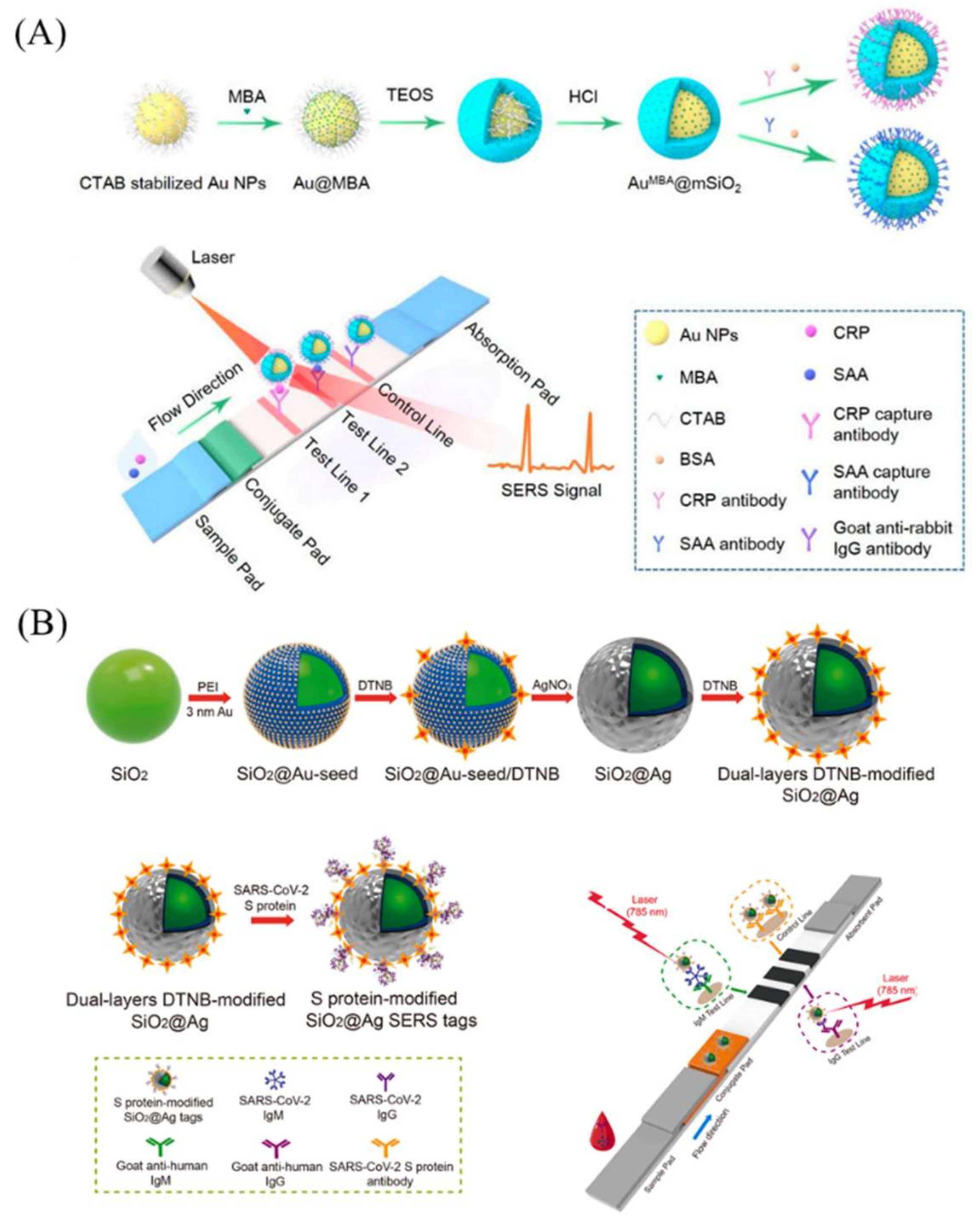
Recent Advances in Silica-Nanomaterial-Assisted Lateral Flow Assay
Abstract
:1. Introduction
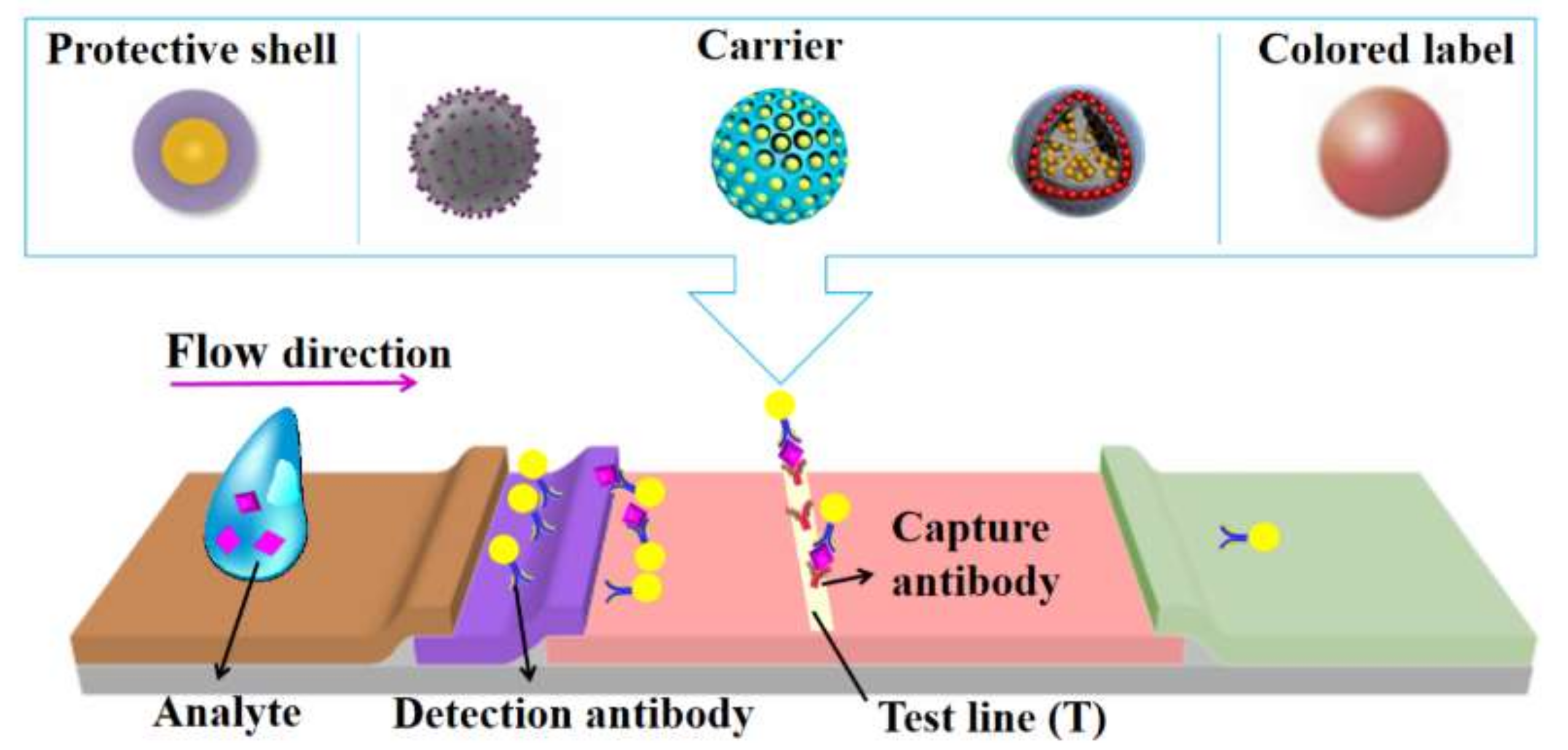
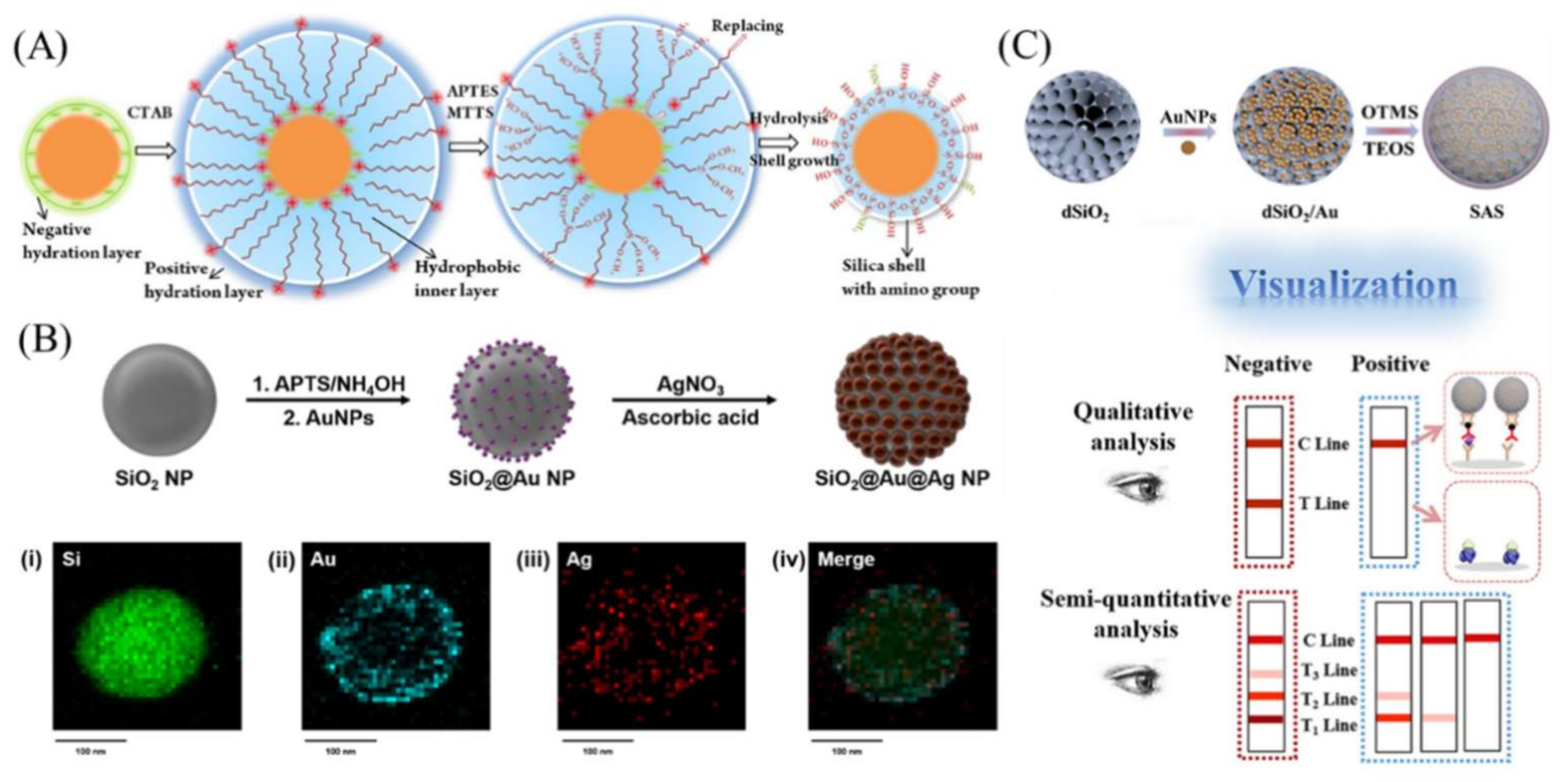
2. Colorimetric LFAs Using Silica Nanomaterials
3. Fluorescent LFAs Using Silica Nanomaterials
3.1. Quantum Dots
