Estimation of Aortic Stiffness with Bramwell–Hill Equation: A Comparative Analysis with Carotid–Femoral Pulse Wave Velocity
Abstract
:1. Introduction
2. Methods
2.1. Carotid–Femoral Pulse Wave Velocity
2.2. Ultrasound
2.3. Pulse Pressure
- Using the PulsePen, the central pressure was derived from carotid pressure waves, which have been shown to be similar to those recorded in the ascending aorta [20].
2.4. Bramwell–Hill Equation
- represents the density of the blood;
- A is the mean area of the blood vessel;
- is the difference between the maximum and minimum area of the blood vessel during a cardiac cycle;
- is the difference between the central systolic and diastolic pressures;
- is the estimated PWV obtained from the measurement of pulsatility () and pressure variation ().
2.5. Experimental Data
2.6. Statistical Analysis
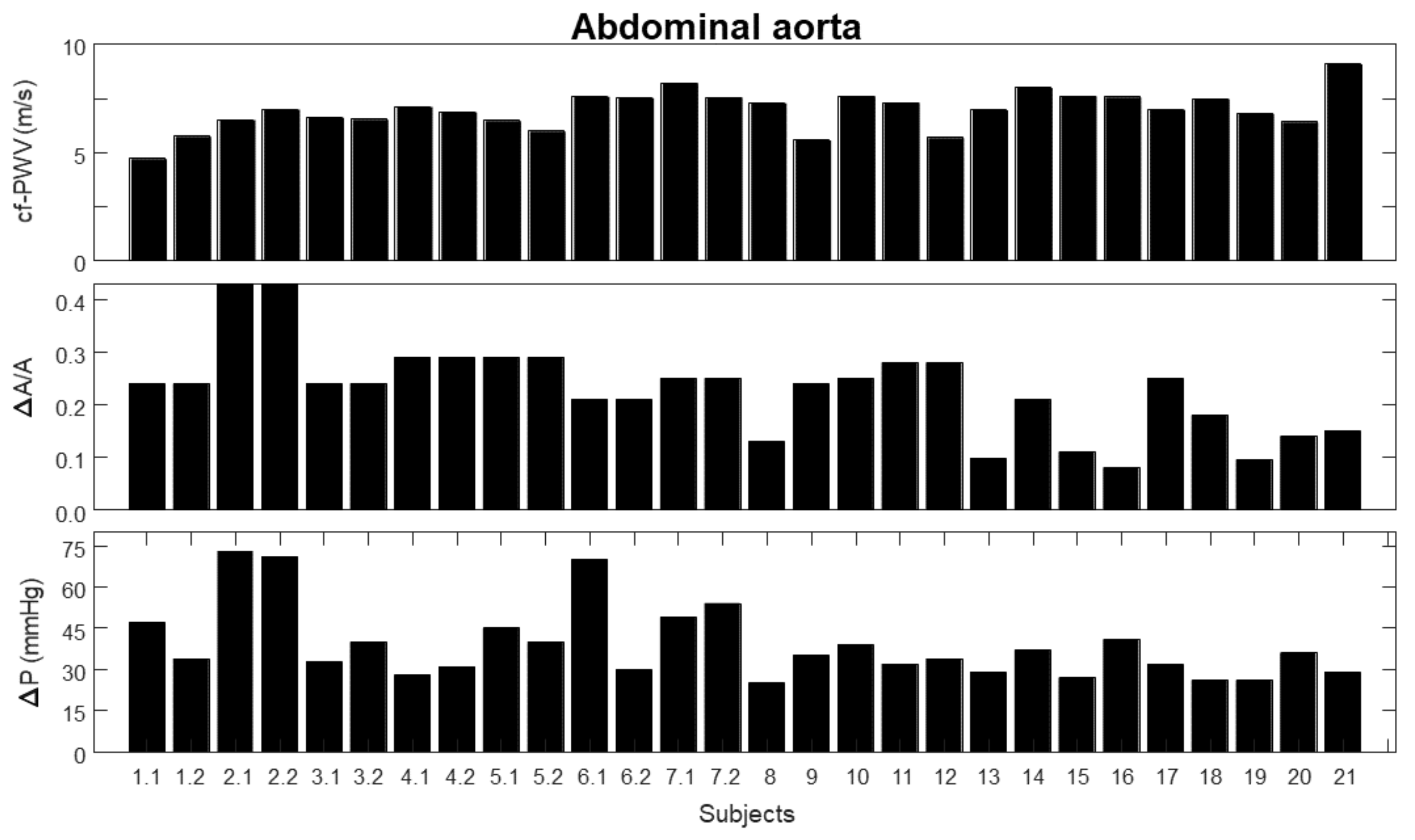
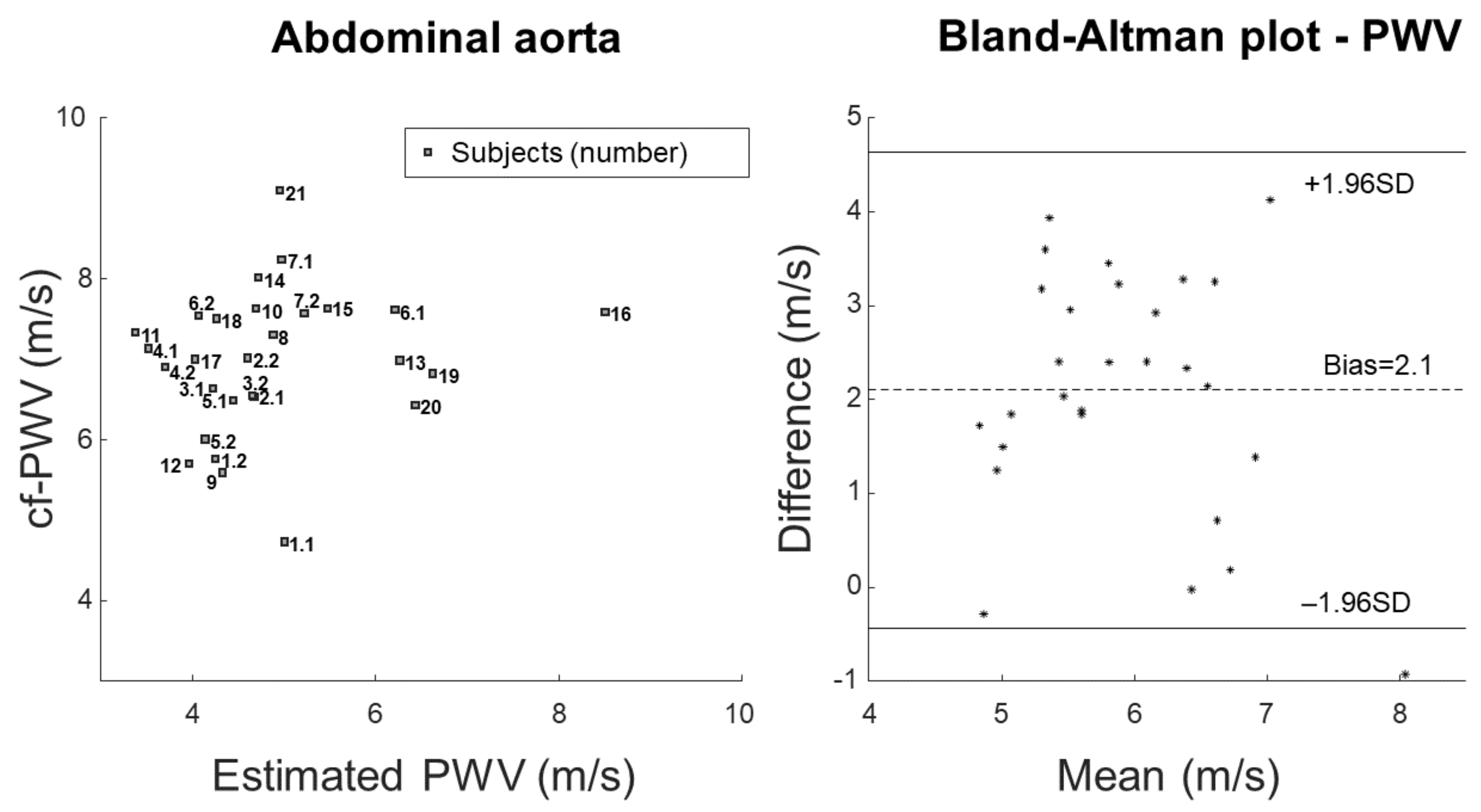
