Assessment and Scientific Progresses in the Analysis of Olfactory Evoked Potentials
Abstract
1. Introduction
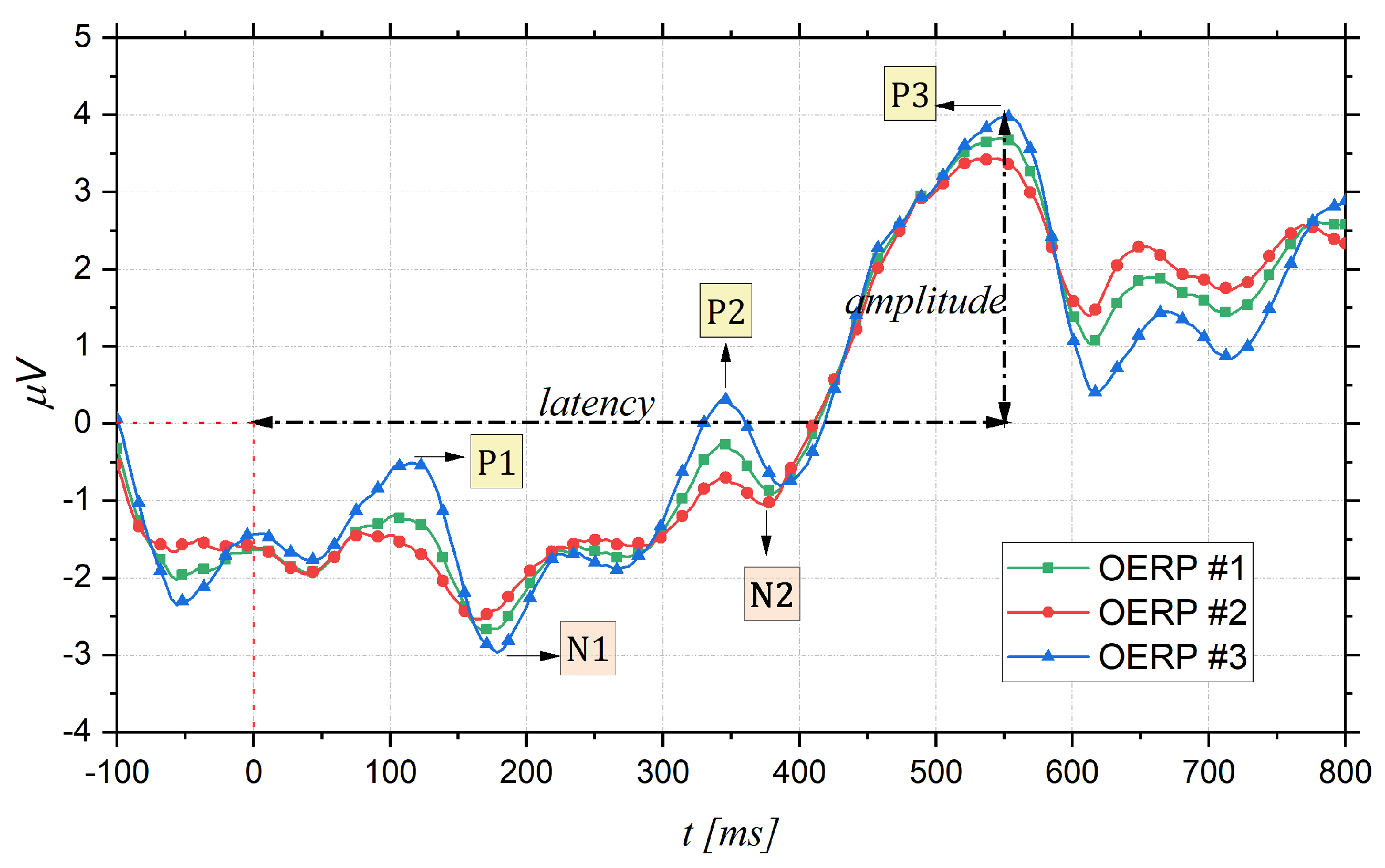
2. Olfactory Event Related Potentials
- Latency: time interval between the stimulus onset (fixed to 0 ms) and the point of maximum value (peak) of the component;
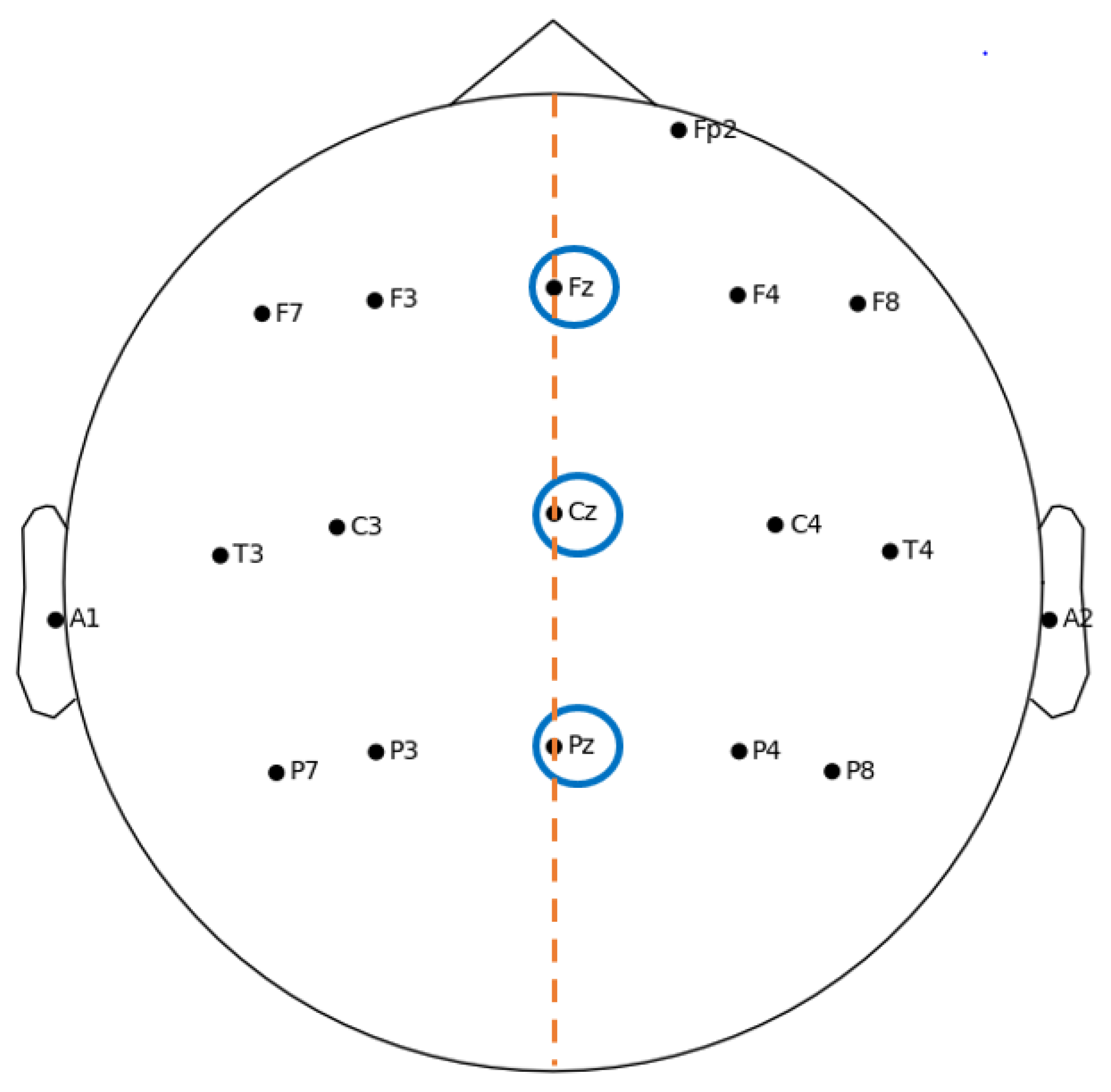
- Topography: position on the cranial surface where the maximum amplitude of the component can be registered, thus allowing identification of which cortical area is active following a particular stimulus;
- Amplitude: vertical distance measured from the baseline (fixed to 0 V) to the maximum peak.
