.473 | 1.011 to 1.936 | **** | <0.0001 | |
| Tb10 vs. Tb2 | 1.673 | 1.211 to 2.136 | **** | <0.0001 | |
| Tb10 vs. Tb0 (HEC16) | 1.657 | 1.194 to 2.119 | **** | <0.0001 | |
| Tb10 vs. Control | 2.033 | 1.571 to 2.496 | **** | <0.0001 | |
| Tb8 vs. Tb6 | 1.067 | 0.6040 to 1.529 | **** | <0.0001 | |
| Tb8 vs. Tb4 | 1.607 | 1.144 to 2.069 | **** | <0.0001 | |
| Tb8 vs. Tb2 | 1.807 | 1.344 to 2.269 | **** | <0.0001 | |
| Tb8 vs. Tb0 (HEC16) | 1.790 | 1.327 to 2.253 | **** | <0.0001 | |
| Tb8 vs. Control | 2.167 | 1.704 to 2.629 | **** | <0.0001 | |
| Tb6 vs. Tb4 | 0.5400 | 0.07730 to 1.003 | * | 0.0178 | |
| Tb6 vs. Tb2 | 0.7400 | 0.2773 to 1.203 | ** | 0.0013 | |
| Tb6 vs. Tb0 (HEC16) | 0.7233 | 0.2606 to 1.186 | ** | 0.0016 | |
| Tb6 vs. Control | 1.100 | 0.6373 to 1.563 | **** | <0.0001 | |
| Tb4 vs. Tb2 | 0.2000 | −0.2627 to 0.6627 | ns | 0.7538 | |
| Tb4 vs. Tb0 (HEC16) | 0.1833 | −0.2794 to 0.6460 | ns | 0.8166 | |
| Tb4 vs. Control | 0.5600 | 0.09730 to 1.023 | * | 0.0136 | |
| Tb2 vs. Tb0 (HEC16) | −0.01667 | −0.4794 to 0.4460 | ns | >0.9999 | |
| Tb2 vs. Control | 0.3600 | −0.1027 to 0.8227 | ns | 0.1809 | |
| Tb0 (HEC16) vs. Control | 0.3767 | −0.08603 to 0.8394 | ns | 0.1486 | |
| Ordinary Two-Way ANOVA | |||||
|---|---|---|---|---|---|
| Alpha | 0.05 | ||||
| Source of Variation | % of total variation | p value | p value summary | ||
| Interaction | 1.265 | <0.0001 | **** | ||
| Substrate type | 97.92 | <0.0001 | **** | ||
| Week | 0.3163 | 0.0020 | ** | ||
| ANOVA table | |||||
| SS | DF | MS | F (DFn. DFd) | p value | |
| Interaction | 0.9577 | 4 | 0.2394 | F (4, 20) = 12.68 | p < 0.0001 |
| Substrate type | 74.11 | 4 | 18.53 | F (4, 20) = 981.3 | p < 0.0001 |
| Week | 0.2394 | 1 | 0.2394 | F (1, 20) = 12.68 | p = 0.0020 |
| Residual | 0.3776 | 20 | 0.01888 | ||
| Tukey’s multiple comparisons test | |||||
| Number of families | 1 | ||||
| Number of comparisons per family | 45 | ||||
| Alpha | 0.05 | ||||
| Mean Diff. | 95% CI of diff. | Summary | Adjusted p Value | ||
| Masonite:week1 vs. Masonite:week2 | 0.000 | −0.3973 to 0.3973 | ns | >0.9999 | |
| Masonite:week1 vs. HEC16:week1 | −0.1933 | −0.5906 to 0.2039 | ns | 0.7709 | |
| Masonite:week1 vs. HEC16:week2 | −0.1933 | −0.5906 to 0.2039 | ns | 0.7709 | |
| Masonite:week1 vs. HEC16/S6:week1 | −0.2167 | −0.6139 to 0.1806 | ns | 0.6504 | |
| Masonite:week1 vs. HEC16/S6:week2 | −1.110 | −1.507 to −0.7127 | **** | <0.0001 | |
| Masonite:week1 vs. HEC16/Tb8:week1 | −4.110 | −4.507 to −3.713 | **** | <0.0001 | |
| Masonite:week1 vs. HEC16/Tb8:week2 | −4.110 | −4.507 to −3.713 | **** | <0.0001 | |
| Masonite:week1 vs. Control:week1 | −0.04333 | −0.4406 to 0.3539 | ns | >0.9999 | |
| Masonite:week1 vs. Control:week2 | −0.04333 | −0.4406 to 0.3539 | ns | >0.9999 | |
| Masonite:week2 vs. HEC16:week1 | −0.1933 | −0.5906 to 0.2039 | ns | 0.7709 | |
| Masonite:week2 vs. HEC16:week2 | −0.1933 | −0.5906 to 0.2039 | ns | 0.7709 | |
| Masonite:week2 vs. HEC16/S6:week1 | −0.2167 | −0.6139 to 0.1806 | ns | 0.6504 | |
| Masonite:week2 vs. HEC16/S6:week2 | −1.110 | −1.507 to −0.7127 | **** | <0.0001 | |
| Masonite:week2 vs. HEC16/Tb8:week1 | −4.110 | −4.507 to −3.713 | **** | <0.0001 | |
| Masonite:week2 vs. HEC16/Tb8:week2 | −4.110 | −4.507 to −3.713 | **** | <0.0001 | |
| Masonite:week2 vs. Control:week1 | −0.04333 | −0.4406 to 0.3539 | ns | >0.9999 | |
| Masonite:week2 vs. Control:week2 | −0.04333 | −0.4406 to 0.3539 | ns | >0.9999 | |
| HEC16:week1 vs. HEC16:week2 | 0.000 | −0.3973 to 0.3973 | ns | >0.9999 | |
| HEC16:week1 vs. HEC16/S6:week1 | −0.02333 | −0.4206 to 0.3739 | ns | >0.9999 | |
| HEC16:week1 vs. HEC16/S6:week2 | −0.9167 | −1.314 to −0.5194 | **** | <0.0001 | |
| HEC16:week1 vs. HEC16/Tb8:week1 | −3.917 | −4.314 to −3.519 | **** | <0.0001 | |