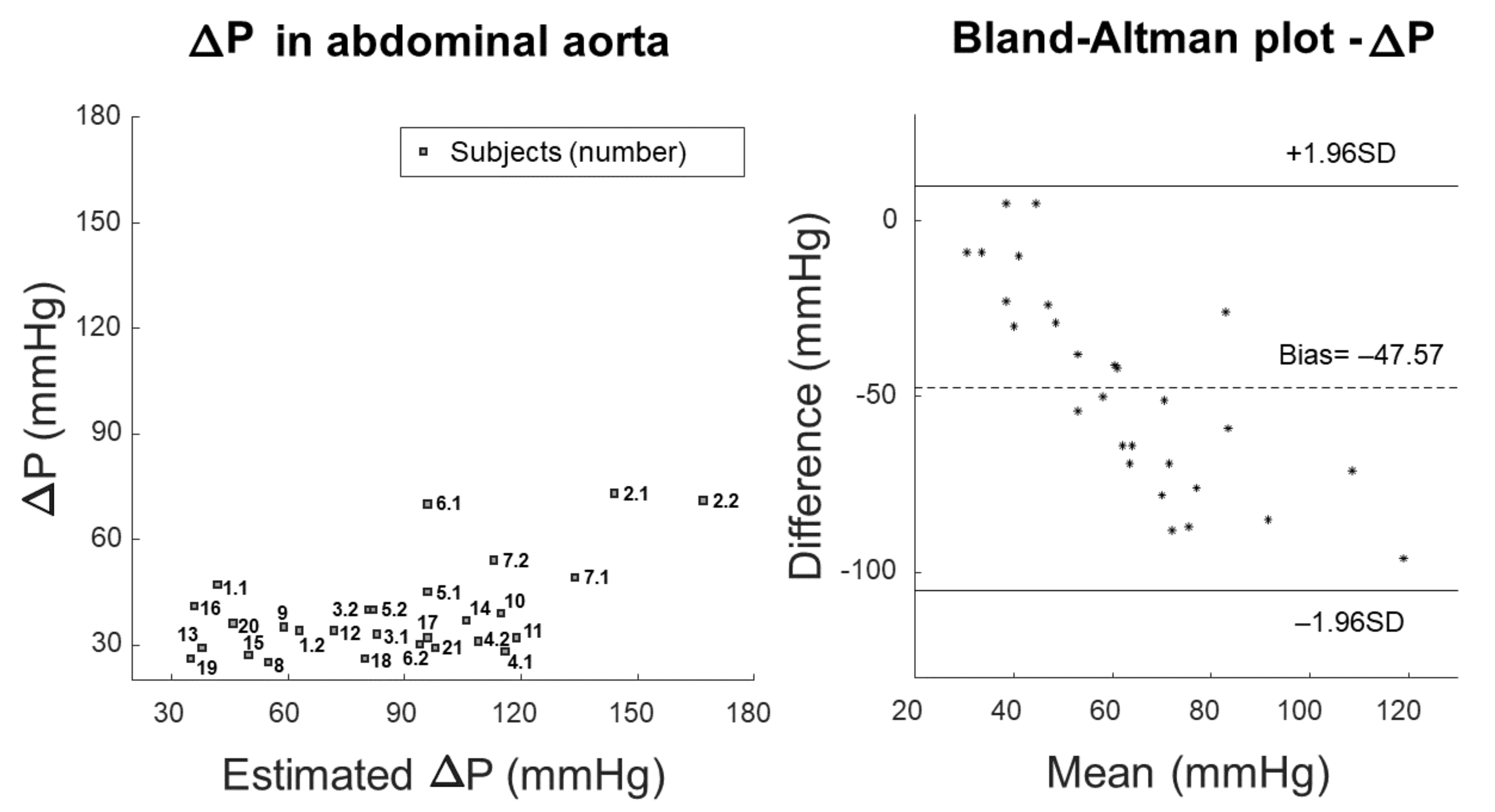
3. Results
- 1.
- carotid–femoral pulse wave velocity (cf-PWV);
- 2.
- pulsatility (), indicated as the ratio between the range (i.e., maximum minus minimum value) and the mean value of the area of a portion of the abdominal aorta measured by automated edge tracking from ultrasound scans;
- 3.
- pressure variation (), measured using either PulsePen (first seven subjects) or SphygmoCor (remaining subjects).
