An Optimized Method to Decellularize Human Trabecular Meshwork
Abstract
:1. Introduction
2. Materials and Methods
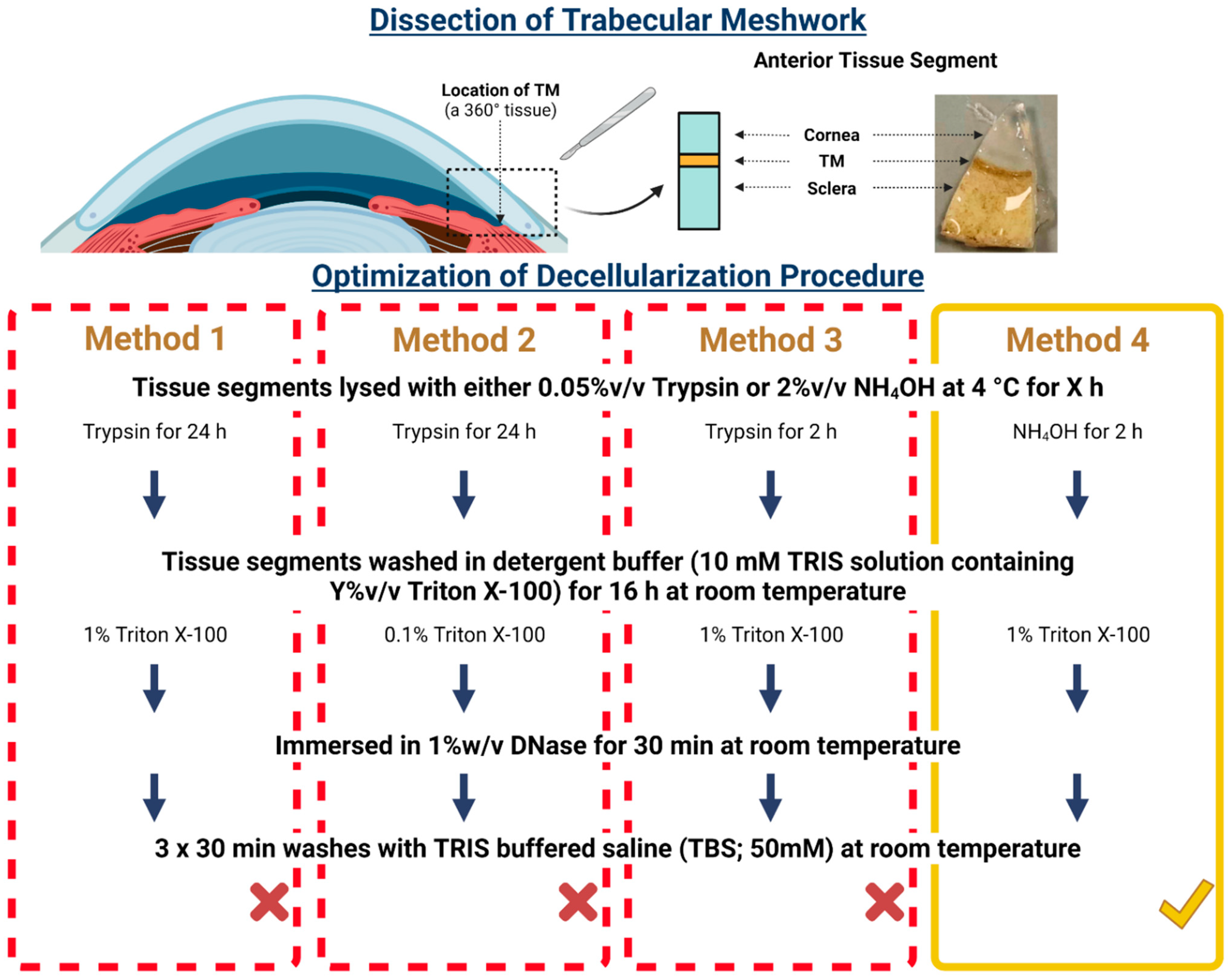
2.1. Decellularization of Bovine Trabecular Meshwork
2.1.1. Sourcing Bovine Eyes
2.1.2. Determining a Non-Destructive Decellularization Method Using Bovine Trabecular Meshwork
2.1.3. Immunocytochemical Analysis of BTM
2.1.4. Imaging BTM with Scanning Electron Microscopy
2.1.5. Quantification of DNA Content for BTM
2.1.6. Histological Evaluation of BTM
2.1.7. Statistical Analysis of BTM
2.2. Decellularization of the Human Trabecular Meshwork
2.2.1. Sourcing Human Eyes
2.2.2. Decellularization of the Human Trabecular Meshwork Using the Optimized Non-Destructive Method
2.2.3. X-ray Computed Tomography Imaging of HTM
2.2.4. Statistical Analysis of HTM
3. Results
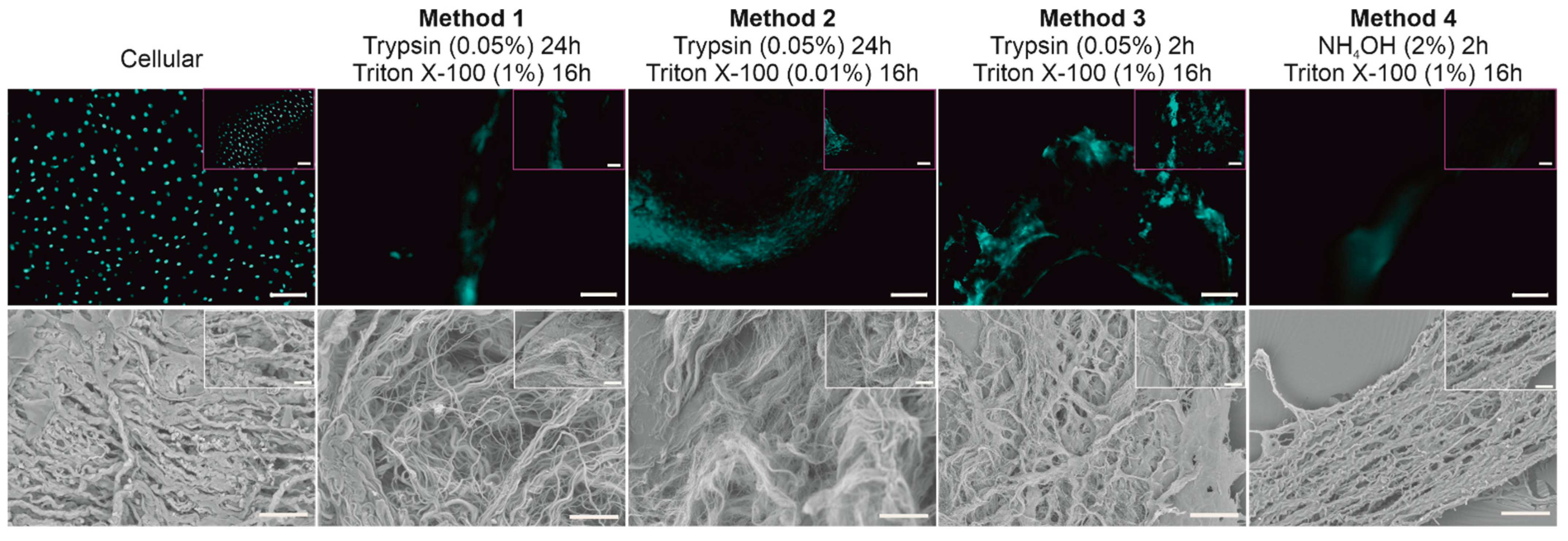
3.1. Determining an Optimal Method for BTM Decellularization
3.1.1. Immunocytochemical Evaluation of BTM
3.1.2. Structural Evaluation of BTM Using SEM
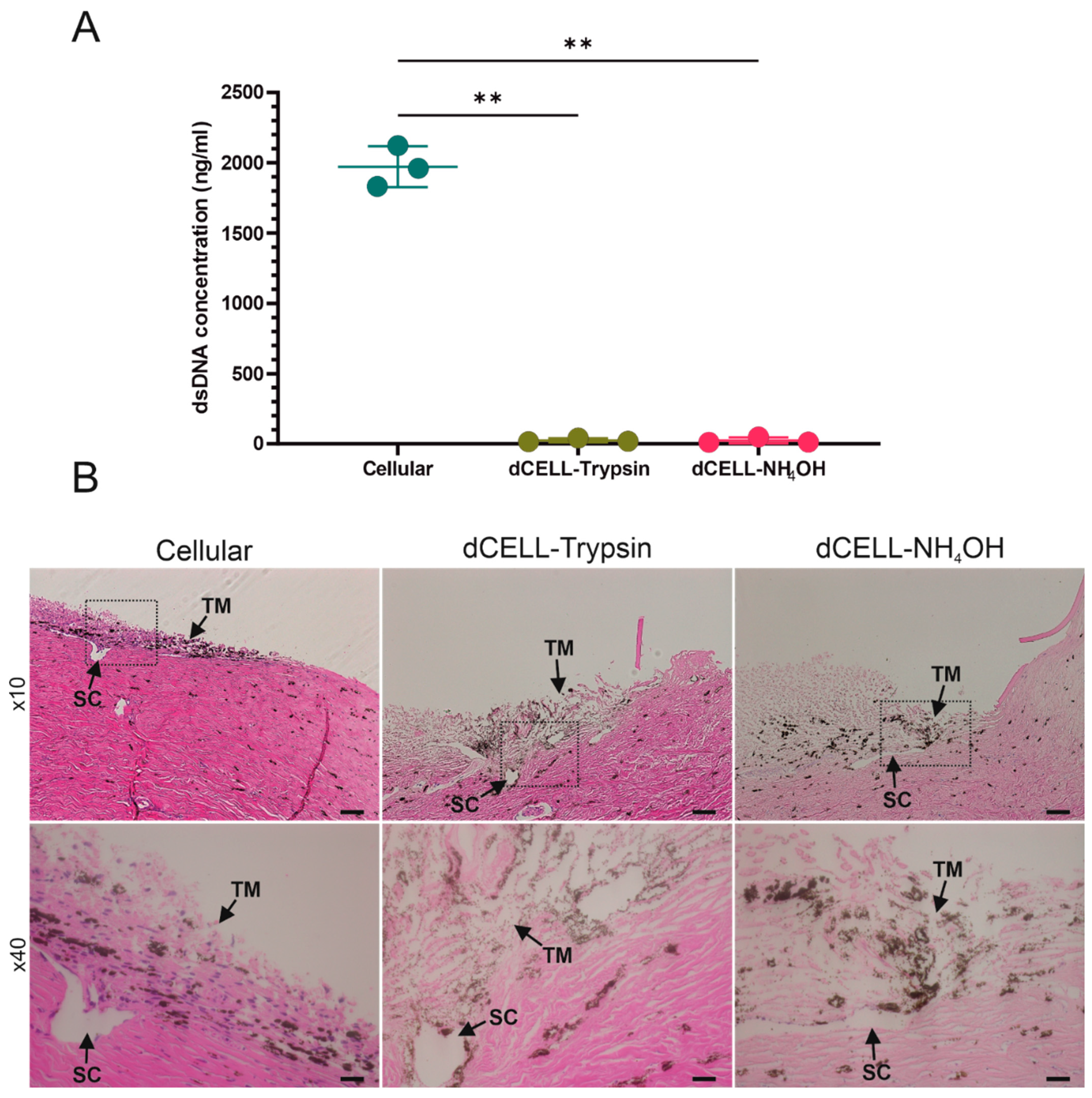
3.1.3. Quantifying DNA Content of BTM
3.1.4. Histological Evaluation of BTM
3.2. Decellularization of HTM Using Optimized Method
3.2.1. Quantifying DNA Content of HTM (Anterior Tissue Segment)
3.2.2. Histological Evaluation of HTM (Anterior Tissue Segment)
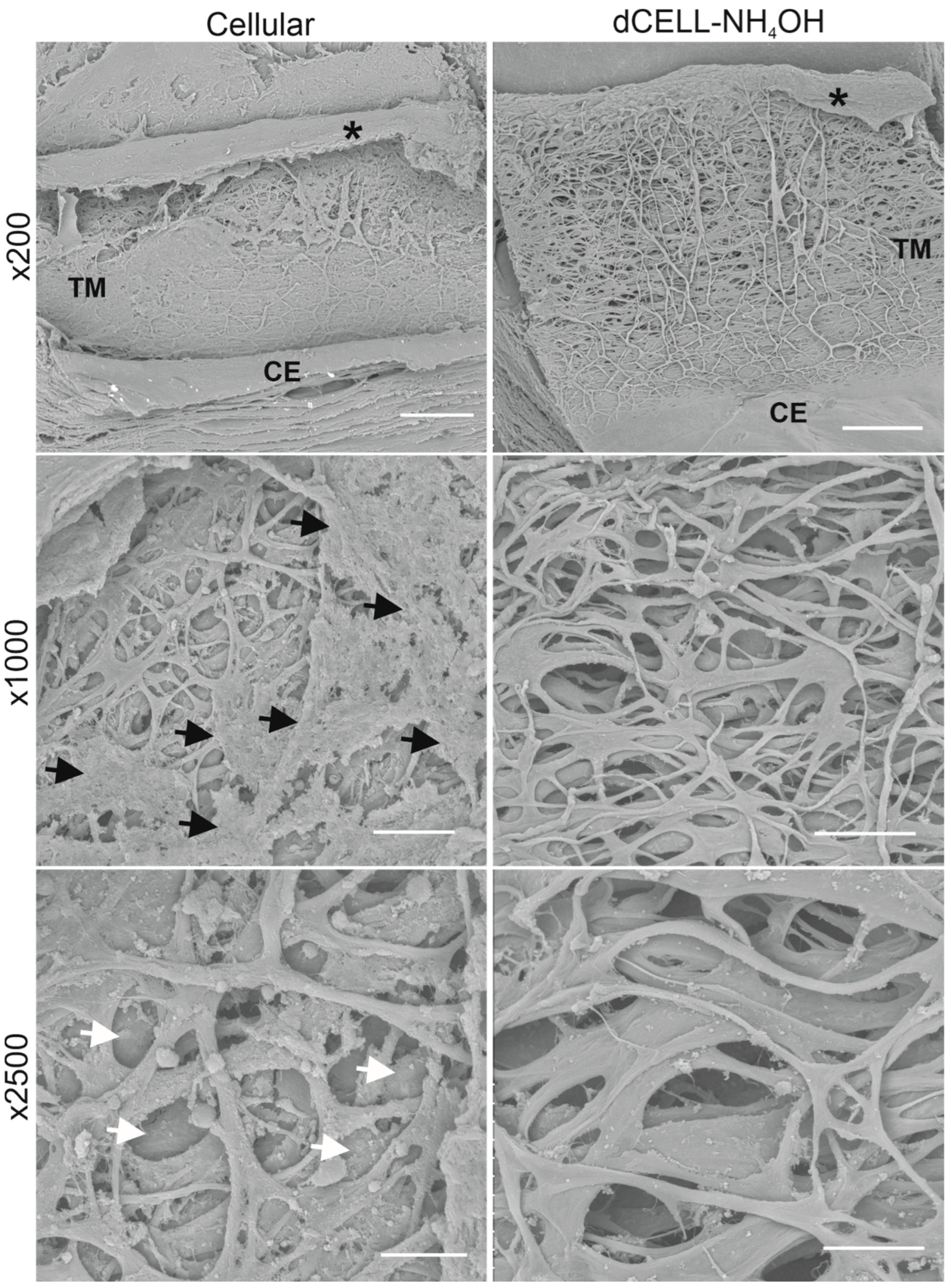
3.2.3. Structural Evaluation of HTM Using SEM
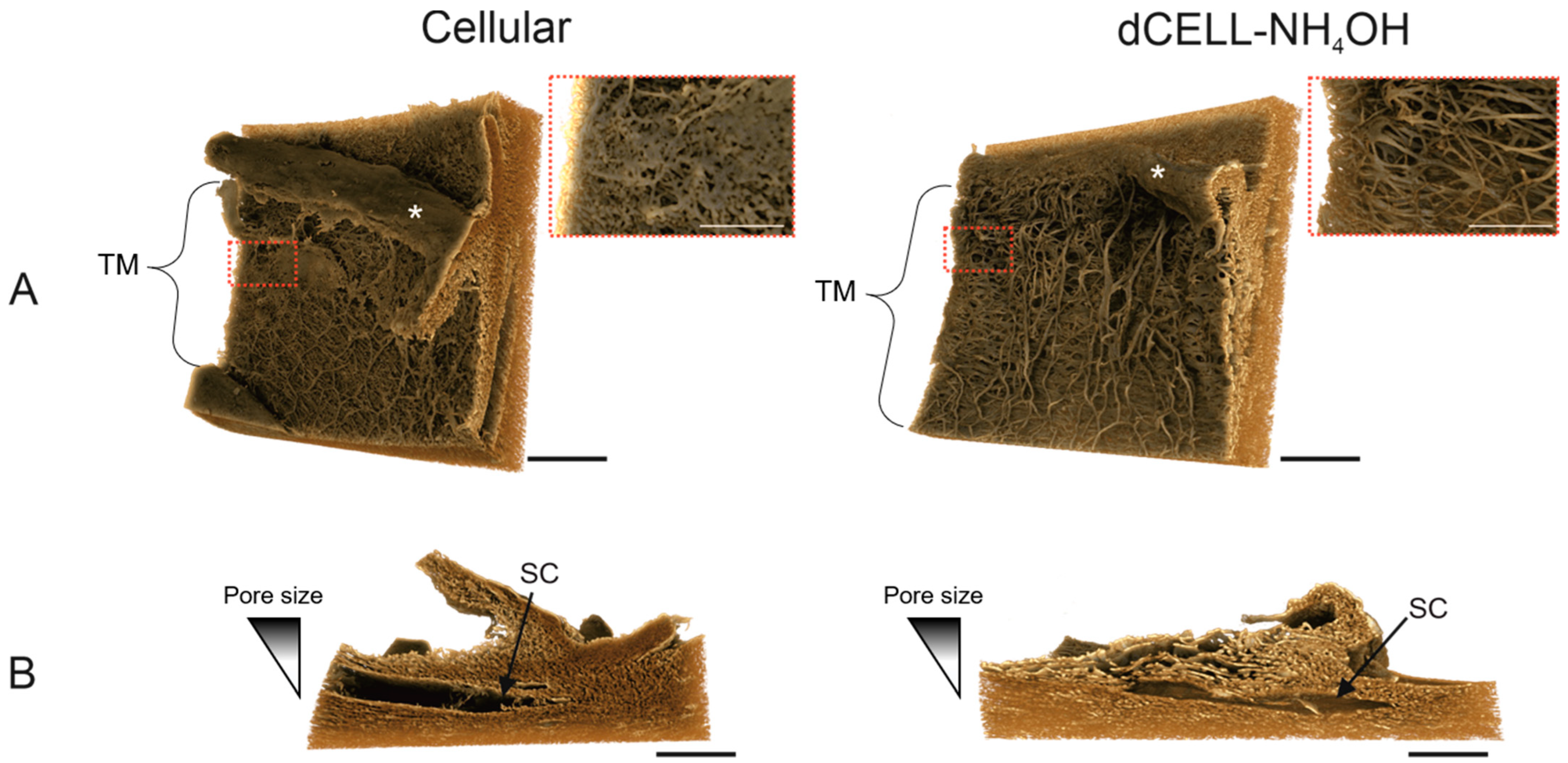
3.2.4. X-CT Imaging and 3-Dimensional Reconstruction of the HTM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040 A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Saccà, S.C.; Cutolo, C.A.; Rossi, T. Glaucoma: An Overview. In Handbook of Nutrition, Diet, and the Eye; Elsevier: London, UK, 2019; pp. 167–187. [Google Scholar]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Tee Khaw, P. Primary Open-Angle Glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef]
