Modular Bioreactor Design for Directed Tendon/Ligament Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. MSC Strain-Induction Analysis
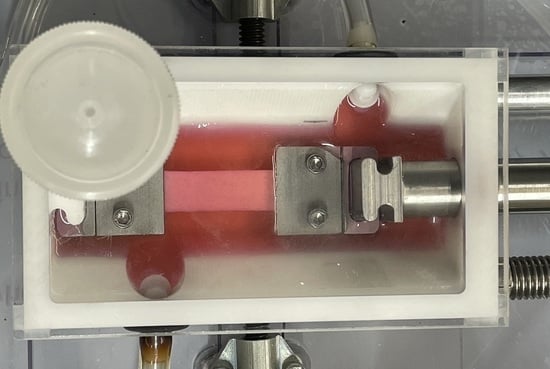
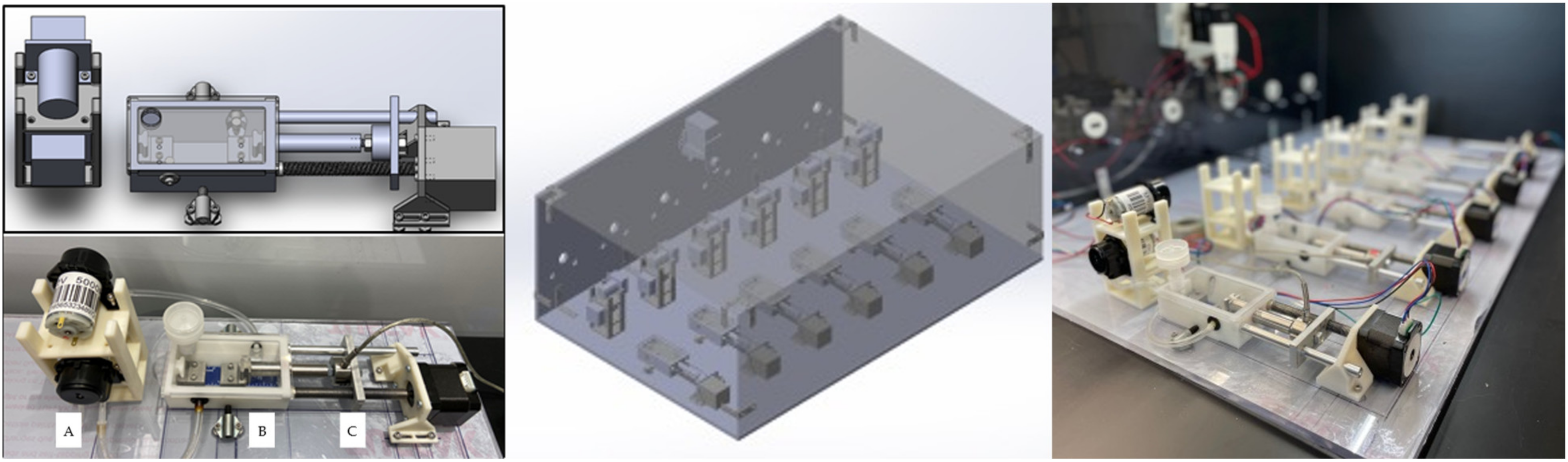
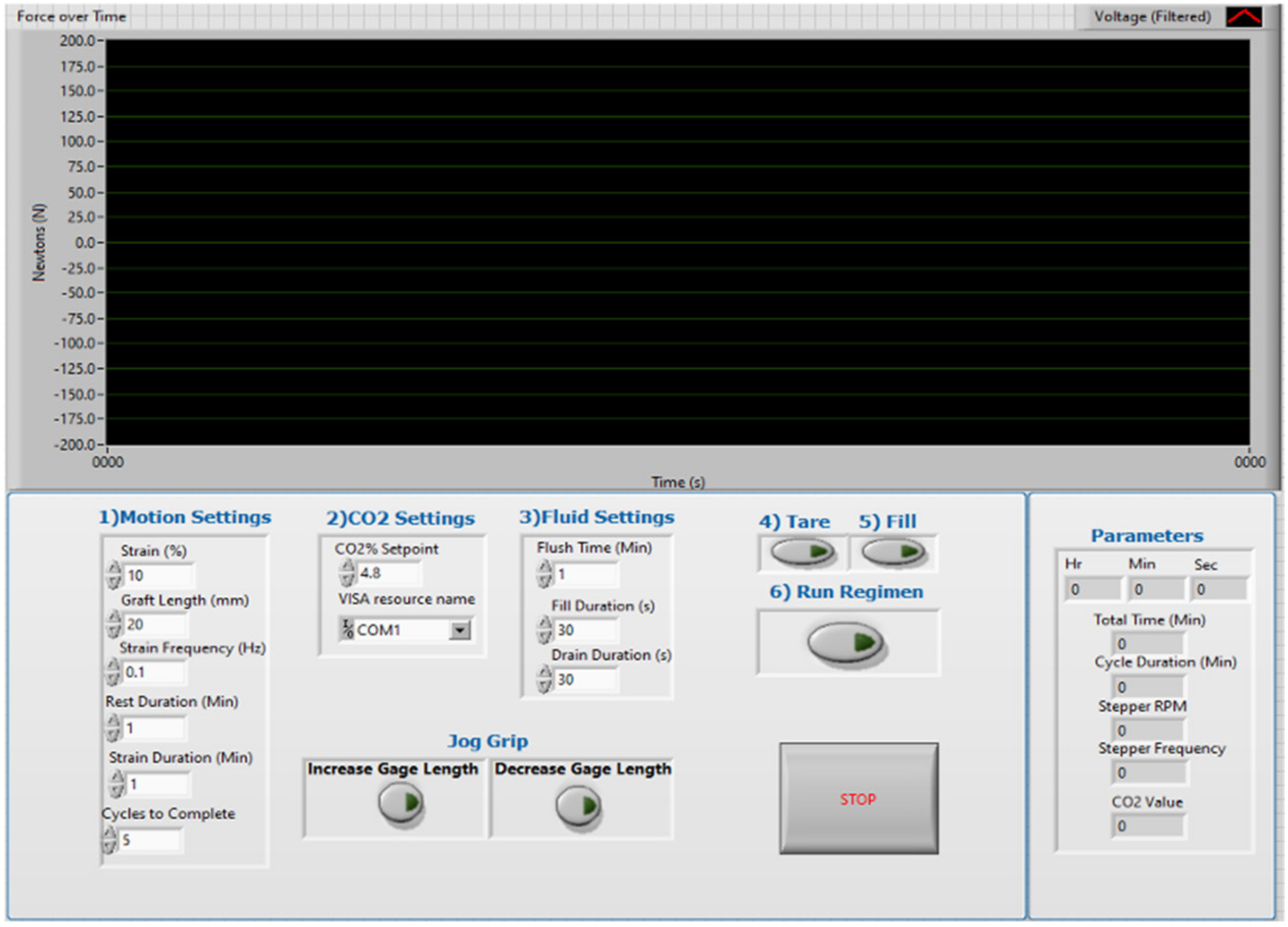
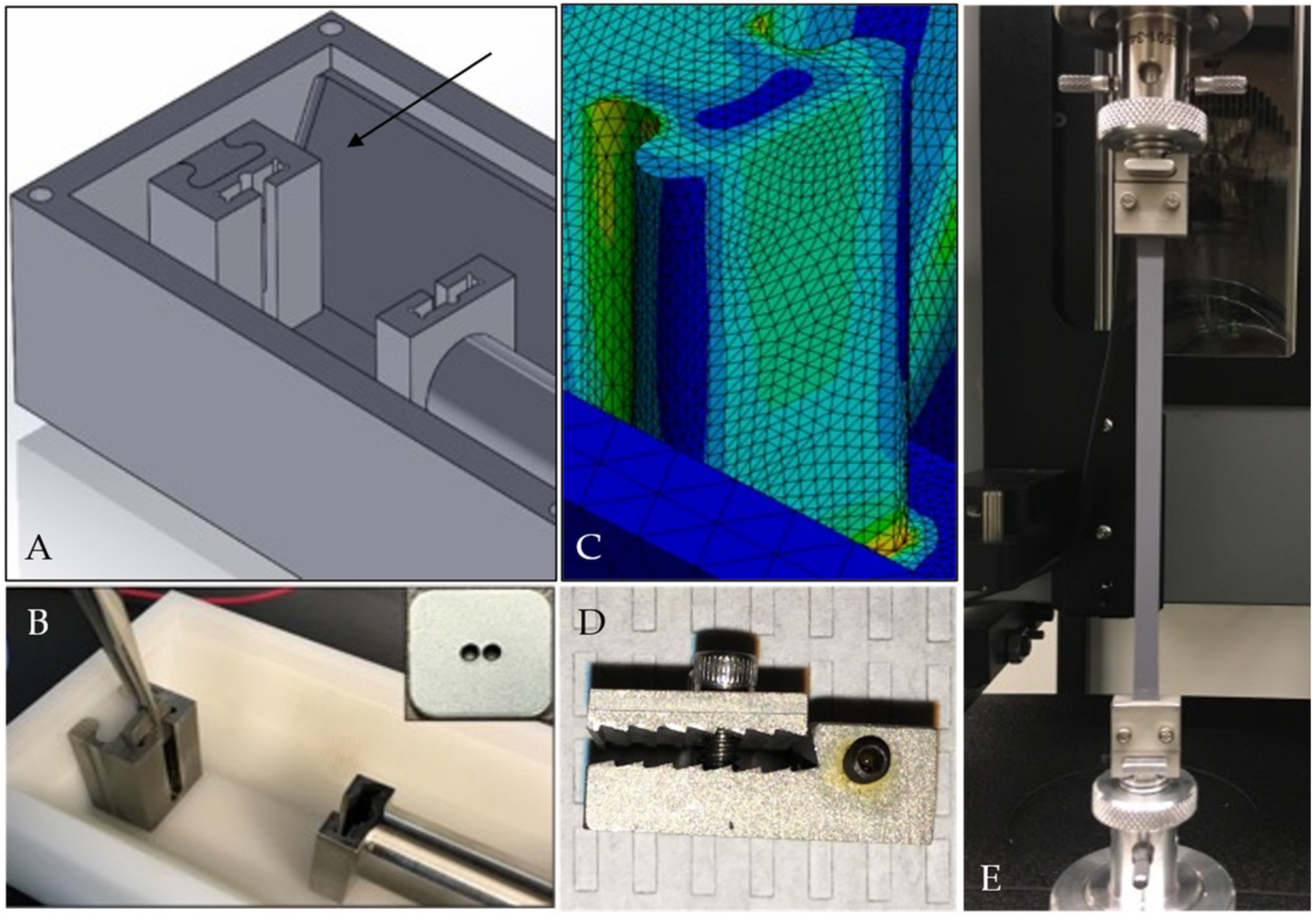
2.2. Bioreactor System Design
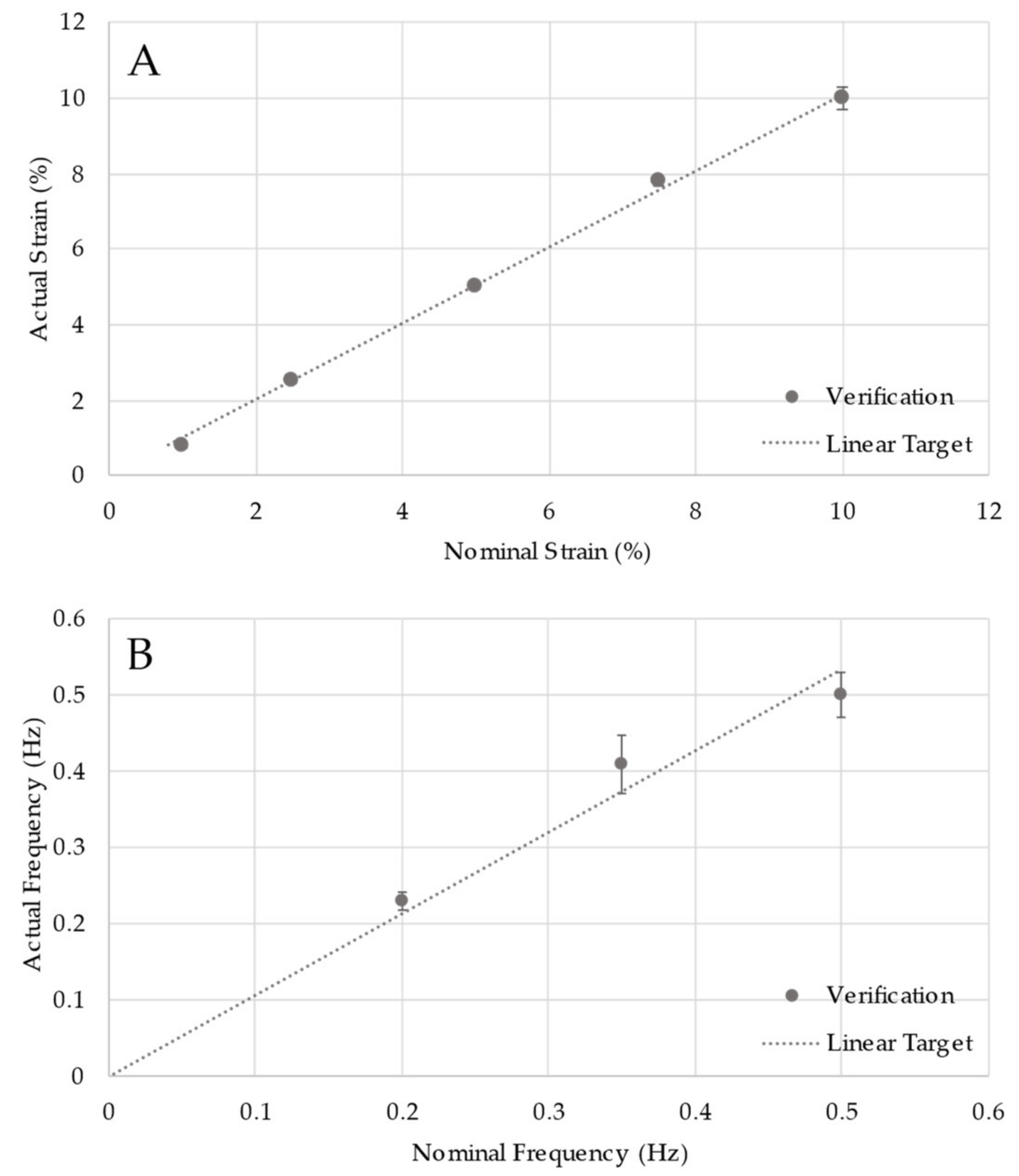
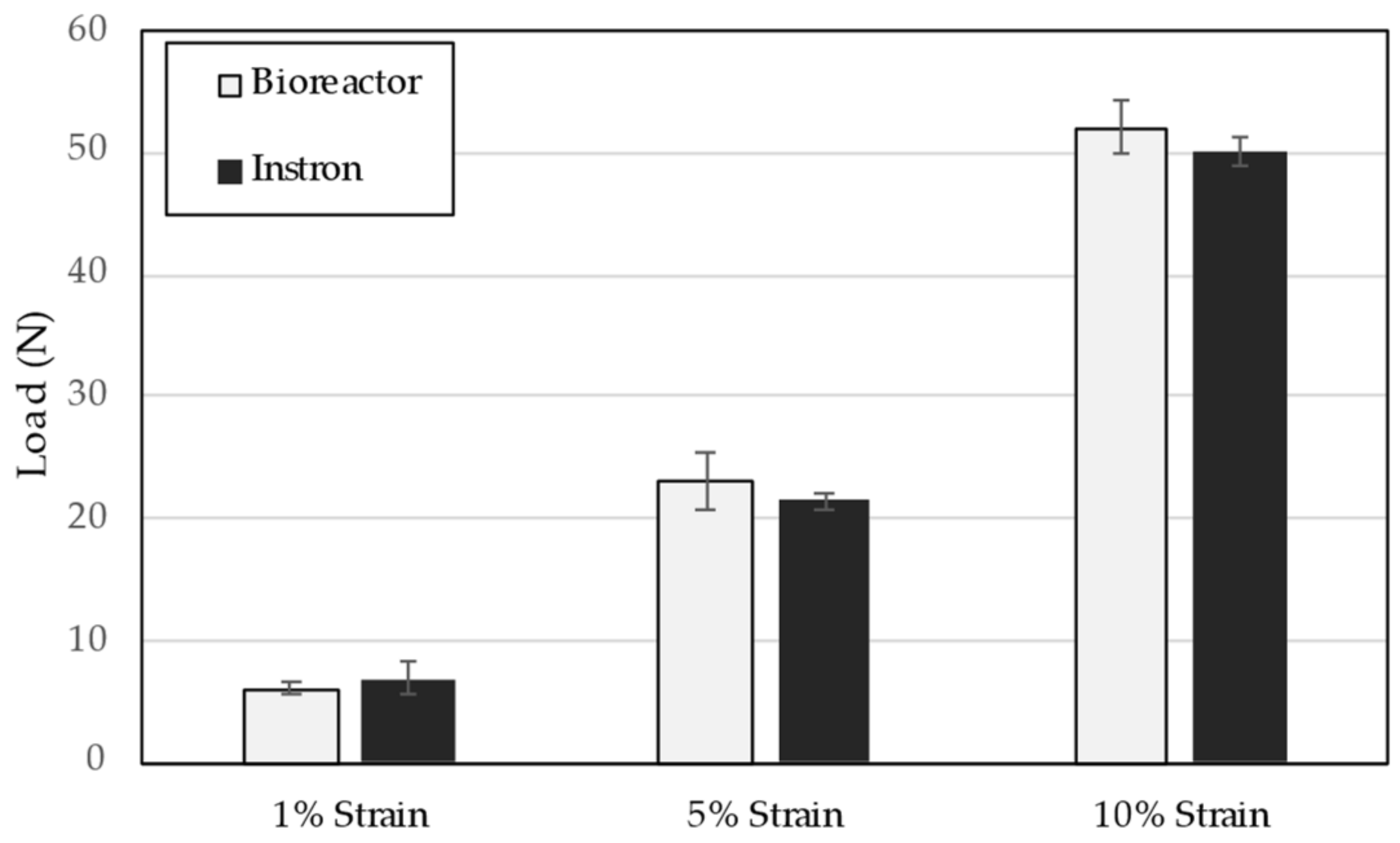
2.3. Design Verification Testing
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butler, D.L.; Juncosa, N.; Dressler, M.R. Functional efficacy of tendon repair processes. Annu. Rev. Biomed. Eng. 2004, 6, 303–329. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Alam, N.; McGrouther, A.D.; Wong, J.K. Tendon grafts: Their Natural History, Biology and Future Development. J. Hand Surg. Eur. Vol. 2015, 40, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.A.; Dolan, E.E. Ligament injury and healing: An Overview of Current Clinical Concepts. J. Prolother. 2011, 3, 836–846. [Google Scholar]
- Kannus, P.; Jarvinen, M. Conservatively treated tears of the anterior cruciate ligament. Long-term results. J. Bone Jt. Surg. Am. 1987, 69, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Engebretsen, L.; Benum, P.; Sundalsvoll, S. Primary suture of the anterior cruciate ligament. A 6-year follow-up of 74 cases. Acta Orthop. Scand. 1989, 60, 561–564. [Google Scholar] [CrossRef]
- van der List, J.P.; DiFelice, G.S. Primary repair of the anterior cruciate ligament: A Paradigm Shift. Surgeon 2017, 15, 161–168. [Google Scholar] [CrossRef]
- Krause, M.; Freudenthaler, F.; Frosch, K.H.; Achtnich, A.; Petersen, W.; Akoto, R. Operative Versus Conservative Treatment of Anterior Cruciate Ligament Rupture. Dtsch. Arztebl. Int. 2018, 115, 855–862. [Google Scholar] [CrossRef]