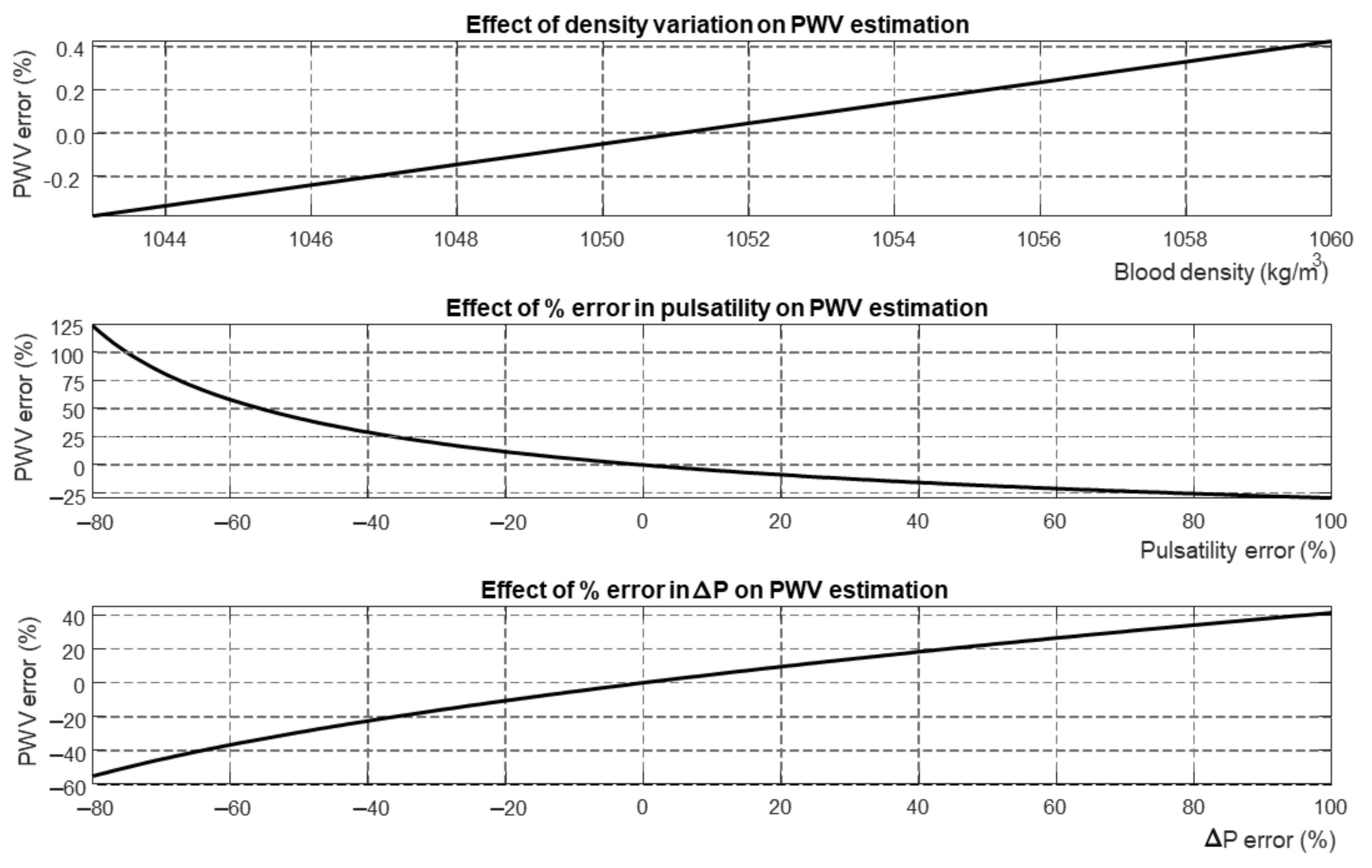
4. Discussion
4.1. Outcomes
4.2. Significance
4.3. Limitations
4.4. Future Perspectives
5. Conclusions and Further Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| cf-PWV | carotid–femoral Pulse Wave Velocity |
| PWV | Pulse Wave Velocity |
References
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.F. Aortic stiffness, pressure and flow pulsatility, and target organ damage. J. Appl. Physiol. 2018, 125, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Segers, P.; Rietzschel, E.R.; Chirinos, J.A. How to Measure Arterial Stiffness in Humans. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1034–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutouyrie, P.; Bruno, R.M. The Clinical Significance and Application of Vascular Stiffness Measurements. Am. J. Hypertens. 2019, 32, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Townsend, R.R.; Wilkinson, I.B.; Schiffrin, E.L.; Avolio, A.P.; Chirinos, J.A.; Cockcroft, J.R.; Heffernan, K.S.; Lakatta, E.G.; McEniery, C.M.; Mitchell, G.F.; et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension 2015, 66, 698–722. [Google Scholar] [CrossRef] [Green Version]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.; Protogerou, A.D.; et al. Artery Society, European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar]
- Salvi, P.; Scalise, F.; Rovina, M.; Moretti, F.; Salvi, L.; Grillo, A.; Gao, L.; Baldi, C.; Faini, A.; Furlanis, G.; et al. Noninvasive Estimation of Aortic Stiffness Through Different Approaches. Hypertension 2019, 74, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Bramwell, J.C.; Hill, A.V. The velocity of the pulse wave in man. Proc. R. Soc. Lond. 1922, 93, 298–306. [Google Scholar]
- Engelen, L.; Bossuyt, J.; Ferreira, I.; van Bortel, L.M.; Reesink, K.D.; Segers, P.; Stehouwer, C.D.; Laurent, S.; Boutouyrie, P. Reference values for local arterial stiffness. Part A: Carotid artery. J. Hypertens. 2015, 33, 1981–1996. [Google Scholar] [CrossRef]
- Uejima, T.; Dunstan, F.D.; Arbustini, E.; Loboz-Grudzien, K.; Hughes, A.D.; Carerj, S.; Favalli, V.; Antonini-Canterin, F.; Vriz, O.; Vinereanu, D.; et al. Age-specific reference values for carotid arterial stiffness estimated by ultrasonic wall tracking. J. Hum. Hypertens. 2020, 34, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Bossuyt, J.; Engelen, L.; Ferreira, I.; Stehouwer, C.D.; Boutouyrie, P.; Laurent, S.; Segers, P.; Reesink, K.; Van Bortel, L.M. Reference values for local arterial stiffness. Part B: Femoral artery. J. Hypertens. 2015, 33, 1997–2009. [Google Scholar] [CrossRef]