- Fan, X.; Bilir, E.K.; Kingston, O.A.; Oldershaw, R.A.; Kearns, V.R.; Willoughby, C.E.; Sheridan, C.M. Replacement of the Trabecular Meshwork Cells—A Way Ahead in Iop Control? Biomolecules 2021, 11, 1371. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Leung, C.K.S.; Crowston, J.G.; Medeiros, F.A.; Friedman, D.S.; Wiggs, J.L.; Martin, K.R. Primary Open-Angle Glaucoma. Nat. Rev. Dis. Prim. 2016, 2, 1–19. [Google Scholar] [CrossRef]
- Garcia-Valenzuela, E.; Shareef, S.; Walsh, J.; Sharma, S.C. Programmed Cell Death of Retinal Ganglion Cells during Experimental Glaucoma. Exp. Eye Res. 1995, 61, 33–44. [Google Scholar] [CrossRef]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous Humor Dynamics: A Review. Open Ophthalmol. J. 2010, 4, 52. [Google Scholar] [CrossRef] [Green Version]
- Grierson, I.; Howes, R.C. Age-Related Depletion of the Cell Population in the Human Trabecular Meshwork. Eye 1987, 1, 204–210. [Google Scholar] [CrossRef] [Green Version]
- Alvarado, J.; Murphy, C.; Polansky, J.; Juster, R. Age-Related Changes in Trabecular Meshwork Cellularity. Investig. Ophthalmol. Vis. Sci. 1981, 21, 714–727. [Google Scholar]
- Alvarado, J.; Murphy, C.; Juster, R. Trabecular Meshwork Cellularity in Primary Open-Angle Glaucoma and Nonglaucomatous Normals. Ophthalmology 1984, 91, 564–579. [Google Scholar] [CrossRef]
- Tamm, E.R.; Fuchshofer, R. What Increases Outflow Resistance in Primary Open-Angle Glaucoma? Surv. Ophthalmol. 2007, 52, S101–S104. [Google Scholar] [CrossRef] [PubMed]