| HEC16:week1 vs. HEC16/Tb8:week2 | −3.917 | −4.314 to −3.519 | **** | <0.0001 | |
| HEC16:week1 vs. Control:week1 | 0.1500 | −0.2473 to 0.5473 | ns | 0.9325 | |
| HEC16:week1 vs. Control:week2 | 0.1500 | −0.2473 to 0.5473 | ns | 0.9325 | |
| HEC16:week2 vs. HEC16/S6:week1 | −0.02333 | −0.4206 to 0.3739 | ns | >0.9999 | |
| HEC16:week2 vs. HEC16/S6:week2 | −0.9167 | −1.314 to −0.5194 | **** | <0.0001 | |
| HEC16:week2 vs. HEC16/Tb8:week1 | −3.917 | −4.314 to −3.519 | **** | <0.0001 | |
| HEC16:week2 vs. HEC16/Tb8:week2 | −3.917 | −4.314 to −3.519 | **** | <0.0001 | |
| HEC16:week2 vs. Control:week1 | 0.1500 | −0.2473 to 0.5473 | ns | 0.9325 | |
| HEC16:week2 vs. Control:week2 | 0.1500 | −0.2473 to 0.5473 | ns | 0.9325 | |
| HEC16/S6:week1 vs. HEC16/S6:week2 | −0.8933 | −1.291 to −0.4961 | **** | <0.0001 | |
| HEC16/S6:week1 vs. HEC16/Tb8:week1 | −3.893 | −4.291 to −3.496 | **** | <0.0001 | |
| HEC16/S6:week1 vs. HEC16/Tb8:week2 | −3.893 | −4.291 to −3.496 | **** | <0.0001 | |
| HEC16/S6:week1 vs. Control:week1 | 0.1733 | −0.2239 to 0.5706 | ns | 0.8583 | |
| HEC16/S6:week1 vs. Control:week2 | 0.1733 | −0.2239 to 0.5706 | ns | 0.8583 | |
| HEC16/S6:week2 vs. HEC16/Tb8:week1 | −3.000 | −3.397 to −2.603 | **** | <0.0001 | |
| HEC16/S6:week2 vs. HEC16/Tb8:week2 | −3.000 | −3.397 to −2.603 | **** | <0.0001 | |
| HEC16/S6:week2 vs. Control:week1 | 1.067 | 0.6694 to 1.464 | **** | <0.0001 | |
| HEC16/S6:week2 vs. Control:week2 | 1.067 | 0.6694 to 1.464 | **** | <0.0001 | |
| HEC16/Tb8:week1 vs. HEC16/Tb8:week2 | 0.000 | −0.3973 to 0.3973 | ns | >0.9999 | |
| HEC16/Tb8:week1 vs. Control:week1 | 4.067 | 3.669 to 4.464 | **** | <0.0001 | |
| HEC16/Tb8:week1 vs. Control:week2 | 4.067 | 3.669 to 4.464 | **** | <0.0001 | |
| HEC16/Tb8:week2 vs. Control:week1 | 4.067 | 3.669 to 4.464 | **** | <0.0001 | |
| HEC16/Tb8:week2 vs. Control:week2 | 4.067 | 3.669 to 4.464 | **** | <0.0001 | |
| Control:week1 vs. Control:week2 | 0.000 | −0.3973 to 0.3973 | ns | >0.9999 | |
| Ordinary Two-Way ANOVA | |||||
|---|---|---|---|---|---|
| Alpha | 0.05 | ||||
| Source of Variation | % of total variation | p value | p value summary | ||
| Interaction | 13.35 | <0.0001 | **** | ||
| Substrate | 7.908 | <0.0001 | **** | ||
| Week | 78.48 | <0.0001 | **** | ||
| ANOVA table | SS | DF | MS | F (DFn. DFd) | p value |
| Interaction | 10.38 | 2 | 5.192 | F (2, 12) = 309.2 | p < 0.0001 |
| Substrate | 6.150 | 1 | 6.150 | F (1, 12) = 366.2 | p < 0.0001 |
| Week | 61.04 | 2 | 30.52 | F (2, 12) = 1817 | p < 0.0001 |
| Residual | 0.2015 | 12 | 0.01679 | ||
| Tukey’s multiple comparisons test | |||||
| Number of families | 1 | ||||
| Number of comparisons per family | 15 | ||||
| Alpha | 0.05 | ||||
| Mean Diff. | 95% CI of diff. | Summary | Adjusted p Value | ||
| 0–15 days: HEC16 vs. 0–15 days: HEC16/S6/TB8 | −0.2809 | −0.6363 to 0.07451 | ns | 0.1569 | |
| 0–15 days: HEC16 vs. 0–15 days: Control | 2.548 | 2.193 to 2.904 | **** | <0.0001 | |
| 0–15 days: HEC16 vs. 15–30 days: HEC16 | −0.1927 | −0.5481 to 0.1627 | ns | 0.4884 | |
| 0–15 days: HEC16 vs. 15–30 days: HEC16/S6/TB8 | −3.595 | −3.951 to −3.240 | **** | <0.0001 | |
| 0–15 days: HEC16 vs. 15–30 days: Control | 2.548 | 2.193 to 2.904 | **** | <0.0001 | |
| 0–15 days: HEC16/S6/TB8 vs. 0–15 days: Control | 2.829 | 2.474 to 3.185 | **** | <0.0001 | |
| 0–15 days: HEC16/S6/TB8 vs. 15–30 days: HEC16 | 0.08818 | −0.2672 to 0.4436 | ns | 0.9552 | |
| 0–15 days: HEC16/S6/TB8 vs. 15–30 days: HEC16/S6/TB8 | −3.315 | −3.670 to −2.959 | **** | <0.0001 | |
| 0–15 days: HEC16/S6/TB8 vs. 15–30 days: Control | 2.829 | 2.474 to 3.185 | **** | <0.0001 | |
| 0–15 days: Control vs. 15–30 days: HEC16 | −2.741 | −3.097 to −2.386 | **** | <0.0001 | |