Reliability of OERPs
3. Registration and Pre-Processing
3.1. ERP Experimental Setup
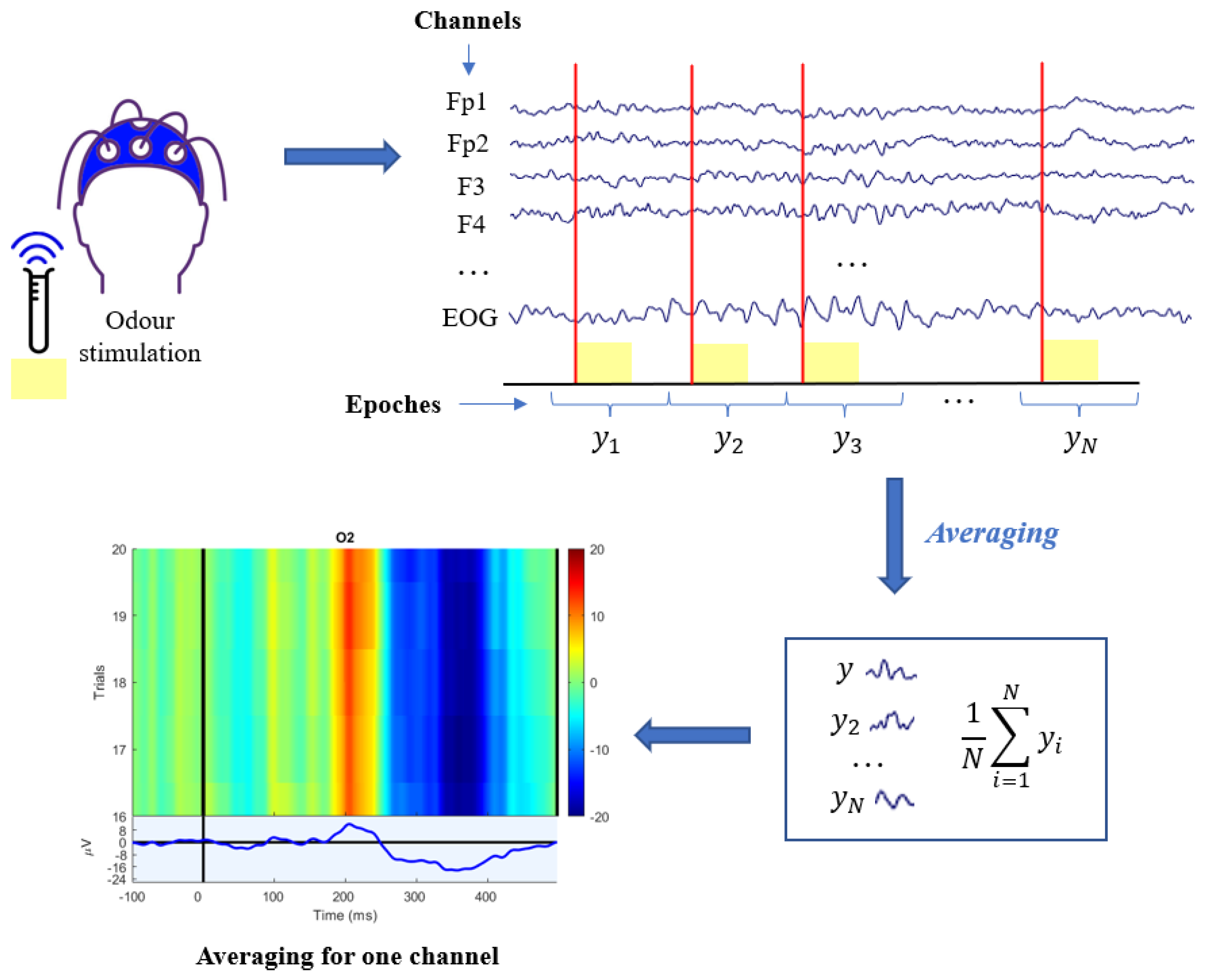
3.2. ERP Processing Techniques
- Variability of the evoked potential: amplitude and latency can vary independently of each other from epochs to epochs. Actually, significant latency jitter can result in a severely distorted and amplitude-reduced ERP average.
- Non-stationarity of the EEG: the basic assumption of averaging is that the background EEG is a random, null averaged, uncorrelated and stationary signal during the recording of the N epochs, but in reality EEG is assimilable to a stochastic model only for short stretches.
- No a priori knowledge about the relationship between EEG and ERP is exploited, so it is necessary to have a large number of sweeps before resorting to the grand averaging algorithm.