- Dikopf, M.S.; Vajaranant, T.S.; Edward, D.P. Topical Treatment of Glaucoma: Established and Emerging Pharmacology. Expert Opin. Pharmacother. 2017, 18, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.H.; Yu, D.Y. Surgical Management of Glaucoma: A Review. Clin. Exp. Ophthalmol. 2012, 40, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Brandão, L.M.; Grieshaber, M.C. Update on Minimally Invasive Glaucoma Surgery (MIGS) and New Implants. J. Ophthalmol. 2013, 2013, 705915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, G.M.; Coleman, A.L. Minimally Invasive Glaucoma Surgery: Current Status and Future Prospects. Clin. Ophthalmol. 2016, 10, 189–206. [Google Scholar]
- Jang, H.K.; Kim, B.S. Modulation of Stem Cell Differentiation with Biomaterials. Int. J. Stem Cells 2010, 3, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Almouemen, N.; Kelly, H.M.; O’Leary, C. Tissue Engineering: Understanding the Role of Biomaterials and Biophysical Forces on Cell Functionality Through Computational and Structural Biotechnology Analytical Methods. Comput. Struct. Biotechnol. J. 2019, 17, 591–598. [Google Scholar] [CrossRef]
- Osmond, M.J.; Krebs, M.D.; Pantcheva, M.B. Human Trabecular Meshwork Cell Behavior Is Influenced by Collagen Scaffold Pore Architecture and Glycosaminoglycan Composition. Biotechnol. Bioeng. 2020, 17, 3150–3159. [Google Scholar] [CrossRef]
- Torrejon, K.Y.; Pu, D.; Bergkvist, M.; Danias, J.; Sharfstein, S.T.; Xie, Y. Recreating a Human Trabecular Meshwork Outflow System on Microfabricated Porous Structures. Biotechnol. Bioeng. 2013, 110, 3205–3218. [Google Scholar] [CrossRef]
- Klapstova, A.; Horakova, J.; Tunak, M.; Shynkarenko, A.; Erben, J.; Hlavata, J.; Bulir, P.; Chvojka, J. A PVDF Electrospun Antifibrotic Composite for Use as a Glaucoma Drainage Implant. Mater. Sci. Eng. C 2021, 119, 111637. [Google Scholar] [CrossRef]