- Sporsheim, A.N.; Gifstad, T.; Lundemo, T.O.; Engebretsen, L.; Strand, T.; Molster, A.; Drogset, J.O. Autologous BPTB ACL Reconstruction Results in Lower Failure Rates Than ACL Repair with and without Synthetic Augmentation at 30 Years of Follow-up: A Prospective Randomized Study. J. Bone Jt. Surg. Am. 2019, 101, 2074–2081. [Google Scholar] [CrossRef]
- Frank, C.B.; Jackson, D.W. The science of reconstruction of the anterior cruciate ligament. J. Bone Jt. Surg. Am. 1997, 79, 1556–1576. [Google Scholar] [CrossRef]
- Spindler, K.P.; Wright, R.W. Clinical practice. Anterior cruciate ligament tear. N. Engl. J. Med. 2008, 359, 2135–2142. [Google Scholar] [CrossRef] [Green Version]
- Gianotti, S.M.; Marshall, S.W.; Hume, P.A.; Bunt, L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: A National Population-Based Study. J. Sci. Med. Sport 2009, 12, 622–627. [Google Scholar] [CrossRef]
- Baer, G.S.; Harner, C.D. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin. Sports Med. 2007, 26, 661–681. [Google Scholar] [CrossRef]
- Macaulay, A.A.; Perfetti, D.C.; Levine, W.N. Anterior cruciate ligament graft choices. Sports Health 2012, 4, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Shaerf, D.A.; Pastides, P.S.; Sarraf, K.M.; Willis-Owen, C.A. Anterior cruciate ligament reconstruction best practice: A Review of Graft Choice. World J. Orthop. 2014, 5, 23–29. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Unexplained deaths following knee surgery—Minnesota, November 2001. Morb. Mortal. Wkly. Rep. 2001, 50, 1035–1036. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Update: Unexplained deaths following knee surgery—Minnesota, 2001. Morb. Mortal. Wkly. Rep. 2001, 50, 1080. [Google Scholar]
- Leung, D.Y.; Glagov, S.; Mathews, M.B. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science 1976, 191, 475–477. [Google Scholar] [CrossRef]
- Wang, J.H.; Thampatty, B.P. An introductory review of cell mechanobiology. Biomech. Model. Mechanobiol. 2006, 5, 1–16. [Google Scholar] [CrossRef]