| 0–15 days: Control vs. 15–30 days: HEC16/S6/TB8 | −6.144 | −6.499 to −5.789 | **** | <0.0001 | |
| 0–15 days: Control vs. 15–30 days: Control | 0.000 | −0.3554 to 0.3554 | ns | >0.9999 | |
| 15–30 days: HEC16 vs. 15–30 days: HEC16/S6/TB8 | −3.403 | −3.758 to −3.047 | **** | <0.0001 | |
| 15–30 days: HEC16 vs. 15–30 days: Control | 2.741 | 2.386 to 3.097 | **** | <0.0001 | |
| 15–30 days: HEC16/S6/TB8 vs. 15–30 days: Control | 6.144 | 5.789 to 6.499 | **** | <0.0001 | |
References
- World Health Organization. World Malaria Report 2016; World Health Organization: Geneva, Switzerland, 2016.
- Jiggins, F.M. The spread of Wolbachia through mosquito populations. PLOS Biol. 2017, 15, e2002780. [Google Scholar] [CrossRef] [PubMed]
- Domingues, M.; Santos, P.L. Essential oil of pennyroyal (Mentha pulegium): Composition and applications as alternatives to pesticides—New tendencies. Ind. Crop. Prod. 2019, 139, 111534. [Google Scholar] [CrossRef]
- Benelli, G.; Canale, A.; Conti, B. Eco-friendly Control Strategies Against the Asian Tiger Mosquito, Aedes albopictus (Diptera: Culicidae): Repellency and Toxic Activity of Plant Essential Oils and Extracts. Pharmacol. Online 2014, 1, 44–50. [Google Scholar]
- Immediato, D.; Figueredo, L.A.; Iatta, R.; Camarda, A.; de Luna, R.L.N.; Giangaspero, A.; Filho, S.B.; Otranto, D.; Cafarchia, C. Essential oils and Beauveria bassiana against Dermanyssus gallinae (Acari: Dermanyssidae): Towards new natural acaricides. Vet. Parasitol. 2016, 229, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Cafarchia, C.; Immediato, D.; Iatta, R.; Ramos, R.A.N.; Lia, R.P.; Porretta, D.; Figueredo, L.A.; Dantas-Torres, F.; Otranto, D. Native strains of Beauveria bassiana for the control of Rhipicephalus sanguineus sensu lato. Parasites Vectors 2015, 8, 80. [Google Scholar] [CrossRef][Green Version]
- Immediato, D.; Camarda, A.; Iatta, R.; Puttilli, M.R.; Ramos, R.A.N.; Di Paola, G.; Giangaspero, A.; Otranto, D.; Cafarchia, C. Laboratory evaluation of a native strain of Beauveria bassiana for controlling Dermanyssus gallinae (De Geer, 1778) (Acari: Dermanyssidae). Vet. Parasitol. 2015, 212, 478–482. [Google Scholar] [CrossRef]
- Lee, J.Y.; Woo, R.M.; Choi, C.J.; Shin, T.Y.; Gwak, W.S.; Woo, S.D. Beauveria bassiana for the simultaneous control of Aedes albopictus and Culex pipiens mosquito adults shows high conidia persistence and productivity. AMB Express 2019, 9, 206. [Google Scholar] [CrossRef]
- Diffenbaugh, N.S.; Krupke, C.H.; White, M.; Alexander, C.E. Global warming presents new challenges for maize pest management. Environ. Res. Lett. 2008, 3, 044007. [Google Scholar] [CrossRef]
- Ahmad, L.; Mahdi, S.S. (Eds.) Precision Pest Management, in Satellite Farming: An Information and Technology Based Agriculture; Springer International Publishing: Cham, Switzerland, 2018; pp. 119–127. [Google Scholar]
- Vacanti, C. The history of tissue engineering. J. Cell. Mol. Med. 2007, 10, 569–576. [Google Scholar] [CrossRef]
- Aguado, B.A.; Grim, J.C.; Rosales, A.M.; Watson-Capps, J.J.; Anseth, K.S. Engineering precision biomaterials for personalized medicine. Sci. Transl. Med. 2018, 10, eaam8645. [Google Scholar] [CrossRef]
- Buczkowski, G.; Roper, E.; Chin, D. Polyacrylamide Hydrogels: An Effective Tool for Delivering Liquid Baits to Pest Ants (Hymenoptera: Formicidae). J. Econ. Èntomol. 2014, 107, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Slippers, B.; Hurley, B.P.; Allison, J. Harnessing the potential of Precision Pest Management in plantation forests. South. For. J. For. Sci. 2020, 82, 197–201. [Google Scholar] [CrossRef]
- Dara, S.K. The New Integrated Pest Management Paradigm for the Modern Age. J. Integr. Pest Manag. 2019, 10, 12. [Google Scholar] [CrossRef]