4. Application Fields
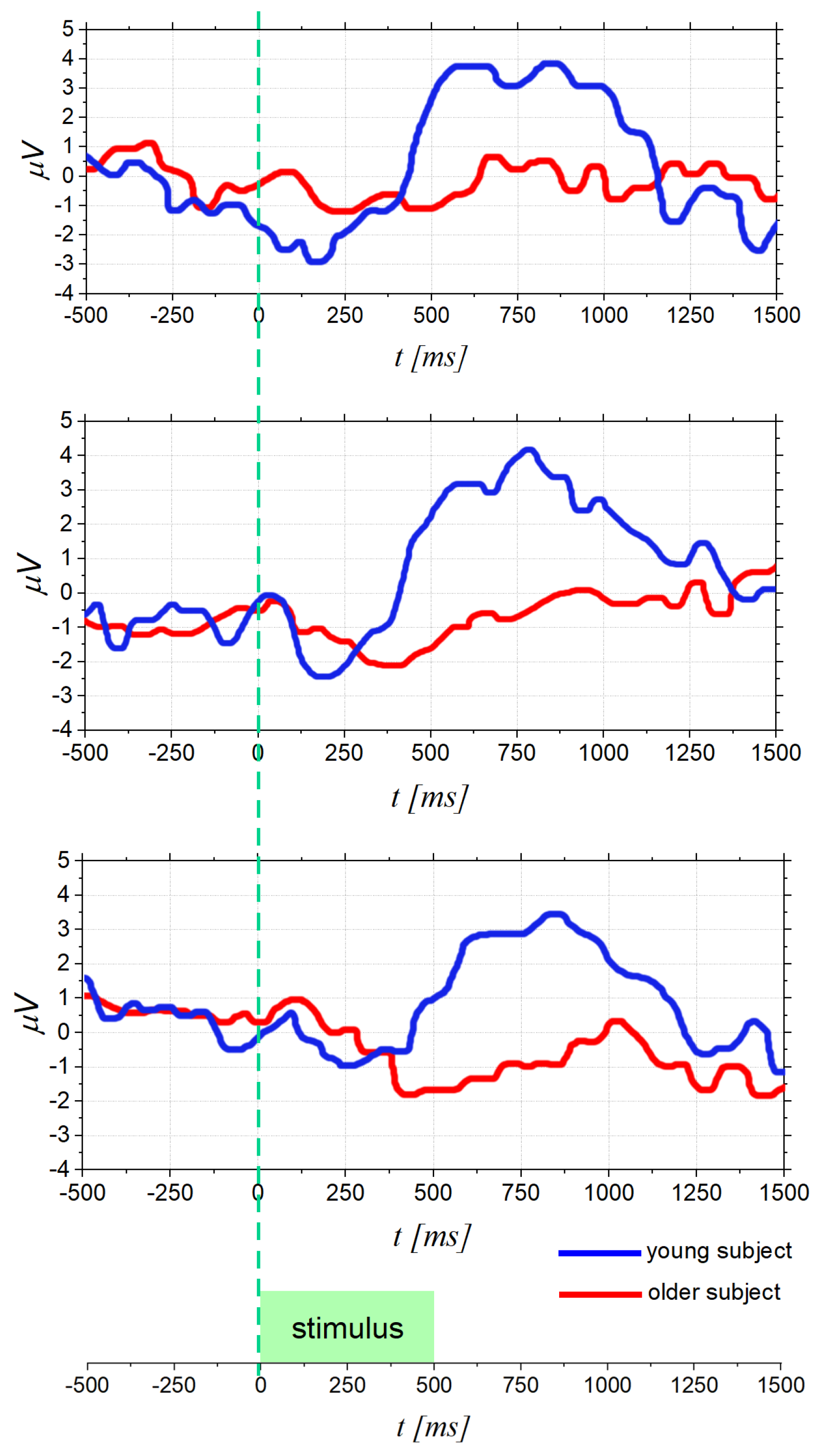
4.1. Olfactory Function Decline in Elderly People
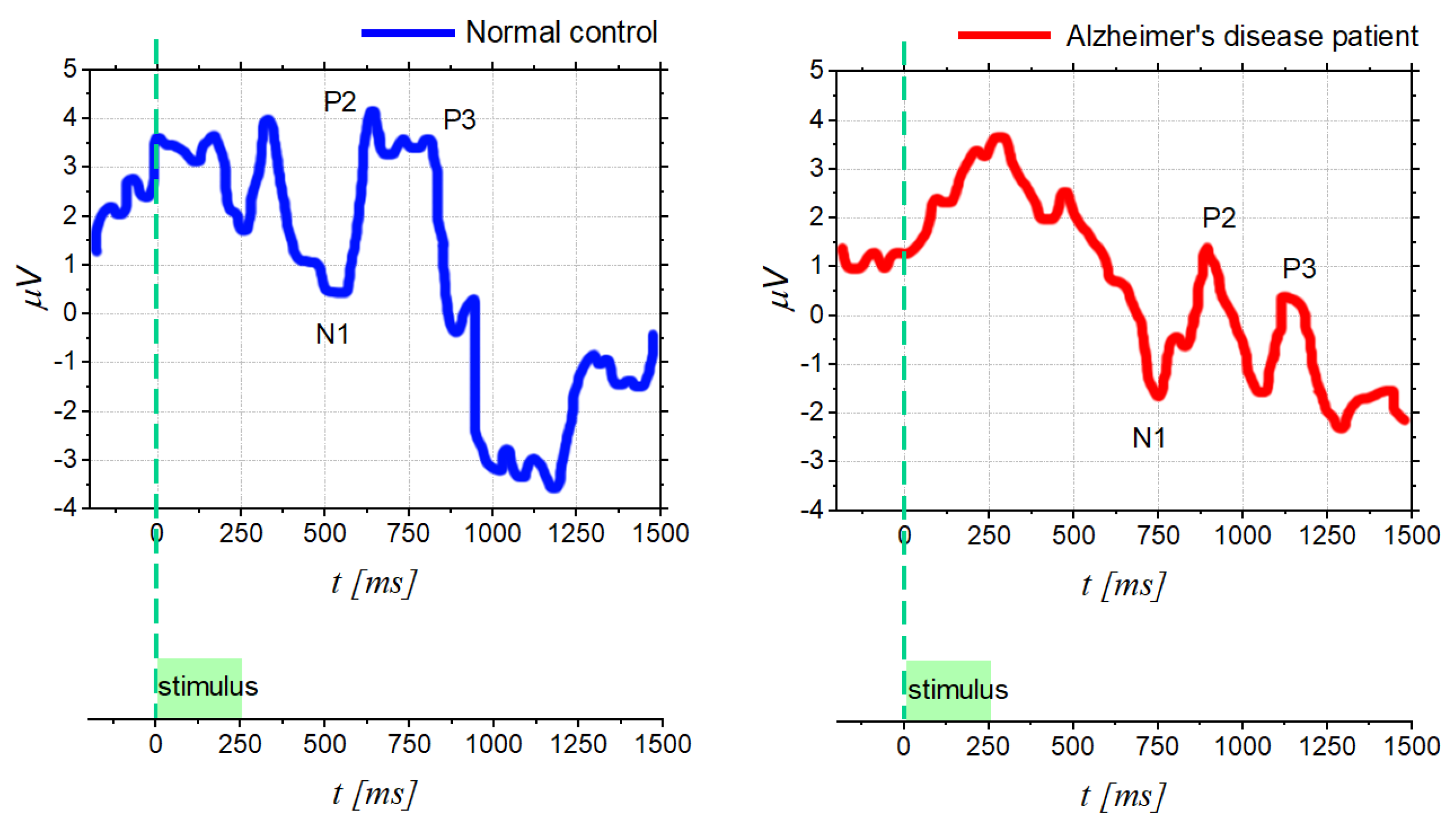
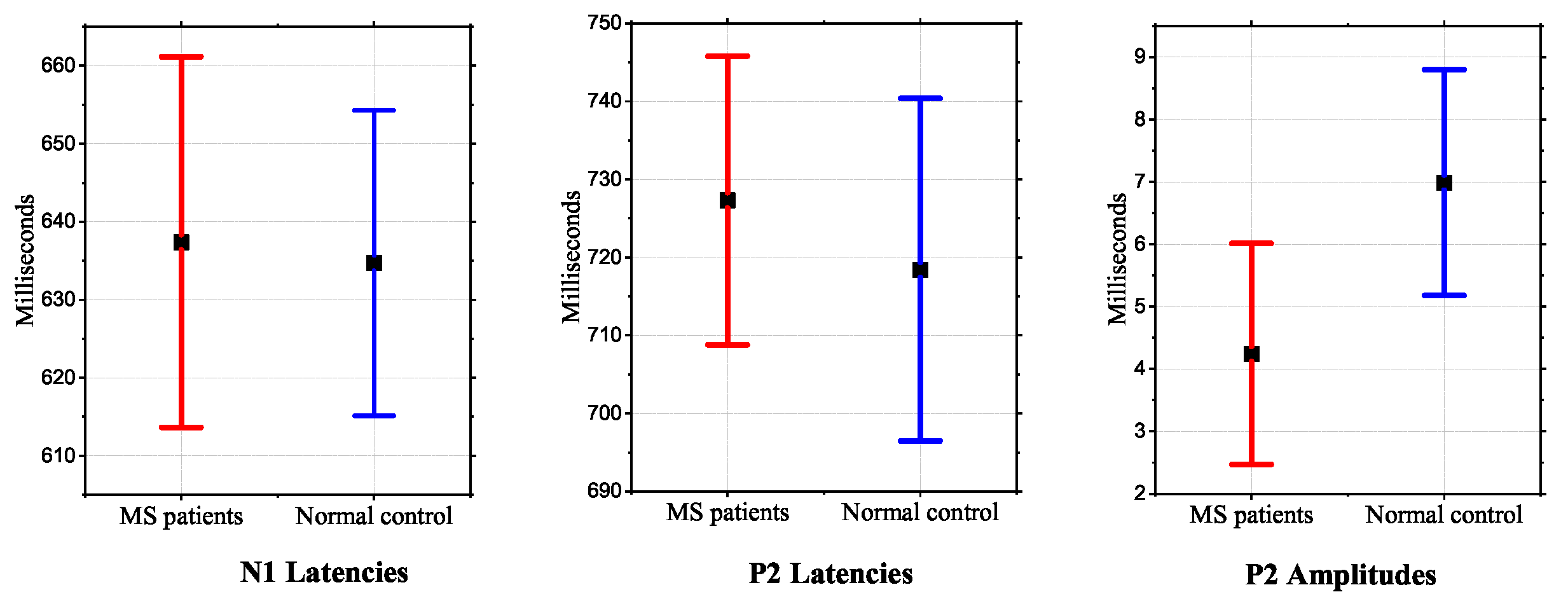
4.2. Diagnostic in Neuro-Degenerative and Neuro-Psychiatric Diseases
4.3. Analysis of Emotions for Informative Purposes
5. Challenges and Future Goals
5.1. Standardized Methods
5.2. Improved Processing Techniques
5.3. Machine Learning and Deep Learning
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Albrecht, J.; Wiesmann, M. The human olfactory system. Anatomy and physiology. Der Nervenarzt 2006, 77, 931–939. [Google Scholar] [CrossRef]
- Sarafoleanu, C.; Mella, C.; Georgescu, M.; Perederco, C. The importance of the olfactory sense in the human behavior and evolution. J. Med. Life 2009, 2, 196. [Google Scholar]
- Vaglio, S. Chemical communication and mother-infant recognition. Commun. Integr. Biol. 2009, 2, 279–281. [Google Scholar] [CrossRef]
- Raudenbush, B.; Grayhem, R.; Sears, T.; Wilson, I. Effects of peppermint and cinnamon odor administration on simulated driving alertness, mood and workload. N. Am. J. Psychol. 2009, 11, 245–256. [Google Scholar]
- Promoting Social Interaction through Emotional Body Odours, 2019–2024. Available online: https://potionh2020.com/ (accessed on 20 May 2022).