- Banes, A.J.; Gilbert, J.; Taylor, D.; Monbureau, O. A new vacuum-operated stress-providing instrument that applies static or variable duration cyclic tension or compression to cells in vitro. J. Cell Sci. 1985, 75, 35–42. [Google Scholar] [CrossRef]
- Juncosa-Melvin, N.; Matlin, K.S.; Holdcraft, R.W.; Nirmalanandhan, V.S.; Butler, D.L. Mechanical stimulation increases collagen type I and collagen type III gene expression of stem cell-collagen sponge constructs for patellar tendon repair. Tissue Eng. 2007, 13, 1219–1226. [Google Scholar] [CrossRef]
- Wang, T.; Gardiner, B.S.; Lin, Z.; Rubenson, J.; Kirk, T.B.; Wang, A.; Xu, J.; Smith, D.W.; Lloyd, D.G.; Zheng, M.H. Bioreactor design for tendon/ligament engineering. Tissue Eng. Part B Rev. 2013, 19, 133–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyment, N.A.; Barrett, J.G.; Awad, H.A.; Bautista, C.A.; Banes, A.J.; Butler, D.L. A brief history of tendon and ligament bioreactors: Impact and Future Prospects. J. Orthop. Res. 2020, 38, 2318–2330. [Google Scholar] [CrossRef] [PubMed]
- Garvin, J.; Qi, J.; Maloney, M.; Banes, A.J. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 2003, 9, 967–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.K.; Tuan, R.S. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng. Part A 2008, 14, 1615–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connor, J.; McQuillan, D.J.; Sandor, M.; Wan, H.; Lombardi, J.; Bachrach, N.; Harper, J.; Xu, H. Retention of structural and biochemical integrity in a biological mesh supports tissue remodeling in a primate abdominal wall model. Regen. Med. 2009, 4, 185–195. [Google Scholar] [CrossRef]
- Begum, T.; Farrelly, P.J.; Craigie, R.J. Non-cross-linked porcine acellular dermal matrix (Strattice Tissue Matrix) in pediatric reconstructive surgery. J. Pediatr. Surg. 2016, 51, 461–464. [Google Scholar] [CrossRef]