3.2. Other Fluorescent Materials Using Silica Nanomaterials
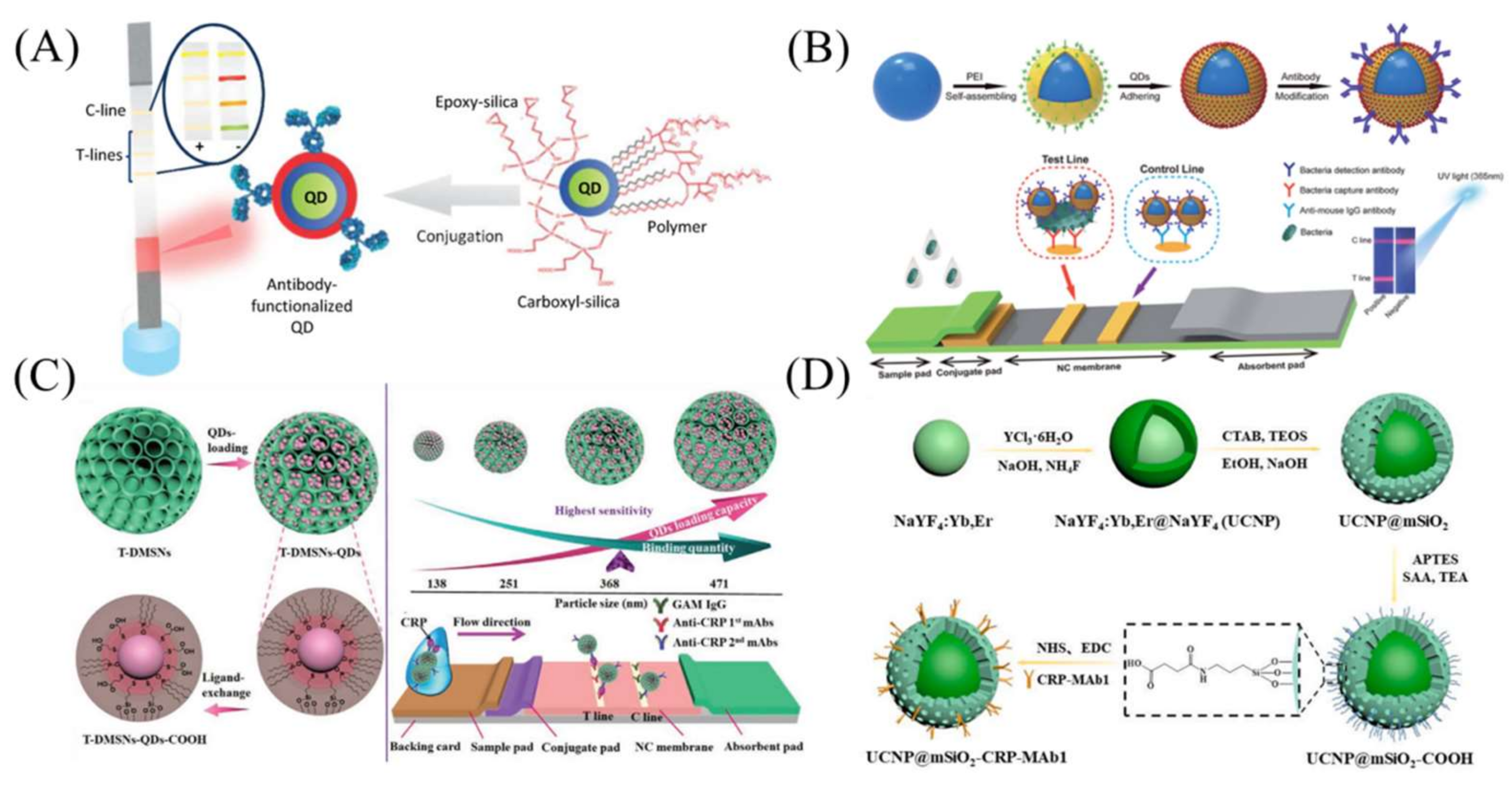
3.3. Dual-Model LFA Using Silica Nanomaterials
4. Chemiluminescent LFAs (CL-LFAs) Using Silica Nanomaterials
5. Surface-Enhanced Raman Scattering LFAs (SERS-LFAs)
6. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ching, K.H. Lateral Flow Immunoassay. In ELISA: Methods and Protocols; Hnasko, R., Ed.; Springer: New York, NY, USA, 2015; pp. 127–137. [Google Scholar]
- Lateral Flow Assay Market Size And Forecast. Available online: https://www.verifiedmarketresearch.com/product/lateral-flow-assay-market/ (accessed on 25 April 2022).