- Mesin, L.; Albani, S.; Policastro, P.; Pasquero, P.; Porta, M.; Melchiorri, C.; Leonardi, G.; Albera, C.; Scacciatella, P.; Pellicori, P.; et al. Assessment of Phasic Changes of Vascular Size by Automated Edge Tracking-State of the Art and Clinical Perspectives. Front. Cardiovasc. Med. 2022, 8, 775635. [Google Scholar] [CrossRef]
- Evangelista, A.; Flachskampf, F.A.; Erbel, R.; Antonini-Canterin, F.; Vlachopoulos, C.; Rocchi, G.; Sicari, R.; Nihoyannopoulos, P.; Zamorano, J. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur. J. Echocardiogr. 2010, 11, 645–658. [Google Scholar] [CrossRef] [Green Version]
- Mesin, L.; Pasquero, P.; Roatta, S. Tracking and Monitoring Pulsatility of a Portion of Inferior Vena Cava from Ultrasound Imaging in Long Axis. Ultrasound Med. Biol. 2019, 45, 1338–1343. [Google Scholar] [CrossRef]
- Albani, S.; Pinamonti, B.; Giovinazzo, T.; de Scordilli, M.; Fabris, E.; Stolfo, D.; Perkan, A.; Gregorio, C.; Barbati, G.; Geri, P.; et al. Accuracy of right atrial pressure estimation using a multi-parameter approach derived from inferior vena cava semi-automated edge-tracking echocardiography: A pilot study in patients with cardiovascular disorders. Int. J. Cardiovasc. Imaging 2020, 36, 1213–1225. [Google Scholar] [CrossRef]
- Mesin, L.; Giovinazzo, T.; D’Alessandro, S.; Roatta, S.; Raviolo, A.; Chiacchiarini, F.; Porta, M.; Pasquero, P. Improved repeatability of the estimation of pulsatility of inferior vena cava. Ultrasound Med. Biol. 2019, 45, 2830–2843. [Google Scholar] [CrossRef]
- Mesin, L.; Albani, S.; Sinagra, G. Non-invasive Estimation of Right Atrial Pressure using the Pulsatility of Inferior Vena Cava. Ultrasound Med. Biol. 2019, 45, 1331–1337. [Google Scholar] [CrossRef]
- Mesin, L.; Pasquero, P.; Roatta, S.; Porta, M. Automated Volume Status Assessment Using Inferior Vena Cava Pulsatility. Electronics 2020, 9, 1671. [Google Scholar] [CrossRef]
- Salvi, P.; Grillo, A.; Parati, G. Noninvasive estimation of central blood pressure and analysis of pulse waves by applanation tonometry. Hypertens. Res. 2015, 38, 646–648. [Google Scholar] [CrossRef]
- Salvi, P.; Lio, G.; Labat, C.; Ricci, E.; Pannier, B.; Benetos, A. Validation of a new non-invasive portable tonometer for determining arterial pressure wave and pulse wave velocity: The PulsePen device. J. Hypertens. 2004, 22, 2285–2293. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Nevo, E.; Fetics, B.; Pak, P.H.; Yin, F.C.; Maughan, W.L.; Kass, D.A. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation 1997, 95, 1827–1836. [Google Scholar] [CrossRef]
- Pauca, A.L.; O’Rourke, M.F.; Kon, N.D. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 2001, 38, 932–937. [Google Scholar] [CrossRef] [Green Version]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 8, 307–310. [Google Scholar] [CrossRef]