- Fouet, C.; Kamdem, C. Integrated Mosquito Management: Is Precision Control a Luxury or Necessity? Trends Parasitol. 2019, 35, 85–95. [Google Scholar] [CrossRef]
- Powell, J.R. Mosquitoes on the move. Science 2016, 354, 971–972. [Google Scholar] [CrossRef] [PubMed]
- Dormont, L.; Mulatier, M.; Carrasco, D.; Cohuet, A. Mosquito Attractants. J. Chem. Ecol. 2021, 47, 351–393. [Google Scholar] [CrossRef]
- Amraoui, F.; Failloux, A.-B. Chikungunya: An unexpected emergence in Europe. Curr. Opin. Virol. 2016, 21, 146–150. [Google Scholar] [CrossRef]
- Bellini, R.; Michaelakis, A.; Petrić, D.; Schaffner, F.; Alten, B.; Angelini, P.; Aranda, C.; Becker, N.; Carrieri, M.; Di Luca, M.; et al. Practical management plan for invasive mosquito species in Europe: I. Asian tiger mosquito (Aedes albopictus). Travel Med. Infect. Dis. 2020, 35, 101691. [Google Scholar] [CrossRef]
- Benedict, M.Q.; Levine, R.S.; Hawley, W.A.; Lounibos, L.P. Spread of The Tiger: Global Risk of Invasion by The Mosquito Aedes albopictus. Vector-Borne Zoonotic Dis. 2007, 7, 76–85. [Google Scholar] [CrossRef]
- Braks, M.; Medlock, J.M.; Hubalek, Z.; Hjertqvist, M.; Perrin, Y.; Lancelot, R.; Duchyene, E.; Hendrickx, G.; Stroo, A.; Heyman, P.; et al. Vector-Borne Disease Intelligence: Strategies to Deal with Disease Burden and Threats. Front. Public Health 2014, 2, 280. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Reiner, R.C., Jr.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Mweresa, C.K.; Mukabana, W.R.; Van Loon, J.J.A.; Dicke, M.; Takken, W. Use of semiochemicals for surveillance and control of hematophagous insects. Chemoecology 2020, 30, 277–286. [Google Scholar] [CrossRef]
- Bukhari, T.; Takken, W.; Koenraadt, C.J.M. Development of Metarhizium anisopliae and Beauveria bassiana formulations for control of malaria mosquito larvae. Parasites Vectors 2011, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Wilke, A.B.B.; Carvajal, A.; Medina, J.; Anderson, M.; Nieves, V.J.; Ramirez, M.; Vasquez, C.; Petrie, W.; Cardenas, G.; Beier, J.C. Assessment of the effectiveness of BG-Sentinel traps baited with CO2 and BG-Lure for the surveillance of vector mosquitoes in Miami-Dade County, Florida. PLoS ONE 2019, 14, e0212688. [Google Scholar] [CrossRef] [PubMed]
- Wooding, M.; Naudé, Y.; Rohwer, E.; Bouwer, M. Controlling mosquitoes with semiochemicals: A review. Parasites Vectors 2020, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Friuli, M.; Cafarchia, C.; Lia, R.P.; Otranto, D.; Pombi, M.; Demitri, C. From tissue engineering to mosquitoes: Biopolymers as tools for developing a novel biomimetic approach to pest management/vector control. Parasites Vectors 2022, 15, 79. [Google Scholar] [CrossRef]
- Freed, L.; Vunjak-Novakovic, G.; Biron, R.J.; Eagles, D.B.; Lesnoy, D.C.; Barlow, S.K.; Langer, R. Biodegradable Polymer Scaffolds for Tissue Engineering. Nat. Biotechnol. 1994, 12, 689–693. [Google Scholar] [CrossRef]
- Grayson, W.L.; Martens, T.P.; Eng, G.M.; Radisic, M.; Vunjak-Novakovic, G. Biomimetic approach to tissue engineering. Semin. Cell Dev. Biol. 2009, 20, 665–673. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920. [Google Scholar] [CrossRef]
- Friuli, M.; Nitti, P.; Madaghiele, M.; Demitri, C. A possible method to avoid skin effect in polymeric scaffold produced through thermally induced phase separation. Results Eng. 2021, 12, 100282. [Google Scholar] [CrossRef]
- Felizatti, A.P.; Manzano, R.M.; Rodrigues, I.M.W.; Silva, M.F.D.G.F.D.; Fernandes, J.B.; Forim, M.R. Encapsulation of B. bassiana in Biopolymers: Improving Microbiology of Insect Pest Control. Front. Microbiol. 2021, 12, 704812. [Google Scholar] [CrossRef] [PubMed]