- Crouch, D.J.; Sheridan, C.M.; D’Sa, R.A.; Willoughby, C.E.; Bosworth, L.A. Exploiting Biomaterial Approaches to Manufacture an Artificial Trabecular Meshwork: A Progress Report. Biomater. Biosyst. 2021, 1, 100011. [Google Scholar] [CrossRef]
- Ma, P.X. Biomimetic Materials for Tissue Engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Ding, B.; Li, B. Biomimetic Electrospun Nanofibrous Structures for Tissue Engineering. Mater. Today 2013, 16, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Ammar, D.A.; Lei, T.C.; Gibson, E.A.; Kahook, M.Y. Two-Photon Imaging of the Trabecular Meshwork. Mol. Vis. 2010, 16, 935–944. [Google Scholar]
- Buller, C.; Johnson, D. Segmental Variability of the Trabecular Meshwork in Normal and Glaucomatous Eyes. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3841–3851. [Google Scholar]
- Hansson, H.; Jerndal, T. Scanning Electron Microscopic Studies on the Iridocorneal Angle Tissue in Normal Human Eyes. J. Japanese Ophthalmol. Soc. 1972, 76, 659–663. [Google Scholar]
- Sanchez-Diaz, P.C. Anatomy of the Eye and Orbit—The Clinical Essentials. Optom. Vis. Sci. 2018, 95, 627. [Google Scholar] [CrossRef]
- Dang, Y.; Waxman, S.; Wang, C.; Jensen, A.; Loewen, R.T.; Bilonick, R.A.; Loewen, N.A. Freeze-Thaw Decellularization of the Trabecular Meshwork in an Ex Vivo Eye Perfusion Model. PeerJ 2017, 2017, e3629. [Google Scholar] [CrossRef] [Green Version]
- Poornejad, N.; Frost, T.S.; Scott, D.R.; Elton, B.B.; Reynolds, P.R.; Roeder, B.L.; Cook, A.D. Freezing/Thawing without Cryoprotectant Damages Native but Not Decellularized Porcine Renal Tissue. Organogenesis 2015, 11, 30–45. [Google Scholar] [CrossRef] [Green Version]