- Khalil, H.H.; Kalkat, M.; Malahias, M.N.; Rhobaye, S.; Ashour, T.; Djearaman, M.G.; Naidu, B. Chest Wall Reconstruction with Porcine Acellular Dermal Matrix (Strattice) and Autologous Tissue Transfer for High Risk Patients with Chest Wall Tumors. Plast. Reconstr. Surg. Glob. Open 2018, 6, e170. [Google Scholar] [CrossRef]
- Maxwell, D.W.; Hart, A.M.; Keifer, O.P., Jr.; Halani, S.H.; Losken, A. A Comparison of Acellular Dermal Matrices in Abdominal Wall Reconstruction. Ann. Plast. Surg. 2019, 82, 435–440. [Google Scholar] [CrossRef]
- Maxwell, G.P.; Gabriel, A. Non-cross-linked porcine acellular dermal matrix in revision breast surgery: Long-Term Outcomes and Safety with Neopectoral Pockets. Aesthet. Surg. J. 2014, 34, 551–559. [Google Scholar] [CrossRef]
- Sandor, M.; Xu, H.; Connor, J.; Lombardi, J.; Harper, J.R.; Silverman, R.P.; McQuillan, D.J. Host response to implanted porcine-derived biologic materials in a primate model of abdominal wall repair. Tissue Eng. Part A 2008, 14, 2021–2031. [Google Scholar] [CrossRef]
- Xu, H.; Wan, H.; Sandor, M.; Qi, S.; Ervin, F.; Harper, J.R.; Silverman, R.P.; McQuillan, D.J. Host response to human acellular dermal matrix transplantation in a primate model of abdominal wall repair. Tissue Eng. Part A 2008, 14, 2009–2019. [Google Scholar] [CrossRef]
- Wagner, C.T.; Bourke, S.L.; McQuillan, D.J. Differential regulation of acellular dermal matrix transition. Matrix Biol. 2006, 25, S11. [Google Scholar] [CrossRef]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 942–965. [Google Scholar] [CrossRef]
- Seifer, B.J.; Wagner, C.T. Strain gradient development in 3-dimensional extracellular matrix scaffolds during in vitro mechanical stimulation. Comput. Methods Biomech. Biomed. Eng. 2016, 1–10. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5439. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Design Control. In Code of Federal Regulations; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2021; pp. 165–166, 21 CFR §820.30. [Google Scholar]