- Kulinets, I.; Amato, S.F.; Ezzell, R.M. 1—Biomaterials and their applications in medicine. In Regulatory Affairs for Biomaterials and Medical Devices; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2015; pp. 1–10. [Google Scholar]
- Nicodemus, G.D.; Bryant, S.J. Cell Encapsulation in Biodegradable Hydrogels for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Khetan, S.; Burdick, J. Au—Burdick, Cellular Encapsulation in 3D Hydrogels for Tissue Engineering. JoVE 2009, 32, e1590. [Google Scholar]
- Batista, D.P.C.; de Oliveira, I.N.; Ribeiro, A.R.B.; Fonseca, E.J.S.; Santos-Magalhães, N.S.; de Sena-Filho, J.G.; Teodoro, A.V.; Grillo, L.A.M.; de Almeida, R.S.; Dornelas, C.B. Encapsulation and release of Beauveria bassiana from alginate–bentonite nanocomposite. RSC Adv. 2017, 7, 26468–26477. [Google Scholar] [CrossRef]
- Blanford, S.; Jenkins, N.E.; Christian, R.; Chan, B.H.; Nardini, L.; Osae, M.; Koekemoer, L.; Coetzee, M.; Read, A.F.; Thomas, M.B. Storage and persistence of a candidate fungal biopesticide for use against adult malaria vectors. Malar. J. 2012, 11, 354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blanford, S.; Shi, W.; Christian, R.; Marden, J.H.; Koekemoer, L.L.; Brooke, B.D.; Coetzee, M.; Read, A.F.; Thomas, M.B. Lethal and Pre-Lethal Effects of a Fungal Biopesticide Contribute to Substantial and Rapid Control of Malaria Vectors. PLoS ONE 2011, 6, e23591. [Google Scholar] [CrossRef] [PubMed]
- Friuli, M.; Nitti, P.; Aneke, C.I.; Demitri, C.; Cafarchia, C.; Otranto, D. Freeze-drying of Beauveria bassiana suspended in Hydroxyethyl cellulose based hydrogel as possible method for storage: Evaluation of survival, growth and stability of conidial concentration before and after processing. Results Eng. 2021, 12, 100283. [Google Scholar] [CrossRef]
- Jürgens, A.; Wee, S.-L.; Shuttleworth, A.; Johnson, S. Chemical mimicry of insect oviposition sites: A global analysis of convergence in angiosperms. Ecol. Lett. 2013, 16, 1157–1167. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Kulikowski, R. Potential of Hydrogel Application for Plant Protection. Ecol. Chem. Eng. 2009, 16, 1191–1198. [Google Scholar]
- Sannino, A.; Madaghiele, M.; Demitri, C.; Scalera, F.; Esposito, A.; Esposito, V.; Maffezzoli, A. Development and characterization of cellulose-based hydrogels for use as dietary bulking agents. J. Appl. Polym. Sci. 2009, 115, 1438–1444. [Google Scholar] [CrossRef]
- Benelli, G.; Wilke, A.B.B.; Beier, J.C. Aedes albopictus (Asian Tiger Mosquito). Trends Parasitol. 2020, 36, 942–943. [Google Scholar] [CrossRef] [PubMed]
- Keswani, C.; Singh, S.P.; Singh, H.B. Beauveria bassiana: Status, Mode of action, Applications and Safety issues. Biotech Today 2013, 3, 16. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, S.; Yu, J.S.; Kim, J.C.; Nai, Y.-S.; Kim, J.S. Biological control of Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae) using Metarhizium anisopliae JEF-003 millet grain. J. Asia-Pac. Èntomol. 2015, 18, 217–221. [Google Scholar] [CrossRef]
- Caputo, B.; Ienco, A.; Cianci, D.; Pombi, M.; Petrarca, V.; Baseggio, A.; Devine, G.J.; Della Torre, A. The “Auto-Dissemination” Approach: A Novel Concept to Fight Aedes albopictus in Urban Areas. PLoS Negl. Trop. Dis. 2012, 6, e1793. [Google Scholar] [CrossRef]
- Degefa, T.; Yewhalaw, D.; Zhou, G.; Lee, M.-C.; Atieli, H.; Githeko, A.K.; Yan, G. Evaluation of the performance of new sticky pots for outdoor resting malaria vector surveillance in western Kenya. Parasites Vectors 2019, 12, 278. [Google Scholar] [CrossRef]
- Eiras, A.E.; Costa, L.H.; Batista-Pereira, L.G.; Paixão, K.S.; Batista, E.P.A. Semi-field assessment of the Gravid Aedes Trap (GAT) with the aim of controlling Aedes (Stegomyia) aegypti populations. PLoS ONE 2021, 16, e0250893. [Google Scholar] [CrossRef]