- Limaye, A. Drishti: A Volume Exploration and Presentation Tool. In Proceedings of the SPIE 8506, Developments in X-ray Tomography VIII, San Diego, CA, USA, 12–16 August 2012; p. 85060X. [Google Scholar]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of Tissues and Organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Gilbert, T.W. Strategies for Tissue and Organ Decellularization. J. Cell. Biochem. 2012, 113, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of Tissue Decellularization Used for Preparation of Biologic Scaffolds and in Vivo Relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, D.; Yee, M.; Sheng, Z.L.J.; Amirul, A.; Naing, M.W. Decellularization Systems and Devices: State-of-the-Art. Acta Biomater. 2020, 115, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, D.E. A Review of Decellularized Extracellular Matrix Biomaterials for Regenerative Engineering Applications. Regen. Eng. Transl. Med. 2019, 5, 155–166. [Google Scholar] [CrossRef]
- Bosworth, L.A.; Doherty, K.G.; Hsuan, J.D.; Cray, S.P.; D’sa, R.A.; Molina, C.P.; Badylak, S.F.; Williams, R.L. Material Characterisation and Stratification of Conjunctival Epithelial Cells on Electrospun Poly(ε-Caprolactone) Fibres Loaded with Decellularised Tissue Matrices. Pharmaceutics 2021, 13, 318. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Stolz, D.B.; Biancaniello, F.; Simmons-Byrd, A.; Badylak, S.F. Production and Characterization of ECM Powder: Implications for Tissue Engineering Applications. Biomaterials 2005, 26, 1431–1435. [Google Scholar] [CrossRef]
- Moroni, F.; Mirabella, T. Decellularized Matrices for Cardiovascular Tissue Engineering. Am. J. Stem Cells 2014, 3, 1–20. [Google Scholar]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [Green Version]