- In Vitro Diagnostics EUAs-Antigen Diagnostic Tests for SARS-CoV-2. Available online: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-antigen-diagnostic-tests-sars-cov-2 (accessed on 25 April 2022).
- Kim, K.; Han, D.K.; Choi, N.; Kim, S.H.; Joung, Y.; Kim, K.; Ho, N.T.; Joo, S.-W.; Choo, J. Surface-Enhanced Raman Scattering-Based Dual-Flow Lateral Flow Assay Sensor for the Ultrasensitive Detection of the Thyroid-Stimulating Hormone. Anal. Chem. 2021, 93, 6673–6681. [Google Scholar] [CrossRef]
- Liu, C.; Jia, Q.; Yang, C.; Qiao, R.; Jing, L.; Wang, L.; Xu, C.; Gao, M. Lateral Flow Immunochromatographic Assay for Sensitive Pesticide Detection by Using Fe3O4 Nanoparticle Aggregates as Color Reagents. Anal. Chem. 2011, 83, 6778–6784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, X.; Liu, X.; Li, J.; Wang, C.; Wang, S. Polyethyleneimine-interlayered silica-core quantum dot-shell nanocomposites for sensitive detection of Salmonella typhimurium via a lateral flow immunoassay. RSC Adv. 2020, 10, 2483–2489. [Google Scholar] [CrossRef] [Green Version]
- Ragnesola, B.; Jin, D.; Lamb, C.C.; Shaz, B.H.; Hillyer, C.D.; Luchsinger, L.L. COVID-19 antibody detection using lateral flow assay tests in a cohort of convalescent plasma donors. BMC Res. Notes 2020, 13, 372. [Google Scholar] [CrossRef]
- Guteneva, N.V.; Znoyko, S.L.; Orlov, A.V.; Nikitin, M.P.; Nikitin, P.I. Rapid lateral flow assays based on the quantification of magnetic nanoparticle labels for multiplexed immunodetection of small molecules: Application to the determination of drugs of abuse. Microchim. Acta 2019, 186, 621. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, S.; Nara, S. Evaluation of gold nanoparticle based lateral flow assays for diagnosis of enterobacteriaceae members in food and water. Food Chem. 2015, 170, 470–483. [Google Scholar] [CrossRef]
- Sajid, M.; Kawde, A.-N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef] [Green Version]
- Xiong, E.; Jiang, L.; Tian, T.; Hu, M.; Yue, H.; Huang, M.; Lin, W.; Jiang, Y.; Zhu, D.; Zhou, X. Simultaneous Dual-Gene Diagnosis of SARS-CoV-2 Based on CRISPR/Cas9-Mediated Lateral Flow Assay. Angew. Chem. Int. Ed. 2021, 60, 5307–5315. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, L.; Qin, Z.; Sackrison, J.; Bischof, J.C. Ultrasensitive and Highly Specific Lateral Flow Assays for Point-of-Care Diagnosis. ACS Nano 2021, 15, 3593–3611. [Google Scholar] [CrossRef]
- Serebrennikova, K.; Samsonova, J.; Osipov, A. Hierarchical Nanogold Labels to Improve the Sensitivity of Lateral Flow Immunoassay. Nano-Micro Lett. 2017, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yang, J.; Li, Q.; Wang, Y.; Wang, Y.; Li, G.; Shi, J.; Ding, P.; Guo, J.; Deng, R.; et al. A strip test for the optical determination of influenza virus H3 subtype using gold nanoparticle coated polystyrene latex microspheres. Microchim. Acta 2020, 187, 306. [Google Scholar] [CrossRef] [PubMed]
- Loynachan, C.N.; Thomas, M.R.; Gray, E.R.; Richards, D.A.; Kim, J.; Miller, B.S.; Brookes, J.C.; Agarwal, S.; Chudasama, V.; McKendry, R.A.; et al. Platinum Nanocatalyst Amplification: Redefining the Gold Standard for Lateral Flow Immunoassays with Ultrabroad Dynamic Range. ACS Nano 2018, 12, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Hwang, J.; Kim, K.; Jeon, J.; Lee, S.; Ko, J.; Lee, J.; Kang, M.; Chung, D.R.; Choo, J. Quantitative Serodiagnosis of Scrub Typhus Using Surface-Enhanced Raman Scattering-Based Lateral Flow Assay Platforms. Anal. Chem. 2019, 91, 12275–12282. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Chan, W.C.W.; Boulware, D.R.; Akkin, T.; Butler, E.K.; Bischof, J.C. Significantly Improved Analytical Sensitivity of Lateral Flow Immunoassays by Using Thermal Contrast. Angew. Chem. Int. Ed. 2012, 51, 4358–4361. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, J.; Sharma, A.; Jang, J. Vertical flow-based paper immunosensor for rapid electrochemical and colorimetric detection of influenza virus using a different pore size sample pad. Biosens. Bioelectron. 2019, 126, 36–43. [Google Scholar] [CrossRef]
- Foubert, A.; Beloglazova, N.V.; Gordienko, A.; Tessier, M.D.; Drijvers, E.; Hens, Z.; De Saeger, S. Development of a Rainbow Lateral Flow Immunoassay for the Simultaneous Detection of Four Mycotoxins. J. Agric. Food Chem. 2017, 65, 7121–7130. [Google Scholar] [CrossRef]