- Delaine-Smith, R.M.; Reilly, G.C. The effects of mechanical loading on mesenchymal stem cell differentiation and matrix production. Vitam. Horm. 2011, 87, 417–480. [Google Scholar]
- Chandrashekar, N.; Mansouri, H.; Slauterbeck, J.; Hashemi, J. Sex-based differences in the tensile properties of the human anterior cruciate ligament. J. Biomech. 2006, 39, 2943–2950. [Google Scholar] [CrossRef]
- Functional Tissue Engineering Conference Group. Evaluation criteria for musculoskeletal and craniofacial tissue engineering constructs: A Conference Report. Tissue Eng. Part A 2008, 14, 2089–2104. [Google Scholar] [CrossRef] [Green Version]
- Miyasaka, K.C.; Daniel, D.M.; Stone, M.L. The incidence of knee ligament injuries in the general population. Am. J. Knee Surg. 1991, 4, 3–8. [Google Scholar]
- Brown, C.H., Jr.; Carson, E.W. Revision anterior cruciate ligament surgery. Clin. Sports Med. 1999, 18, 109–171. [Google Scholar] [CrossRef]
- Mall, N.A.; Chalmers, P.N.; Moric, M.; Tanaka, M.J.; Cole, B.J.; Bach, B.R., Jr.; Paletta, G.A., Jr. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am. J. Sports Med. 2014, 42, 2363–2370. [Google Scholar] [CrossRef]
- Toanen, C.; Demey, G.; Ntagiopoulos, P.G.; Ferrua, P.; Dejour, D. Is There Any Benefit in Anterior Cruciate Ligament Reconstruction in Patients Older Than 60 Years? Am. J. Sports Med. 2017, 45, 832–837. [Google Scholar] [CrossRef]
- Tepolt, F.A.; Feldman, L.; Kocher, M.S. Trends in pediatric ACL reconstruction from the PHIS database. J. Pediatr. Orthop. 2018, 38, e490–e494. [Google Scholar] [CrossRef]
- Longo, U.G.; Salvatore, G.; Ruzzini, L.; Risi Ambrogioni, L.; de Girolamo, L.; Vigano, M.; Facchini, F.; Cella, E.; Candela, V.; Ciccozzi, M.; et al. Trends of anterior cruciate ligament reconstruction in children and young adolescents in Italy show a constant increase in the last 15 years. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 1728–1733. [Google Scholar] [CrossRef]