- Daramola, O.O.; Becker, S.S. An algorithmic approach to the evaluation and treatment of olfactory disorders. Curr. Opin. Otolaryngol. Head Neck Surg. 2015, 23, 8–14. [Google Scholar] [CrossRef]
- Croy, I.; Nordin, S.; Hummel, T. Olfactory disorders and quality of life—An updated review. Chem. Senses 2014, 39, 185–194. [Google Scholar] [CrossRef]
- Hummel, T.; Whitcroft, K.; Andrews, P.; Altundag, A.; Cinghi, C.; Costanzo, R.; Damm, M.; Frasnelli, J.; Gudziol, H.; Gupta, N.; et al. Position paper on olfactory dysfunction. Rhinology. Suppl. 2017, 54. [Google Scholar] [CrossRef]
- Santos, D.V.; Reiter, E.R.; DiNardo, L.J.; Costanzo, R.M. Hazardous Events Associated With Impaired Olfactory Function. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 317–319. [Google Scholar] [CrossRef]
- Dan, X.; Wechter, N.; Gray, S.; Mohanty, J.G.; Croteau, D.L.; Bohr, V.A. Olfactory dysfunction in aging and neurodegenerative diseases. Ageing Res. Rev. 2021, 70, 101416. [Google Scholar] [CrossRef]
- Raviv, J.R.; Kern, R. Chronic sinusitis and olfactory dysfunction. Otolaryngol. Clin N. Am. 2004, 37, 1143–1157. [Google Scholar] [CrossRef]
- Potter, M.; Chen, J.; Lobban, N.; Doty, R. Olfactory dysfunction from acute upper respiratory infections: Relationship to season of onset. Int. Forum Allergy Rhinol. 2020, 10, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Mascagni, P.; Consonni, D.; Bregante, G.; Chiappino, G.; Toffoletto, F. Olfactory function in workers exposed to moderate airborne cadmium levels. Neurotoxicology 2003, 24, 717–724. [Google Scholar] [CrossRef]
- Gobba, F. Olfactory toxicity: Long-term effects of occupational exposures. Int. Arch. Occup. Environ. Health 2006, 79, 322–331. [Google Scholar] [CrossRef]
- Haxel, B.R.; Grant, L.; Mackay-Sim, A. Olfactory dysfunction after head injury. J. Head Trauma Rehabil. 2008, 23, 407–413. [Google Scholar] [CrossRef]
- Barresi, M.; Ciurleo, R.; Giacoppo, S.; Cuzzola, V.F.; Celi, D.; Bramanti, P.; Marino, S. Evaluation of olfactory dysfunction in neurodegenerative diseases. J. Neurol. Sci. 2012, 323, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Vilas, D.; Langdon, C.; Alobid, I.; López-Chacón, M.; Haehner, A.; Hummel, T.; Mullol, J. Olfactory dysfunction in neurodegenerative diseases. Curr. Allergy Asthma Rep. 2018, 18, 1–19. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Network, H.L.B. SARS-CoV-2 entry genes are most highly expressed in nasal goblet and ciliated cells within human airways. arXiv 2020, arXiv:2003.06122v1. [Google Scholar]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Ugurlu, B.N.; Akdogan, O.; Yilmaz, Y.A.; Yapar, D.; Aktar Ugurlu, G.; Yerlikaya, H.S.; Aslan Felek, S. Quantitative evaluation and progress of olfactory dysfunction in COVID-19. Eur. Arch. Oto-Rhino. 2021, 278, 2363–2369. [Google Scholar] [CrossRef]
- Chatterjee, S. Olfactory marketing and market innovation: A systematic literature review and agenda for future research direction. Int. J. Manag. Concepts Philos. 2022, 15, 37–56. [Google Scholar] [CrossRef]
- Doty, R.L.; Shaman, P.; Dann, M. Development of the University of Pennsylvania Smell Identification Test: A standardized microencapsulated test of olfactory function. Physiol. Behav. 1984, 32, 489–502. [Google Scholar] [CrossRef]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. ‘Sniffin’sticks’: Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Saltagi, A.K.; Saltagi, M.Z.; Nag, A.K.; Wu, A.W.; Higgins, T.S.; Knisely, A.; Ting, J.Y.; Illing, E.A. Diagnosis of anosmia and hyposmia: A systematic review. Allergy Rhinol. 2021, 12, 21526567211026568. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Rumeau, C.; Gallet, P.; Jankowski, R. Olfactory exploration: State of the art. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016, 133, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Lötsch, J.; Hummel, T. The clinical significance of electrophysiological measures of olfactory function. Behav. Brain Res. 2006, 170, 78–83. [Google Scholar] [CrossRef]
- Allison, T.; Goff, W. Human cerebral evoked responses to odorous stimuli. Electroencephalogr. Clin. Neurophysiol. 1967, 23, 558–560. [Google Scholar] [CrossRef]
- Simmen, D.; Briner, H.R. Olfaction in rhinology-methods of assessing the sense of smell. Rhinology 2006, 44, 98. [Google Scholar]
- Viviana, B.; Tommaso, B.; Nadia, B.; Francesco, F.; Raffaele, F.; Sara, I.; Koch, G.; Carlo, M.; Piccione, F.; Aldo, R.; et al. Pearl and pitfalls in brain functional analysis by event-related potentials: A narrative review by the Italian Psychophysiology and Cognitive Neuroscience Society on methodological limits and clinical reliability—Part II. Neurol. Sci. 2020, 41, 3503–3515. [Google Scholar]
- Kassab, A.; Schaub, F.; Vent, J.; Hüttenbrink, K.B.; Damm, M. Effects of short inter-stimulus intervals on olfactory and trigeminal event-related potentials. Acta Oto-Laryngol. 2009, 129, 1250–1256. [Google Scholar] [CrossRef]