- Preechakasedkit, P.; Osada, K.; Katayama, Y.; Ruecha, N.; Suzuki, K.; Chailapakul, O.; Citterio, D. Gold nanoparticle core–europium(iii) chelate fluorophore-doped silica shell hybrid nanocomposites for the lateral flow immunoassay of human thyroid stimulating hormone with a dual signal readout. Analyst 2018, 143, 564–570. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kim, J.; An, J.; Bock, S.; Pham, X.-H.; Huynh, K.-H.; Choi, Y.; Hahm, E.; Song, H.; Kim, J.-W.; et al. Au–Ag assembled on silica nanoprobes for visual semiquantitative detection of prostate-specific antigen. J. Nanobiotechnol. 2021, 19, 73. [Google Scholar] [CrossRef]
- Huang, L.; Jin, J.; Ao, L.; Jiang, C.; Zhang, Y.; Wen, H.-M.; Wang, J.; Wang, H.; Hu, J. Hierarchical Plasmonic-Fluorescent Labels for Highly Sensitive Lateral Flow Immunoassay with Flexible Dual-Modal Switching. ACS Appl. Mater. Interfaces 2020, 12, 58149–58160. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, G.; Dou, W. Core-shell red silica nanoparticles based immunochromatographic assay for detection of Escherichia coli O157:H7. Anal. Chim. Acta 2018, 1038, 97–104. [Google Scholar] [CrossRef]
- Xu, C.; Lei, C.; Wang, Y.; Yu, C. Dendritic Mesoporous Nanoparticles: Structure, Synthesis and Properties. Angew. Chem. Int. Ed. 2022, 61, e202112752. [Google Scholar]
- Nguyen, V.-T.; Song, S.; Park, S.; Joo, C. Recent advances in high-sensitivity detection methods for paper-based lateral-flow assay. Biosens. Bioelectron. 2020, 152, 112015. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, S.; Guo, J.; Ma, X. Nanomaterial Labels in Lateral Flow Immunoassays for Point-of-Care-Testing. J. Mater. Sci. Technol. 2021, 60, 90–104. [Google Scholar] [CrossRef]
- Ye, H.; Xia, X. Enhancing the sensitivity of colorimetric lateral flow assay (CLFA) through signal amplification techniques. J. Mater. Chem. B 2018, 6, 7102–7111. [Google Scholar] [CrossRef]
- Yadav, S.; Sadique, M.A.; Ranjan, P.; Kumar, N.; Singhal, A.; Srivastava, A.K.; Khan, R. SERS Based Lateral Flow Immunoassay for Point-of-Care Detection of SARS-CoV-2 in Clinical Samples. ACS Appl. Bio Mater. 2021, 4, 2974–2995. [Google Scholar] [CrossRef]
- Huang, C.; Wen, T.; Shi, F.-J.; Zeng, X.-Y.; Jiao, Y.-J. Rapid Detection of IgM Antibodies against the SARS-CoV-2 Virus via Colloidal Gold Nanoparticle-Based Lateral-Flow Assay. ACS Omega 2020, 5, 12550–12556. [Google Scholar] [CrossRef]
- Choi, D.H.; Lee, S.K.; Oh, Y.K.; Bae, B.W.; Lee, S.D.; Kim, S.; Shin, Y.-B.; Kim, M.-G. A dual gold nanoparticle conjugate-based lateral flow assay (LFA) method for the analysis of troponin I. Biosens. Bioelectron. 2010, 25, 1999–2002. [Google Scholar] [CrossRef]
- Mao, X.; Ma, Y.; Zhang, A.; Zhang, L.; Zeng, L.; Liu, G. Disposable Nucleic Acid Biosensors Based on Gold Nanoparticle Probes and Lateral Flow Strip. Anal. Chem. 2009, 81, 1660–1668. [Google Scholar] [CrossRef]
- de Puig, H.; Bosch, I.; Gehrke, L.; Hamad-Schifferli, K. Challenges of the Nano–Bio Interface in Lateral Flow and Dipstick Immunoassays. Trends Biotechnol. 2017, 35, 1169–1180. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Mei, T.; Guo, Q.; Zhou, W.; Li, X.; Chen, J.; Zhou, X.; Sun, N.; Fang, Z. Improved performance of lateral flow immunoassays for alpha-fetoprotein and vanillin by using silica shell-stabilized gold nanoparticles. Microchim. Acta 2018, 186, 2. [Google Scholar] [CrossRef] [PubMed]
- Panferov, V.G.; Safenkova, I.V.; Varitsev, Y.A.; Drenova, N.V.; Kornev, K.P.; Zherdev, A.V.; Dzantiev, B.B. Development of the sensitive lateral flow immunoassay with silver enhancement for the detection of Ralstonia solanacearum in potato tubers. Talanta 2016, 152, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Lei, L.; Xie, W.; Yang, Q.; Li, C.M.; Liu, Y. Copper deposition-induced efficient signal amplification for ultrasensitive lateral flow immunoassay. Sens. Actuators B Chem. 2019, 282, 96–103. [Google Scholar] [CrossRef]
- Rodríguez, M.O.; Covián, L.B.; García, A.C.; Blanco-López, M.C. Silver and gold enhancement methods for lateral flow immunoassays. Talanta 2016, 148, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Cho, I.-H.; Bhunia, A.; Irudayaraj, J. Rapid pathogen detection by lateral-flow immunochromatographic assay with gold nanoparticle-assisted enzyme signal amplification. Int. J. Food Microbiol. 2015, 206, 60–66. [Google Scholar] [CrossRef]