- Hickson, S.S.; Butlin, M.; Graves, M.; Taviani, V.; Avolio, A.P.; McEniery, C.M.; Wilkinson, I.B. The relationship of age with regional aortic stiffness and diameter. JACC Cardiovasc. Imaging 2010, 3, 1247–1255. [Google Scholar] [CrossRef] [Green Version]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Paini, A.; Boutouyrie, P.; Calvet, D.; Tropeano, A.I.; Laloux, B.; Laurent, S. Carotid and aortic stiffness: Determinants of discrepancies. Hypertension 2006, 47, 371–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvi, P.; Valbusa, F.; Kearney-Schwartz, A.; Labat, C.; Grillo, A.; Parati, G.; Benetos, A. Non-Invasive Assessment of Arterial Stiffness: Pulse Wave Velocity, Pulse Wave Analysis and Carotid Cross-Sectional Distensibility: Comparison between Methods. J. Clin. Med. 2022, 11, 2225. [Google Scholar] [CrossRef]
- Vriz, O.; Driussi, C.; La Carrubba, S.; Di Bello, V.; Zito, C.; Carerj, S.; Antonini-Canterin, F. Comparison of sequentially measured Aloka echo-tracking one-point pulse wave velocity with SphygmoCor carotid-femoral pulse wave velocity. SAGE Open Med. 2013, 1, 2050312113507563. [Google Scholar] [CrossRef]
- Kozakova, M.; Morizzo, C.; Guarino, D.; Federico, G.; Miccoli, M.; Giannattasio, C.; Palombo, C. The impact of age and risk factors on carotid and carotid-femoral pulse wave velocity. J. Hypertens. 2015, 33, 1446–1451. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesin, L.; Floris, L.; Policastro, P.; Albani, S.; Scacciatella, P.; Pugliese, N.; Masi, S.; Grillo, A.; Fabris, B.; Antonini-Canterin, F. Estimation of Aortic Stiffness with Bramwell–Hill Equation: A Comparative Analysis with Carotid–Femoral Pulse Wave Velocity. Bioengineering 2022, 9, 265. https://doi.org/10.3390/bioengineering9070265
Mesin L, Floris L, Policastro P, Albani S, Scacciatella P, Pugliese N, Masi S, Grillo A, Fabris B, Antonini-Canterin F. Estimation of Aortic Stiffness with Bramwell–Hill Equation: A Comparative Analysis with Carotid–Femoral Pulse Wave Velocity. Bioengineering. 2022; 9(7):265. https://doi.org/10.3390/bioengineering9070265
Chicago/Turabian StyleMesin, Luca, Luca Floris, Piero Policastro, Stefano Albani, Paolo Scacciatella, Nicola Pugliese, Stefano Masi, Andrea Grillo, Bruno Fabris, and Francesco Antonini-Canterin. 2022. "Estimation of Aortic Stiffness with Bramwell–Hill Equation: A Comparative Analysis with Carotid–Femoral Pulse Wave Velocity" Bioengineering 9, no. 7: 265. https://doi.org/10.3390/bioengineering9070265
APA StyleMesin, L., Floris, L., Policastro, P., Albani, S., Scacciatella, P., Pugliese, N., Masi, S., Grillo, A., Fabris, B., & Antonini-Canterin, F. (2022). Estimation of Aortic Stiffness with Bramwell–Hill Equation: A Comparative Analysis with Carotid–Femoral Pulse Wave Velocity. Bioengineering, 9(7), 265. https://doi.org/10.3390/bioengineering9070265