- Pause, B.M.; Krauel, K. Chemosensory event-related potentials (CSERP) as a key to the psychology of odors. Int. J. Psychophysiol. 2000, 36, 105–122. [Google Scholar] [CrossRef]
- Pause, B.M.; Sojka, B.; Ferstl, R. Central processing of odor concentration is a temporal phenomenon as revealed by chemosensory event-related potentials (CSERP). Chem. Senses 1997, 22, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Barz, S.; Lötsch, J.; Roscher, S.; Kettenmann, B.; Kobal, G. Loss of olfactory function leads to a decrease of trigeminal sensitivity. Chem. Senses 1996, 21, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Rombaux, P.; Huart, C.; Mouraux, A. Fisiologia ed esplorazione dei disturbi dell’olfatto. EMC-Otorinolaringoiatr. 2016, 15, 1–11. [Google Scholar] [CrossRef]
- Wang, L.; Walker, V.E.; Sardi, H.; Fraser, C.; Jacob, T.J. The correlation between physiological and psychological responses to odour stimulation in human subjects. Clin. Neurophysiol. 2002, 113, 542–551. [Google Scholar] [CrossRef]
- Morgan, C.D.; Covington, J.W.; Geisler, M.W.; Polich, J.; Murphy, C. Olfactory event-related potentials: Older males demonstrate the greatest deficits. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1997, 104, 351–358. [Google Scholar] [CrossRef]
- Murphy, C.; Nordin, S.; de Wijk, R.A.; Cain, W.S.; Polich, J. Olfactory-evoked potentials: Assessment of young and elderly, and comparison to psychophysical threshold. Chem. Senses 1994, 19, 47–56. [Google Scholar] [CrossRef]
- Donchin, E.; Heffley, E.; Hillyard, S.A.; Loveless, N.; Maltzman, I.; Öhman, A.; Rösler, F.; Ruchkin, D.; Siddle, D. Cognition and event-related potentials: II. The orienting reflex and P300. Ann. N. Y. Acad. Sci. 1984, 425, 39–57. [Google Scholar] [CrossRef]
- Polich, J.; Hoffman, L.D. P300 and handedness: On the possible contribution of corpus callosal size to ERPs. Psychophysiology 1998, 35, 497–507. [Google Scholar] [CrossRef]
- Krbot Skorić, M.; Adamec, I.; Jerbić, A.B.; Gabelić, T.; Hajnšek, S.; Habek, M. Electroencephalographic response to different odors in healthy individuals: A promising tool for objective assessment of olfactory disorders. Clin. EEG Neurosci. 2015, 46, 370–376. [Google Scholar] [CrossRef]
- Lorig, T.S.; Schwartz, G.E. Brain and odor: I. Alteration of human EEG by odor administration. Psychobiology 1988, 16, 281–284. [Google Scholar] [CrossRef]
- Klemm, W.; Lutes, S.; Hendrix, D.; Warrenburg, S. Topographical EEG maps of human responses to odors. Chem. Senses 1992, 17, 347–361. [Google Scholar] [CrossRef]
- Martin, G.N. Human electroencephalographic (EEG) response to olfactory stimulation: Two experiments using the aroma of food. Int. J. Psychophysiol. 1998, 30, 287–302. [Google Scholar] [CrossRef]
- Aydemir, O. Olfactory recognition based on EEG gamma-band activity. Neural Comput. 2017, 29, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Meng, Q.H.; Zeng, M.; Hou, H.R. Decoding olfactory EEG signals for different odor stimuli identification using wavelet-spatial domain feature. J. Neurosci. Methods 2021, 363, 109355. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Kobal, G.; Lorig, T.S.; Prah, J.D. Suggestions for collection and reporting of chemosensory (olfactory) event-related potentials. Chem. Senses 1993, 18, 751–756. [Google Scholar] [CrossRef]
- Lorig, T.S. The application of electroencephalographic techniques to the study of human olfaction: A review and tutorial. Int. J. Psychophysiol. 2000, 36, 91–104. [Google Scholar] [CrossRef]
- Kobal, G. Elektrophysiologische Untersuchungen des Menschlichen Geruchssinns; Thieme: New York, NY, USA, 1981. [Google Scholar]
- Thesen, T.; Murphy, C. Reliability analysis of event-related brain potentials to olfactory stimuli. Psychophysiology 2002, 39, 733–738. [Google Scholar] [CrossRef]
- Rombaux, P.; Bertrand, B.; Keller, T.; Mouraux, A. Clinical significance of olfactory event-related potentials related to orthonasal and retronasal olfactory testing. Laryngoscope 2007, 117, 1096–1101. [Google Scholar] [CrossRef]
- Brämerson, A.; Millqvist, E.; Ydse, B.; Larsson, C.; Olofsson, J.K.; Bende, M. Event-related potentials in patients with olfactory loss. Acta Oto-Laryngol. 2008, 128, 1126–1131. [Google Scholar] [CrossRef]
- Limphaibool, N.; Iwanowski, P.; Kozubski, W.; Swidziński, T.; Frankowska, A.; Kamińska, I.; Linkowska-Swidzińska, K.; Sekula, A.; Swidziński, P.; Maciejewska-Szaniec, Z.; et al. Subjective and objective assessments of post-traumatic olfactory dysfunction. Front. Neurol. 2020, 11, 970. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, D.; Sun, Z.; Yao, L.; Liu, J.; Wei, Y. Prognostic value of olfactory evoked potentials in patients with post-infectious olfactory dysfunction. Eur. Arch. Oto-Rhino 2021, 278, 3839–3846. [Google Scholar] [CrossRef] [PubMed]
- Jasper, H.H. The ten-twenty electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 370–375. [Google Scholar]
- Bio-Logic Website. Available online: https://fda.report/PMN/K854362 (accessed on 20 May 2022).