- He, Y.; Zhang, S.; Zhang, X.; Baloda, M.; Gurung, A.S.; Xu, H.; Zhang, X.; Liu, G. Ultrasensitive nucleic acid biosensor based on enzyme–gold nanoparticle dual label and lateral flow strip biosensor. Biosens. Bioelectron. 2011, 26, 2018–2024. [Google Scholar] [CrossRef]
- Chapman, R.; Lin, Y.; Burnapp, M.; Bentham, A.; Hillier, D.; Zabron, A.; Khan, S.; Tyreman, M.; Stevens, M.M. Multivalent Nanoparticle Networks Enable Point-of-Care Detection of Human Phospholipase-A2 in Serum. ACS Nano 2015, 9, 2565–2573. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Huang, M.; Shi, Z.; Huang, L.; Jin, J.; Jiang, C.; Yu, W.; Guo, Z.; Wang, J. Ultrasensitive Competitive Lateral Flow Immunoassay with Visual Semiquantitative Inspection and Flexible Quantification Capabilities. Anal. Chem. 2022, 94, 2996–3004. [Google Scholar] [CrossRef]
- Kamel, M.; Salah, F.; Demerdash, Z.; Maher, S.; Atta, S.; Badr, A.; Afifi, A.; El Baz, H. Development of new lateral-flow immunochromatographic strip using colloidal gold and mesoporous silica nanoparticles for rapid diagnosis of active schistosomiasis. Asian Pac. J. Trop. Biomed. 2019, 9, 315–322. [Google Scholar]
- Zhu, C.; Zhao, G.; Dou, W. Immunochromatographic assay using brightly colored silica nanoparticles as visible label for point-of-care detection of clenbuterol. Sens. Actuators B Chem. 2018, 266, 392–399. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, Z.; Deng, K.; Li, Z.; Zhou, Y.; Qiu, H.; Gao, Y.; Che, S.; Tang, Z. Gold Nanorod@Chiral Mesoporous Silica Core–shell Nanoparticles with Unique Optical Properties. J. Am. Chem. Soc. 2013, 135, 9659–9664. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Yin, R.; Yu, Q.; Qiu, W.; Li, K.; Qian, L.; Li, H.; Shen, B.; Liu, G. Sensitive detection of microRNA-21 in cancer cells and human serum with Au@Si nanocomposite and lateral flow assay. Anal. Chim. Acta 2021, 1147, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Goebl, J.; Ge, J.; Yin, Y. Self-assembly and tunable plasmonic property of gold nanoparticles on mercapto-silica microspheres. J. Mater. Chem. 2009, 19, 4597–4602. [Google Scholar] [CrossRef]
- Xu, H.; Chen, J.; Birrenkott, J.; Zhao, J.X.; Takalkar, S.; Baryeh, K.; Liu, G. Gold-Nanoparticle-Decorated Silica Nanorods for Sensitive Visual Detection of Proteins. Anal. Chem. 2014, 86, 7351–7359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.; Dou, W.; Liu, J.; Zhao, G.; Yang, S.; Zhu, D.; Zhao, Y.; Li, L. A sensitive and quantitative immunochromatographic assay for HBsAg based on novel red silica nanoparticles. Anal. Methods 2019, 11, 268–275. [Google Scholar] [CrossRef]
- Gong, X.; Cai, J.; Zhang, B.; Zhao, Q.; Piao, J.; Peng, W.; Gao, W.; Zhou, D.; Zhao, M.; Chang, J. A review of fluorescent signal-based lateral flow immunochromatographic strips. J. Mater. Chem. B 2017, 5, 5079–5091. [Google Scholar] [CrossRef]
- Bruno, J.G. Application of DNA Aptamers and Quantum Dots to Lateral Flow Test Strips for Detection of Foodborne Pathogens with Improved Sensitivity versus Colloidal Gold. Pathogens 2014, 3, 341–355. [Google Scholar] [CrossRef]
- Juntunen, E.; Myyryläinen, T.; Salminen, T.; Soukka, T.; Pettersson, K. Performance of fluorescent europium(III) nanoparticles and colloidal gold reporters in lateral flow bioaffinity assay. Anal. Biochem. 2012, 428, 31–38. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Wang, J.; Tang, Z.; Pounds, J.G.; Lin, Y. Rapid and Sensitive Detection of Protein Biomarker Using a Portable Fluorescence Biosensor Based on Quantum Dots and a Lateral Flow Test Strip. Anal. Chem. 2010, 82, 7008–7014. [Google Scholar] [CrossRef]
- Goryacheva, O.A.; Guhrenz, C.; Schneider, K.; Beloglazova, N.V.; Goryacheva, I.Y.; De Saeger, S.; Gaponik, N. Silanized Luminescent Quantum Dots for the Simultaneous Multicolor Lateral Flow Immunoassay of Two Mycotoxins. ACS Appl. Mater. Interfaces 2020, 12, 24575–24584. [Google Scholar] [CrossRef]
- Wang, C.; Shi, D.; Wan, N.; Yang, X.; Liu, H.; Gao, H.; Zhang, M.; Bai, Z.; Li, D.; Dai, E.; et al. Development of spike protein-based fluorescence lateral flow assay for the simultaneous detection of SARS-CoV-2 specific IgM and IgG. Analyst 2021, 146, 3908–3917. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liao, T.; Wang, J.; Ao, L.; Su, W.; Hu, J. Brilliant Pitaya-Type Silica Colloids with Central–Radial and High-Density Quantum Dots Incorporation for Ultrasensitive Fluorescence Immunoassays. Adv. Funct. Mater. 2018, 28, 1705380. [Google Scholar] [CrossRef]