- Kasbekar, S.; Kaye, S.B.; Williams, R.L.; Stewart, R.M.K.; Leow-Dyke, S.; Rooney, P. Development of Decellularized Conjunctiva as a Substrate for the Ex Vivo Expansion of Conjunctival Epithelium. J. Tissue Eng. Regen. Med. 2018, 12, 973–982. [Google Scholar] [CrossRef]
- Ponce Márquez, S.; Martínez, V.S.; McIntosh Ambrose, W.; Wang, J.; Gantxegui, N.G.; Schein, O.; Elisseeff, J. Decellularization of Bovine Corneas for Tissue Engineering Applications. Acta Biomater. 2009, 5, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Maqueda, M.; Mosquera, J.L.; García-Arumí, J.; Veiga, A.; Duarri, A. Repopulation of Decellularized Retinas with HiPSC-Derived Retinal Pigment Epithelial and Ocular Progenitor Cells Shows Cell Engraftment, Organization and Differentiation. Biomaterials 2021, 276, 121049. [Google Scholar] [CrossRef] [PubMed]
- Flügel, C.; Tamm, E.; Lütjen-Drecoll, E. Different Cell Populations in Bovine Trabecular Meshwork: An Ultrastructural and Immunocytochemical Study. Exp. Eye Res. 1991, 52, 681–690. [Google Scholar] [CrossRef] [Green Version]
- Wade, N.C.; Grierson, I.; O’Reilly, S.; Hoare, M.J.; Cracknell, K.P.B.; Paraoan, L.I.; Brotchie, D.; Clark, A.F. Cross-Linked Actin Networks (CLANs) in Bovine Trabecular Meshwork Cells. Exp. Eye Res. 2009, 89, 648–659. [Google Scholar] [CrossRef]
- Zia, S.; Mozafari, M.; Natasha, G.; Tan, A.; Cui, Z.; Seifalian, A.M. Hearts Beating through Decellularized Scaffolds: Whole-Organ Engineering for Cardiac Regeneration and Transplantation. Crit. Rev. Biotechnol. 2015, 36, 705–715. [Google Scholar] [CrossRef]
- Reing, J.E.; Brown, B.N.; Daly, K.A.; Freund, J.M.; Gilbert, T.W.; Hsiong, S.X.; Huber, A.; Kullas, K.E.; Tottey, S.; Wolf, M.T.; et al. The Effects of Processing Methods upon Mechanical and Biologic Properties of Porcine Dermal Extracellular Matrix Scaffolds. Biomaterials 2010, 31, 8626–8633. [Google Scholar] [CrossRef] [Green Version]