- Facchinelli, L.; Valerio, L.; Pombi, M.; Reiter, P.; Costantini, C.; Della Torre, A. Development of a novel sticky trap for container-breeding mosquitoes and evaluation of its sampling properties to monitor urban populations of Aedes albopictus. Med. Vet. Èntomol. 2007, 21, 183–195. [Google Scholar] [CrossRef]
- Johnson, B.J.; Ritchie, S.A.; Fonseca, D.M. The State of the Art of Lethal Oviposition Trap-Based Mass Interventions for Arboviral Control. Insects 2017, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.J.; Amador, M.; Barrera, R. An improved autocidal gravid ovitrap for the control and surveillance of Aedes aegypti. Parasites Vectors 2013, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.A.; Long, S.A.; McCaffrey, N.; Key, C.; Lonergan, G.; Williams, C.R. A Biodegradable Lethal Ovitrap for Control of Container-Breeding Aedes. J. Am. Mosq. Control Assoc. 2008, 24, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Hawley, W.A. The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. Suppl. 1988, 1, 1–39. [Google Scholar]
- Dom, N.C.; Mokhtar, M.A.M.; Australia, C.T. Development and oviposition preferences of field collected Aedes albopictus based on different water characteristics. Malays. J. Fundam. Appl. Sci. 2019, 15, 61–64. [Google Scholar] [CrossRef][Green Version]
- Day, J.F. Mosquito Oviposition Behavior and Vector Control. Insects 2016, 7, 65. [Google Scholar] [CrossRef]
- Jinguji, H.; Fujiwara, Y.; Ohtsu, K.; Morimoto, M. Effects of Sodium Chloride on Oviposition Behavior of Aedes albopictus. J. Am. Mosq. Control Assoc. 2020, 36, 253–256. [Google Scholar] [CrossRef]
- Madzlan, F.; Dom, N.C.; Tiong, C.S.; Zakaria, N. Breeding Characteristics of Aedes Mosquitoes in Dengue Risk Area. Procedia-Soc. Behav. Sci. 2016, 234, 164–172. [Google Scholar] [CrossRef]
- Meena, A.; Choudhary, N. Container breeding preference of Aedes albopictus in urban environment. Int. J. Mosq. Res. 2019, 6, 44–47. [Google Scholar]
- Reiskind, M.H.; Zarrabi, A.A. Water surface area and depth determine oviposition choice in Aedes albopictus (Diptera: Culicidae). J. Med. Èntomol. 2012, 49, 71–76. [Google Scholar] [CrossRef]
- Reiter, P.; Amador, M.A.; Colon, N. Enhancement of the CDC ovitrap with hay infusions for daily monitoring of Aedes aegypti populations. J. Am. Mosq. Control Assoc. 1991, 7, 52–55. [Google Scholar] [PubMed]
- Thavara, U.; Tawatsin, A.; Chompoosri, J. Evaluation of attractants and egg-laying substrate preference for oviposition by Aedes albopictus (Diptera: Culicidae). J. Vector Ecol. 2004, 29, 66–72. [Google Scholar] [PubMed]
- Madeira, N.G.; Macharelli, C.A.; Carvalho, L.R. Variation of the Oviposition Preferences of Aedes aegypti in Function of Substratum and Humidity. Memórias Inst. Oswaldo Cruz 2002, 97, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Okal, M.N.; Francis, B.; Herrera-Varela, M.; Fillinger, U.; Lindsay, S.W. Water vapour is a pre-oviposition attractant for the malaria vector Anopheles gambiae sensu stricto. Malar. J. 2013, 12, 365. [Google Scholar] [CrossRef] [PubMed]
- Pereira-dos-Santos, T.; Roiz, D.; Lourenço-de-Oliveira, R.; Paupy, C. A Systematic Review: Is Aedes albopictus an Efficient Bridge Vector for Zoonotic Arboviruses? Pathogens 2020, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Bentley, M.D.; Day, J.F. Chemical Ecology and Behavioral Aspects of Mosquito Oviposition. Annu. Rev. Entomol. 1989, 34, 401–421. [Google Scholar] [CrossRef]
- Alcalay, Y.; Tsurim, I.; Ovadia, O. Female mosquitoes disperse further when they develop under predation risk. Behav. Ecol. 2018, 29, 1402–1408. [Google Scholar] [CrossRef]
- Kono, H. Characterization and properties of carboxymethyl cellulose hydrogels crosslinked by polyethylene glycol. Carbohydr. Polym. 2014, 106, 84–93. [Google Scholar] [CrossRef]