- Gao, F.; Lei, C.; Liu, Y.; Song, H.; Kong, Y.; Wan, J.; Yu, C. Rational Design of Dendritic Mesoporous Silica Nanoparticles’ Surface Chemistry for Quantum Dot Enrichment and an Ultrasensitive Lateral Flow Immunoassay. ACS Appl. Mater. Interfaces 2021, 13, 21507–21515. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, Y.; Lei, C.; Liu, C.; Song, H.; Gu, Z.; Jiang, P.; Jing, S.; Wan, J.; Yu, C. The Role of Dendritic Mesoporous Silica Nanoparticles’ Size for Quantum Dots Enrichment and Lateral Flow Immunoassay Performance. Small Methods 2021, 5, 2000924. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, C.; Yao, Y.; Lei, C.; Li, S.; Yuan, L.; Song, H.; Yang, Y.; Wan, J.; Yu, C. Quantum dots’ size matters for balancing their quantity and quality in label materials to improve lateral flow immunoassay performance for C-reactive protein determination. Biosens. Bioelectron. 2022, 199, 113892. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Y.; Liao, T.; Xu, K.; Jiang, C.; Zhuo, D.; Wang, Y.; Wen, H.-M.; Wang, J.; Ao, L.; et al. Compact Magneto-Fluorescent Colloids by Hierarchical Assembly of Dual-Components in Radial Channels for Sensitive Point-of-Care Immunoassay. Small 2021, 17, 2100862. [Google Scholar] [CrossRef]
- Xu, L.-D.; Du, F.-L.; Zhu, J.; Ding, S.-N. Luminous silica colloids with carbon dot incorporation for sensitive immunochromatographic assay of Zika virus. Analyst 2021, 146, 706–713. [Google Scholar] [CrossRef]
- Palo, E.; Tuomisto, M.; Hyppänen, I.; Swart, H.C.; Hölsä, J.; Soukka, T.; Lastusaari, M. Highly uniform up-converting nanoparticles: Why you should control your synthesis even more. J. Lumin. 2017, 185, 125–131. [Google Scholar] [CrossRef]
- Gnach, A.; Bednarkiewicz, A. Lanthanide-doped up-converting nanoparticles: Merits and challenges. Nano Today 2012, 7, 532–563. [Google Scholar] [CrossRef]
- Guo, J.; Chen, S.; Tian, S.; Liu, K.; Ni, J.; Zhao, M.; Kang, Y.; Ma, X.; Guo, J. 5G-enabled ultra-sensitive fluorescence sensor for proactive prognosis of COVID-19. Biosens. Bioelectron. 2021, 181, 113160. [Google Scholar] [CrossRef]
- Li, C.; Liu, J.; Alonso, S.; Li, F.; Zhang, Y. Upconversion nanoparticles for sensitive and in-depth detection of Cu2+ ions. Nanoscale 2012, 4, 6065–6071. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, S.; Tian, S.; Liu, K.; Ma, X.; Guo, J. A sensitive and quantitative prognosis of C-reactive protein at picogram level using mesoporous silica encapsulated core-shell up-conversion nanoparticle based lateral flow strip assay. Talanta 2021, 230, 122335. [Google Scholar] [CrossRef] [PubMed]
- Wiriyachaiporn, N.; Sirikaew, S.; Bamrungsap, S.; Limcharoen, T.; Polkankosit, P.; Roeksrungruang, P.; Ponlamuangdee, K. A simple fluorescence-based lateral flow test platform for rapid influenza B virus screening. Anal. Methods 2021, 13, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yang, M.; Wu, J.; Zhang, W.; Jiang, X. A Self-Contained Chemiluminescent Lateral Flow Assay for Point-of-Care Testing. Anal. Chem. 2018, 90, 9132–9137. [Google Scholar] [CrossRef]
- Jung, H.; Park, S.H.; Lee, J.; Lee, B.; Park, J.; Seok, Y.; Choi, J.-H.; Kim, M.-G.; Song, C.-S.; Lee, J. A Size-Selectively Biomolecule-Immobilized Nanoprobe-Based Chemiluminescent Lateral Flow Immunoassay for Detection of Avian-Origin Viruses. Anal. Chem. 2021, 93, 792–800. [Google Scholar] [CrossRef]
- Tao, L.; Zhang, C.; Zhang, J.; Sun, Y.; Li, X.; Yan, K.; Jin, B.; Zhang, Z.; Yang, K. Sensitive chemiluminescence immunoassay for staphylococcal enterotoxin C1 based on the use of dye-encapsulated mesoporous silica nanoparticles. Microchim. Acta 2016, 183, 2163–2168. [Google Scholar] [CrossRef]
- Climent, E.; Rurack, K. Combining Electrochemiluminescence Detection with Aptamer-Gated Indicator Releasing Mesoporous Nanoparticles Enables ppt Sensitivity for Strip-Based Rapid Tests. Angew. Chem. Int. Ed. 2021, 60, 26287–26297. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Y.; Rao, Y. Ruthenium(II)-Catalyzed Synthesis of Hydroxylated Arenes with Ester as an Effective Directing Group. Org. Lett. 2012, 14, 2874–2877. [Google Scholar] [CrossRef]