- Wilson, W.D.; Tanious, F.A.; Barton, H.J.; Wydra, R.L.; Strekowski, L.; Jones, R.L.; Fox, K. DNA Sequence Dependent Binding Modes of 4′,6-Diamidino-2-Phenylindole (DAPI). Biochemistry 1990, 29, 8452–8461. [Google Scholar] [CrossRef]
- Meyer, S.R.; Chiu, B.; Churchill, T.A.; Zhu, L.; Lakey, J.R.T.; Ross, D.B. Comparison of Aortic Valve Allograft Decellularization Techniques in the Rat. J. Biomed. Mater. Res. A 2006, 79, 254–262. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Y. Mechanical Evaluation of Decellularized Porcine Thoracic Aorta. J. Surg. Res. 2012, 175, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Behrend, M.; Kluge, E.; Von Wasielewski, R.; Klempnauer, J. The Mechanical Influence of Tissue Engineering Techniques on Tracheal Strength: An Experimental Study on Sheep Trachea. J. Investig. Surg. 2002, 15, 227–236. [Google Scholar] [CrossRef]
- Baptista, P.M.; Orlando, G.; Mirmalek-Sani, S.H.; Siddiqui, M.; Atala, A.; Soker, S. Whole Organ Decellularization—A Tool for Bioscaffold Fabrication and Organ Bioengineering. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 6526–6529. [Google Scholar] [CrossRef]
- Raghunathan, V.K.; Morgan, J.T.; Chang, Y.R.; Weber, D.; Phinney, B.; Murphy, C.J.; Russell, P. Transforming Growth Factor Beta 3 Modifies Mechanics and Composition of Extracellular Matrix Deposited by Human Trabecular Meshwork Cells. ACS Biomater. Sci. Eng. 2015, 1, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Raghunathan, V.K.; Morgan, J.T.; Park, S.A.; Weber, D.; Phinney, B.S.; Murphy, C.J.; Russell, P. Dexamethasone Stiffens Trabecular Meshwork, Trabecular Meshwork Cells, and Matrix. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4447–4459. [Google Scholar] [CrossRef] [PubMed]