- Shalapy, A.; Zhao, S.; Zhang, C.; Li, Y.; Geng, H.; Ullah, S.; Wang, G.; Huang, S.; Liu, Y. Adsorption of Deoxynivalenol (DON) from Corn Steep Liquor (CSL) by the Microsphere Adsorbent SA/CMC Loaded with Calcium. Toxins 2020, 12, 208. [Google Scholar] [CrossRef]
- Bakry, N.; Isa, M.I.N.M.; Sarbon, N. Effect of sorbitol at different concentrations on the functional properties of gelatin/carboxymethyl cellulose (CMC)/chitosan composite films. Int. Food Res. J. 2017, 24, 1753–1762. [Google Scholar]
- Gunathilaka, N.; Ranathunge, T.; Udayanga, L.; Wijegunawardena, A.; Abeyewickreme, W. Oviposition preferences of dengue vectors; Aedes aegypti and Aedes albopictus in Sri Lanka under laboratory settings. Bull. Èntomol. Res. 2017, 108, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Hatakeyama, T. Characterization of water in polysaccharide hydrogels by DSC. J. Therm. Anal. 1993, 40, 483–489. [Google Scholar] [CrossRef]
- Fellows, P. Food Processing Technology: Principles and Practice, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1–913. [Google Scholar]
- Yanniotis, S.; Skaltsi, S.; Karaburnioti, S. Effect of moisture content on the viscosity of honey at different temperatures. J. Food Eng. 2006, 72, 372–377. [Google Scholar] [CrossRef]
- Navarro, D.M.A.F.; De Oliveira, P.E.S.; Potting, R.P.J.; Brito, A.C.; Fital, S.J.F.; Sant’Ana, A.E.G. The potential attractant or repellent effects of different water types on oviposition in Aedes aegypti L. (Dipt., Culicidae). J. Appl. Èntomol. 2003, 127, 46–50. [Google Scholar] [CrossRef]
- Brown, P. Aedes Aegypti Oviposition Differences Among Ornamental Bromeliads with Variable Water Levels. J. Fla. Mosq. Control. Assoc. 2021, 66, 1–6. [Google Scholar] [CrossRef]
- Falsone, L.; Brianti, E.; Severini, F.; Giannetto, S.; Romi, R. Oviposition substrate in Asian tiger mosquito surveillance: Do the sizes matter? J. Vector Ecol. 2015, 40, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, D.; Lefèvre, T.; Moiroux, N.; Pennetier, C.; Chandre, F.; Cohuet, A. Behavioural adaptations of mosquito vectors to insecticide control. Curr. Opin. Insect Sci. 2019, 34, 48–54. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Das, M.; Baruah, I.; Veer, V.; Dutta, P. Physicochemical characteristics of habitats in relation to the density of container-breeding mos-quitoes in Asom, India. J. Vector Borne Dis. 2013, 50, 215–219. [Google Scholar]
- Shen, X.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green Chem. 2015, 18, 53–75. [Google Scholar] [CrossRef]
- Gaudin, S.; Lourdin, D.; Le Botlan, D.; Ilari, J.; Colonna, P. Plasticisation and Mobility in Starch-Sorbitol Films. J. Cereal Sci. 1999, 29, 273–284. [Google Scholar] [CrossRef]
- Pombi, M.; Guelbeogo, W.M.; Kreppel, K.; Calzetta, M.; Traoré, A.; Sanou, A.; Ranson, H.; Ferguson, H.M.; Sagnon, N.; Della Torre, A. The Sticky Resting Box, a new tool for studying resting behaviour of Afrotropical malaria vectors. Parasites Vectors 2014, 7, 247. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.M.; Chua, T.H.; Sulaiman, W.-Y.; Joanne, S.; Lim, Y.A.-L.; Sekaran, S.D.; Chinna, K.; Venugopalan, B.; Vythilingam, I. A new paradigm for Aedes spp. surveillance using gravid ovipositing sticky trap and NS1 antigen test kit. Parasites Vectors 2017, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Li, X.; Zhang, Y.; Miao, S.; Chen, L.; Li, L.; Liang, Y. Understanding the effect of freeze-drying on microstructures of starch hydrogels. Food Hydrocoll. 2020, 101, 105509. [Google Scholar] [CrossRef]
- Friuli, M.; Demitri, C.; Sannino, A.; Otranto, D.; Cafarchia, C.; Pombi, M.; Lia, R.P.L. Insecticidal Device; Ufficio Italiano Marchi e Brevetti, M. srl: Rome, Italy, 2021; pp. 1–40.