- Schweitzer, R.; Chyung, J.H.; Murtaugh, L.C.; Brent, A.E.; Rosen, V.; Olson, E.N.; Lassar, A.; Tabin, C.J. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 2001, 128, 3855–3866. [Google Scholar] [CrossRef] [PubMed]
- Shukunami, C.; Takimoto, A.; Oro, M.; Hiraki, Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev. Biol. 2006, 298, 234–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usami, Y.; Gunawardena, A.T.; Iwamoto, M.; Enomoto-Iwamoto, M. Wnt signaling in cartilage development and diseases: Lessons from Animal Studies. Lab. Investig. 2016, 96, 186–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Hu, Y.; Akk, A.; Rai, M.F.; Pan, H.; Wickline, S.A.; Pham, C.T.N. Induction of WNT16 via Peptide-mRNA Nanoparticle-Based Delivery Maintains Cartilage Homeostasis. Pharmaceutics 2020, 12, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gori, F.; Lerner, U.; Ohlsson, C.; Baron, R. A new WNT on the bone: WNT16, Cortical Bone Thickness, Porosity and Fractures. Bonekey Rep. 2015, 4, 669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.F.; Tobias, J.H.; Duncan, E.; Evans, D.M.; Eriksson, J.; Paternoster, L.; Yerges-Armstrong, L.M.; Lehtimaki, T.; Bergstrom, U.; Kahonen, M.; et al. WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet. 2012, 8, e1002745. [Google Scholar] [CrossRef] [Green Version]
- Nalesso, G.; Thomas, B.L.; Sherwood, J.C.; Yu, J.; Addimanda, O.; Eldridge, S.E.; Thorup, A.S.; Dale, L.; Schett, G.; Zwerina, J.; et al. WNT16 antagonises excessive canonical WNT activation and protects cartilage in osteoarthritis. Ann. Rheum. Dis. 2017, 76, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Hsu, S.H.; Huang, G.S. Substrate-dependent Wnt signaling in MSC differentiation within biomaterial-derived 3D spheroids. Biomaterials 2013, 34, 4725–4738. [Google Scholar] [CrossRef]