- Hong, D.; Jo, E.-J.; Kim, K.; Song, M.-B.; Kim, M.-G. Ru(bpy)32+-Loaded Mesoporous Silica Nanoparticles as Electrochemiluminescent Probes of a Lateral Flow Immunosensor for Highly Sensitive and Quantitative Detection of Troponin I. Small 2020, 16, 2004535. [Google Scholar] [CrossRef]
- Hong, D.; Kim, K.; Jo, E.-J.; Kim, M.-G. Electrochemiluminescence-Incorporated Lateral Flow Immunosensors Using Ru(bpy)32+-Labeled Gold Nanoparticles for the Full-Range Detection of Physiological C-Reactive Protein Levels. Anal. Chem. 2021, 93, 7925–7932. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, J.; Yu, B.; You, T. A novel self-enhanced electrochemiluminescence immunosensor based on hollow Ru-SiO2@PEI nanoparticles for NSE analysis. Sci. Rep. 2016, 6, 22234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.-T.; Wang, Y.-L.; Zhang, J.-J.; Dong, Y.-X.; Liu, F.-R.; Ren, S.-W.; Liu, Y.-M. Immuno-Electrochemiluminescent Imaging of a Single Cell Based on Functional Nanoprobes of Heterogeneous Ru(bpy)32+@SiO2/Au Nanoparticles. Anal. Chem. 2018, 90, 10334–10339. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Hu, X.; Hu, C.; Zhang, Y.; Jia, N. A novel solid-state electrochemiluminescence sensor for melamine with Ru(bpy)32+/mesoporous silica nanospheres/Nafion composite modified electrode. Biosens. Bioelectron. 2013, 41, 911–915. [Google Scholar] [CrossRef]
- Xu, X.; Li, H.; Hasan, D.; Ruoff, R.S.; Wang, A.X.; Fan, D.L. Near-Field Enhanced Plasmonic-Magnetic Bifunctional Nanotubes for Single Cell Bioanalysis. Adv. Funct. Mater. 2013, 23, 4332–4338. [Google Scholar] [CrossRef] [Green Version]
- Surface-Enhanced Raman Spectroscopy. Available online: https://en.wikipedia.org/wiki/Surface-enhanced_Raman_spectroscopy#cite_note-1 (accessed on 9 May 2022).
- Gao, X.; Zheng, P.; Kasani, S.; Wu, S.; Yang, F.; Lewis, S.; Nayeem, S.; Engler-Chiurazzi, E.B.; Wigginton, J.G.; Simpkins, J.W.; et al. Paper-Based Surface-Enhanced Raman Scattering Lateral Flow Strip for Detection of Neuron-Specific Enolase in Blood Plasma. Anal. Chem. 2017, 89, 10104–10110. [Google Scholar] [CrossRef]
- Gao, X.; Boryczka, J.; Kasani, S.; Wu, N. Enabling Direct Protein Detection in a Drop of Whole Blood with an “On-Strip” Plasma Separation Unit in a Paper-Based Lateral Flow Strip. Anal. Chem. 2021, 93, 1326–1332. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Guo, J.; Zhang, Y.; Guo, J.; Wu, X.; Wang, B.; Ma, X. Simultaneous Detection of Inflammatory Biomarkers by SERS Nanotag-Based Lateral Flow Assay with Portable Cloud Raman Spectrometer. Nanomaterials 2021, 11, 1496. [Google Scholar] [CrossRef]
- Jeon, J.; Lee, S.H.; Joung, Y.; Kim, K.; Choi, N.; Choo, J. Improvement of reproducibility and thermal stability of surface-enhanced Raman scattering-based lateral flow assay strips using silica-encapsulated gold nanoparticles. Sens. Actuators B Chem. 2020, 321, 128521. [Google Scholar] [CrossRef]
- Borzenkov, M.; Chirico, G.; D’Alfonso, L.; Sironi, L.; Collini, M.; Cabrini, E.; Dacarro, G.; Milanese, C.; Pallavicini, P.; Taglietti, A.; et al. Thermal and Chemical Stability of Thiol Bonding on Gold Nanostars. Langmuir 2015, 31, 8081–8091. [Google Scholar] [CrossRef]
- Liu, H.; Dai, E.; Xiao, R.; Zhou, Z.; Zhang, M.; Bai, Z.; Shao, Y.; Qi, K.; Tu, J.; Wang, C.; et al. Development of a SERS-based lateral flow immunoassay for rapid and ultra-sensitive detection of anti-SARS-CoV-2 IgM/IgG in clinical samples. Sens. Actuators B Chem. 2021, 329, 129196. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, H.; Xu, C.; Gao, F.; Li, Y.; Lei, C.; Yu, C. Recent Advances in Silica-Nanomaterial-Assisted Lateral Flow Assay. Bioengineering 2022, 9, 266. https://doi.org/10.3390/bioengineering9070266
Zhuang H, Xu C, Gao F, Li Y, Lei C, Yu C. Recent Advances in Silica-Nanomaterial-Assisted Lateral Flow Assay. Bioengineering. 2022; 9(7):266. https://doi.org/10.3390/bioengineering9070266
Chicago/Turabian StyleZhuang, Han, Chun Xu, Fang Gao, Yiwei Li, Chang Lei, and Chengzhong Yu. 2022. "Recent Advances in Silica-Nanomaterial-Assisted Lateral Flow Assay" Bioengineering 9, no. 7: 266. https://doi.org/10.3390/bioengineering9070266
APA StyleZhuang, H., Xu, C., Gao, F., Li, Y., Lei, C., & Yu, C. (2022). Recent Advances in Silica-Nanomaterial-Assisted Lateral Flow Assay. Bioengineering, 9(7), 266. https://doi.org/10.3390/bioengineering9070266