- De Wijk, R.; Cain, W.; Murphy, C. A portable olfactometer for human psychophysics. Chem. Senses 1993, 18, 651. [Google Scholar]
- Covington, J.W.; Geisler, M.W.; Polich, J.; Murphy, C. Normal aging and odor intensity effects on the olfactory event-related potential. Int. J. Psychophysiol. 1999, 32, 205–214. [Google Scholar] [CrossRef]
- Lorig, T.S.; Zald, D.H.; Pardo, J.V. A computer-controlled olfactometer for fMRI and electrophysiological studies of olfaction. Behav. Res. Methods Instrum. Comput. 1999, 31, 370–375. [Google Scholar] [CrossRef]
- Fatourechi, M.; Bashashati, A.; Ward, R.; Birch, G. EMG and EOG artifacts in brain computer interface systems: A survey. Clin. Neurophysiol. 2006, 118, 480–494. [Google Scholar] [CrossRef]
- Comon, P. Independent component analysis, a new concept? Signal Process. 1994, 36, 287–314. [Google Scholar] [CrossRef]
- Hyvärinen, A.; Oja, E. Independent component analysis: Algorithms and applications. Neural Netw. 2000, 13, 411–430. [Google Scholar] [CrossRef]
- Sparacino, G.; Milani, S.; Arslan, E.; Cobelli, E. A Bayesian approach to estimate evoked potentials. Comput. Methods Programs Biomed. 2002, 68, 233–248. [Google Scholar] [CrossRef]
- Blankertz, B.; Lemm, S.; Treder, M.; Haufe, S.; Müller, K.R. Single-trial analysis and classification of ERP components—A tutorial. NeuroImage 2011, 56, 814–825. [Google Scholar] [CrossRef]
- Niedermeyer, E.; da Silva, F.L. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Mouraux, A.; Iannetti, G.D. Across-trial averaging of event-related EEG responses and beyond. Magn. Reson. Imaging 2008, 26, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- D’Avanzo, C.; Schiff, S.; Amodio, P.; Sparacino, G. A Bayesian method to estimate single-trial event-related potentials with application to the study of the P300 variability. J. Neurosci. Methods 2011, 198, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Ito, P. 7 Robustness of ANOVA and MANOVA test procedures. In Analysis of Variance; Elsevier: Amsterdam, The Netherlands, 1980; Volume 1, pp. 199–236. [Google Scholar]
- Schiffman, S. Food recognition by the elderly. J. Gerontol. 1977, 32, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Kamath, V. The influences of age on olfaction: A review. Front. Psychol. 2014, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Luo, Z.; Pinto, J.M.; Shiroma, E.J.; Tranah, G.J.; Wirdefeldt, K.; Fang, F.; Harris, T.B.; Chen, H. Relationship Between Poor Olfaction and Mortality Among Community-Dwelling Older Adults. Ann. Intern. Med. 2019, 170, 673–681. [Google Scholar] [CrossRef]
- Doty, R.L. Olfactory dysfunction in neurodegenerative diseases: Is there a common pathological substrate? Lancet Neurol. 2017, 16, 478–488. [Google Scholar] [CrossRef]
- Murphy, C.; Morgan, C.D.; Geisler, M.W.; Wetter, S.; Covington, J.W.; Madowitz, M.D.; Nordin, S.; Polich, J.M. Olfactory event-related potentials and aging: Normative data. Int. J. Psychophysiol. 2000, 36, 133–145. [Google Scholar] [CrossRef]
- Mesholam, R.I.; Moberg, P.J.; Mahr, R.N.; Doty, R.L. Olfaction in neurodegenerative disease: A meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. Arch. Neurol. 1998, 55, 84–90. [Google Scholar] [CrossRef]
- Regensburger, M.; Prots, I.; Winner, B. Adult hippocampal neurogenesis in Parkinson’s disease: Impact on neuronal survival and plasticity. Neural Plast. 2014, 2014, 454696. [Google Scholar] [CrossRef]
- Son, G.; Jahanshahi, A.; Yoo, S.; Boonstra, J.T.; Hopkins, D.A.; Steinbusch, H.W.M.; Moon, C. Olfactory neuropathology in Alzheimer’s disease: A sign of ongoing neurodegeneration. BMB Rep. 2021, 54, 295–304. [Google Scholar] [CrossRef]
- Kowalewski, J.; Murphy, C. Olfactory ERPs in an odor/visual congruency task differentiate ApoE ϵ4 carriers from non-carriers. Brain Res. 2012, 1442, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Wetter, S.; Murphy, C. Apolipoprotein E ϵ4 positive individuals demonstrate delayed olfactory event-related potentials. Neurobiol. Aging 2001, 22, 439–447. [Google Scholar] [CrossRef]
- Murphy, C.; Solomon, E.S.; Haase, L.; Wang, M.; Morgan, C.D. Olfaction in Aging and Alzheimer’s Disease: Event-related Potentials to a Cross-modal Odor-Recognition Memory Task Discriminate ApoE ϵ4+ and ApoE ϵ4− Individuals. Ann. N. Y. Acad. Sci. 2009, 1170, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Corby, K.; Morgan, C.D.; Murphy, C. Abnormal event-related potentials in young and middle-aged adults with the ApoE ε4 allele. Int. J. Psychophysiol. 2012, 83, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Ansari, K.; Johnson, A. Olfactory function in patients with Parkinson’s disease. J. Chronic Dis. 1975, 28, 493–497. [Google Scholar] [CrossRef]
- Doty, R.L. Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol. 2012, 8, 329–339. [Google Scholar] [CrossRef]
- Doty, R.L.; Bromley, S.M.; Stern, M.B. Olfactory testing as an aid in the diagnosis of Parkinson’s disease: Development of optimal discrimination criteria. Neurodegeneration 1995, 4, 93–97. [Google Scholar] [CrossRef]
- Morgan, C.D.; Murphy, C. Olfactory event-related potentials in Alzheimer’s disease. J. Int. Neuropsychol. Soc. 2002, 8, 753–763. [Google Scholar] [CrossRef]
- Doty, R.L. Handbook of Olfaction and Gustation; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Caminiti, F.; De Salvo, S.; De Cola, M.; Russo, M.; Bramanti, P.; Marino, S.; Ciurleo, R. Detection of Olfactory Dysfunction Using Olfactory Event Related Potentials in Young Patients with Multiple Sclerosis. PLoS ONE 2014, 9, e103151. [Google Scholar] [CrossRef]
- Lascano, A.M.; Lalive, P.H.; Hardmeier, M.; Fuhr, P.; Seeck, M. Clinical evoked potentials in neurology: A review of techniques and indications. J. Neurol. Neurosurg. Psychiatry 2017, 88, 688–696. [Google Scholar] [CrossRef]
- Rosella, C.; Bonanno, L.; De Salvo, S.; Romeo, L.; Rifici, C.; Sessa, E.; D’Aleo, G.; Russo, M.; Bramanti, P.; Marino, S.; et al. Olfactory dysfunction as a prognostic marker for disability progression in Multiple Sclerosis: An olfactory event related potential study. PLoS ONE 2018, 13, e0196006. [Google Scholar]
- Turetsky, B.I.; Moberg, P.J.; Owzar, K.; Johnson, S.C.; Doty, R.L.; Gur, R.E. Physiologic impairment of olfactory stimulus processing in schizophrenia. Biol. Psychiatry 2003, 53, 403–411. [Google Scholar] [CrossRef]