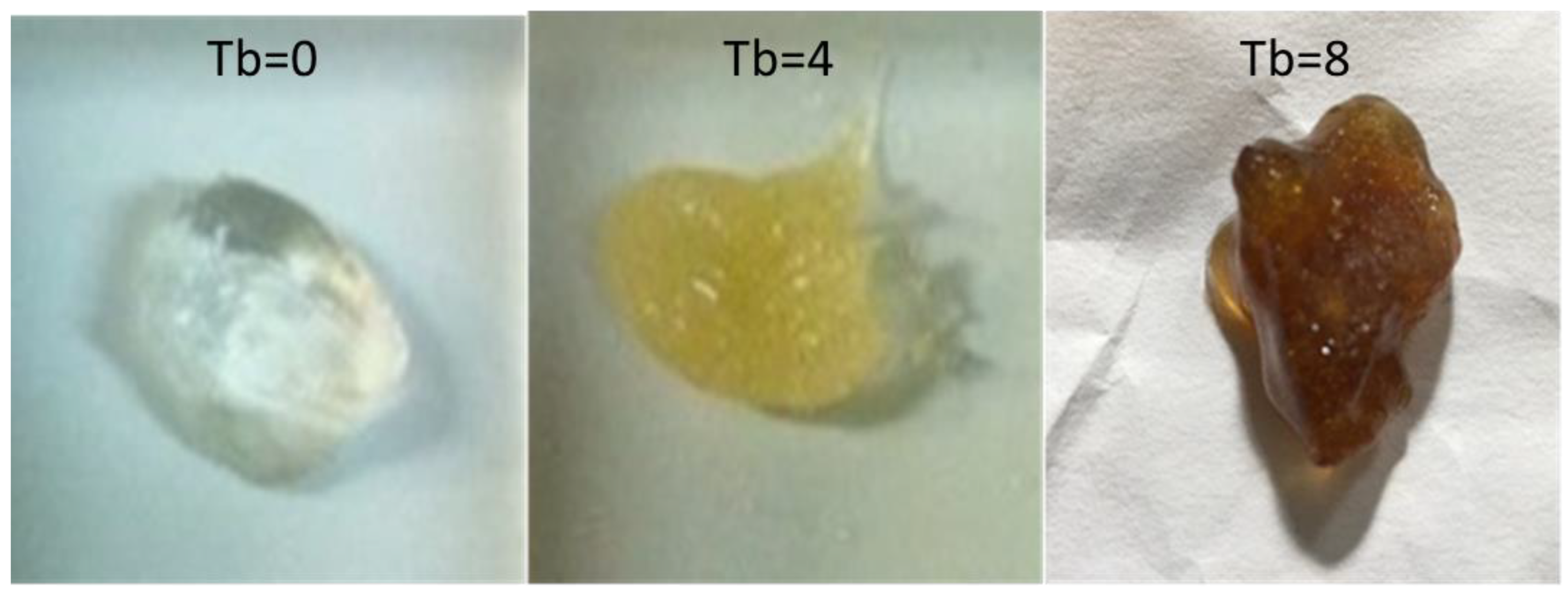




| Oviposition Parameters | |||||||
|---|---|---|---|---|---|---|---|
| Parameters | pH | Salinity [%] | Substrate Composition | Water Content [wt%] | Substrate Texture/Orientation | Morphology | Turbidity |
| Suitable conditions | From mild acidic to basic | Mild | Organic | >0% | From mud to wood-like/Sloped | Rough | Cloudy water |
| Oviposition Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| pH | Salinity [%] | Turbidity [McF] | Sorbitol [wt%] | Composition [wt%] | Water Content [wt%] | Viscosity [Pas] | Yield Stress [Pa] | Morphology |
| Working range | ||||||||
| 4.5–7.5 | <3% | 0–10 | 0–10 | HEC8-20/SA16-30 | >0% | 4.5–18.3/11–33.1 | >50 | Rough |
| Best preparation | ||||||||
| 6.5–7.5 | <1% | 8 | 6 | HEC16/S6/Tb8 | 80% | 14.1 | >50 | Rough |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friuli, M.; Cafarchia, C.; Cataldo, A.; Lia, R.P.; Otranto, D.; Pombi, M.; Demitri, C. Proof of Concept of Biopolymer Based Hydrogels as Biomimetic Oviposition Substrate to Develop Tiger Mosquitoes (Aedes albopictus) Cost-Effective Lure and Kill Ovitraps. Bioengineering 2022, 9, 267. https://doi.org/10.3390/bioengineering9070267
Friuli M, Cafarchia C, Cataldo A, Lia RP, Otranto D, Pombi M, Demitri C. Proof of Concept of Biopolymer Based Hydrogels as Biomimetic Oviposition Substrate to Develop Tiger Mosquitoes (Aedes albopictus) Cost-Effective Lure and Kill Ovitraps. Bioengineering. 2022; 9(7):267. https://doi.org/10.3390/bioengineering9070267
Chicago/Turabian StyleFriuli, Marco, Claudia Cafarchia, Andrea Cataldo, Riccardo Paolo Lia, Domenico Otranto, Marco Pombi, and Christian Demitri. 2022. "Proof of Concept of Biopolymer Based Hydrogels as Biomimetic Oviposition Substrate to Develop Tiger Mosquitoes (Aedes albopictus) Cost-Effective Lure and Kill Ovitraps" Bioengineering 9, no. 7: 267. https://doi.org/10.3390/bioengineering9070267
APA StyleFriuli, M., Cafarchia, C., Cataldo, A., Lia, R. P., Otranto, D., Pombi, M., & Demitri, C. (2022). Proof of Concept of Biopolymer Based Hydrogels as Biomimetic Oviposition Substrate to Develop Tiger Mosquitoes (Aedes albopictus) Cost-Effective Lure and Kill Ovitraps. Bioengineering, 9(7), 267. https://doi.org/10.3390/bioengineering9070267