- Raghunathan, V.K.; Benoit, J.; Kasetti, R.; Zode, G.; Salemi, M.; Phinney, B.S.; Keller, K.E.; Staverosky, J.A.; Murphy, C.J.; Acott, T.; et al. Glaucomatous Cell Derived Matrices Differentially Modulate Non-Glaucomatous Trabecular Meshwork Cellular Behavior. Acta Biomater. 2018, 71, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Hann, C.R.; Bentley, M.D.; Vercnocke, A.; Ritman, E.L.; Fautsch, M.P. Imaging the Aqueous Humor Outflow Pathway in Human Eyes by Three-Dimensional Micro-Computed Tomography (3D Micro-CT). Exp. Eye Res. 2011, 92, 104–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahler, C.K.; Hann, C.R.; Fjield, T.; Haffner, D.; Heitzmann, H.; Fautsch, M.P. Second-Generation Trabecular Meshwork Bypass Stent (Istent Inject) Increases Outflow Facility in Cultured Human Anterior Segments. Am. J. Ophthalmol. 2012, 153, 1206–1213. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crouch, D.J.; Sheridan, C.M.; Behnsen, J.G.; Bosworth, L.A. An Optimized Method to Decellularize Human Trabecular Meshwork. Bioengineering 2022, 9, 194. https://doi.org/10.3390/bioengineering9050194
Crouch DJ, Sheridan CM, Behnsen JG, Bosworth LA. An Optimized Method to Decellularize Human Trabecular Meshwork. Bioengineering. 2022; 9(5):194. https://doi.org/10.3390/bioengineering9050194
Chicago/Turabian StyleCrouch, Devon J., Carl M. Sheridan, Julia G. Behnsen, and Lucy A. Bosworth. 2022. "An Optimized Method to Decellularize Human Trabecular Meshwork" Bioengineering 9, no. 5: 194. https://doi.org/10.3390/bioengineering9050194
APA StyleCrouch, D. J., Sheridan, C. M., Behnsen, J. G., & Bosworth, L. A. (2022). An Optimized Method to Decellularize Human Trabecular Meshwork. Bioengineering, 9(5), 194. https://doi.org/10.3390/bioengineering9050194