- Kayser, J.; Tenke, C.E.; Malaspina, D.; Kroppmann, C.J.; Schaller, J.D.; Deptula, A.; Gates, N.A.; Harkavy-Friedman, J.M.; Gil, R.; Bruder, G.E. Neuronal generator patterns of olfactory event-related brain potentials in schizophrenia. Psychophysiology 2010, 47, 1075–1086. [Google Scholar] [CrossRef]
- Turetsky, B.I.; Kohler, C.G.; Gur, R.E.; Moberg, P.J. Olfactory physiological impairment in first-degree relatives of schizophrenia patients. Schizophr. Res. 2008, 102, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Brämerson, A.; Nordin, S.; Bende, M. Clinical experience with patients with olfactory complaints, and their quality of life. Acta Oto-Laryngol. 2007, 127, 167–174. [Google Scholar] [CrossRef]
- Flohr, E.L.; Erwin, E.; Croy, I.; Hummel, T. Sad man’s nose: Emotion induction and olfactory perception. Emotion 2017, 17, 369. [Google Scholar] [CrossRef]
- Li, D.; Jia, J.; Wang, X. Unpleasant food odors modulate the processing of facial expressions: An event-related potential study. Front. Neurosci. 2020, 686. [Google Scholar] [CrossRef]
- Ghinea, G.; Ademoye, O.A. Olfaction-enhanced multimedia: Perspectives and challenges. Multimed. Tools Appl. 2011, 55, 601–626. [Google Scholar] [CrossRef]
- Chen, D.; Dalton, P. The effect of emotion and personality on olfactory perception. Chem. Senses 2005, 30, 345–351. [Google Scholar] [CrossRef]
- Cohen, J.; Polich, J. On the number of trials needed for P300. Int. J. Psychophysiol. 1997, 25, 249–255. [Google Scholar] [CrossRef]
- Duncan, C.C.; Barry, R.J.; Connolly, J.F.; Fischer, C.; Michie, P.T.; Näätänen, R.; Polich, J.; Reinvang, I.; Van Petten, C. Event-related potentials in clinical research: Guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin. Neurophysiol. 2009, 120, 1883–1908. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Bae, J.; Jin, Y.; Moon, C. Odor habituation can modulate very early olfactory event-related potential. Sci. Rep. 2020, 10, 18117. [Google Scholar] [CrossRef] [PubMed]
- Sanei, S.; Chambers, J.A. EEG Signal Processing; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Huart, C.; Legrain, V.; Hummel, T.; Rombaux, P.; Mouraux, A. Time-frequency analysis of chemosensory event-related potentials to characterize the cortical representation of odors in humans. PLoS ONE 2012, 7, e33221. [Google Scholar] [CrossRef]
- Schriever, V.A.; Han, P.; Weise, S.; Hösel, F.; Pellegrino, R.; Hummel, T. Time frequency analysis of olfactory induced EEG-power change. PLoS ONE 2017, 12, e0185596. [Google Scholar] [CrossRef] [PubMed]
- Güdücü, C.; Olcay, B.O.; Schäfer, L.; Aziz, M.; Schriever, V.; Özgören, M.; Hummel, T. Separating normosmic and anosmic patients based on entropy evaluation of olfactory event-related potentials. Brain Res. 2019, 1708, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Lanata, A.; Guidi, A.; Greco, A.; Valenza, G.; Di Francesco, F.; Scilingo, E.P. Automatic recognition of pleasant content of odours through ElectroEncephaloGraphic activity analysis. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 4519–4522. [Google Scholar]
- Decoding olfactory stimuli in EEG data using nonlinear features: A pilot study. J. Neurosci. Methods 2020, 341, 108780. [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. WIREs Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Izenman, A.J. Linear Discriminant Analysis. In Modern Multivariate Statistical Techniques: Regression, Classification, and Manifold Learning; Springer: New York, NY, USA, 2008; pp. 237–280. [Google Scholar]
- Şeker, M.; Ozerdem, M. Application of Higuchi’s Fractal Dimension for the Statistical Analysis of Human EEG Responses to Odors. In Proceedings of the 41st International Conference on Telecommunications and Signal Processing (TSP), Athens, Greece, 4–6 July 2018; pp. 1–4. [Google Scholar]
- Hamet, P.; Tremblay, J. Artificial intelligence in medicine. Metabolism 2017, 69, S36–S40. [Google Scholar] [CrossRef]
- Hashimoto, D.A.; Rosman, G.; Rus, D.; Meireles, O.R. Artificial intelligence in surgery: Promises and perils. Ann. Surg. 2018, 268, 70. [Google Scholar] [CrossRef]
- Chang, A.C. Intelligence-Based Medicine: Artificial Intelligence and Human Cognition in Clinical Medicine and Healthcare; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Apicella, A.; Arpaia, P.; Frosolone, M.; Moccaldi, N. High-wearable EEG-based distraction detection in motor rehabilitation. Sci. Rep. 2021, 11, 5297. [Google Scholar] [CrossRef]

| Psychophysical Orthonasal Testing (e.g., Sniffin’ Sticks) | OERP | Conclusion |
|---|---|---|
| Normosmia | Present | Normal olfactory function |
| Normosmia | Absent | Possibly normal olfactory function, consider the possibility of a technical problem (e.g., EEG artifacts) |
| Hyposmia | Present | Decreased olfactory function (the presence of OERPs may be correlated with a good prognosis) |
| Hyposmia | Absent | Decreased olfactory function (the absence of OERPs may be correlated with a poor prognosis) |
| Anosmia | Present | Consider patient malingering |
| Anosmia | Absent | Severely altered olfactory function, poor prognosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arpaia, P.; Cataldo, A.; Criscuolo, S.; De Benedetto, E.; Masciullo, A.; Schiavoni, R. Assessment and Scientific Progresses in the Analysis of Olfactory Evoked Potentials. Bioengineering 2022, 9, 252. https://doi.org/10.3390/bioengineering9060252
Arpaia P, Cataldo A, Criscuolo S, De Benedetto E, Masciullo A, Schiavoni R. Assessment and Scientific Progresses in the Analysis of Olfactory Evoked Potentials. Bioengineering. 2022; 9(6):252. https://doi.org/10.3390/bioengineering9060252
Chicago/Turabian StyleArpaia, Pasquale, Andrea Cataldo, Sabatina Criscuolo, Egidio De Benedetto, Antonio Masciullo, and Raissa Schiavoni. 2022. "Assessment and Scientific Progresses in the Analysis of Olfactory Evoked Potentials" Bioengineering 9, no. 6: 252. https://doi.org/10.3390/bioengineering9060252
APA StyleArpaia, P., Cataldo, A., Criscuolo, S., De Benedetto, E., Masciullo, A., & Schiavoni, R. (2022). Assessment and Scientific Progresses in the Analysis of Olfactory Evoked Potentials. Bioengineering, 9(6), 252. https://doi.org/10.3390/bioengineering9060252