- Ling, L.; Nurcombe, V.; Cool, S.M. Wnt signaling controls the fate of mesenchymal stem cells. Gene 2009, 433, 1–7. [Google Scholar] [CrossRef]
- Willert, K.; Nusse, R. Wnt proteins. Cold Spring Harb. Perspect. Biol. 2012, 4, a007864. [Google Scholar] [CrossRef]
- Lim, W.H.; Liu, B.; Cheng, D.; Williams, B.O.; Mah, S.J.; Helms, J.A. Wnt signaling regulates homeostasis of the periodontal ligament. J. Periodontal Res. 2014, 49, 751–759. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, Q.; Chen, J.; Jiang, J.; Mo, X.; He, C.; Zhao, J. Electrodeposition of calcium phosphate onto polyethylene terephthalate artificial ligament enhances graft-bone integration after anterior cruciate ligament reconstruction. Bioact. Mater. 2021, 6, 783–793. [Google Scholar] [CrossRef]
- Badylak, S.F. The extracellular matrix as a biologic scaffold material. Biomaterials 2007, 28, 3587–3593. [Google Scholar] [CrossRef]
- Wagner, C.T.; Owens, R.T.; Harper, J.R.; McQuillan, D.J. Human-derived acellular matrices for dermal replacement. In Biomaterials for Treating Skin Loss; Orgill, D.P., Blanco, C., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 142–173. [Google Scholar]
- Glasberg, S.B.; Light, D. AlloDerm and Strattice in breast reconstruction: A Comparison and Techniques for Optimizing Outcomes. Plast. Reconstr. Surg. 2012, 129, 1223–1233. [Google Scholar] [CrossRef]
- Shridharani, S.M.; Tufaro, A.P. A systematic review of acelluar dermal matrices in head and neck reconstruction. Plast. Reconstr. Surg. 2012, 130, 35S–43S. [Google Scholar] [CrossRef]
- Yurteri-Kaplan, L.A.; Gutman, R.E. The use of biological materials in urogynecologic reconstruction: A Systematic Review. Plast. Reconstr. Surg. 2012, 130, 242S–253S. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.W. Clinical outcomes of biologic mesh: Where Do We Stand? Surg. Clin. N. Am. 2013, 93, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Youngstrom, D.W.; Rajpar, I.; Kaplan, D.L.; Barrett, J.G. A bioreactor system for in vitro tendon differentiation and tendon tissue engineering. J. Orthop. Res. 2015, 33, 911–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, D.L.; Shearn, J.T.; Juncosa, N.; Dressler, M.R.; Hunter, S.A. Functional tissue engineering parameters toward designing repair and replacement strategies. Clin. Orthop. 2004, 427, S190–S199. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.L.; Juncosa-Melvin, N.; Boivin, G.P.; Galloway, M.T.; Shearn, J.T.; Gooch, C.; Awad, H. Functional tissue engineering for tendon repair: A Multidisciplinary Strategy Using Mesenchymal Stem Cells, Bioscaffolds, and Mechanical Stimulation. J. Orthop. Res. 2008, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Breidenbach, A.P.; Gilday, S.D.; Lalley, A.L.; Dyment, N.A.; Gooch, C.; Shearn, J.T.; Butler, D.L. Functional tissue engineering of tendon: Establishing Biological Success Criteria for Improving Tendon Repair. J. Biomech. 2014, 47, 1941–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoffel, M.; Willenberg, W.; Azarnoosh, M.; Fuhrmann-Nelles, N.; Zhou, B.; Markert, B. Towards bioreactor development with physiological motion control and its applications. Med. Eng. Phys. 2017, 39, 106–112. [Google Scholar] [CrossRef]
- Willenberg, W.; Azarnoosh, M.; Stoffel, M.; Markert, B. Experimental and numerical investigation of tendons and tendon cells. PAMM 2016, 16, 113–114. [Google Scholar] [CrossRef]
- Butler, D.L.; Hunter, S.A.; Chokalingam, K.; Cordray, M.J.; Shearn, J.; Juncosa-Melvin, N.; Nirmalanandhan, S.; Jain, A. Using functional tissue engineering and bioreactors to mechanically stimulate tissue-engineered constructs. Tissue Eng. Part A 2009, 15, 741–749. [Google Scholar] [CrossRef] [Green Version]

| Gene | Sense (5′ → 3′) Anti-Sense (5′ → 3′) | NCBI GenBank Accession Number | Product Size (bp) |
|---|---|---|---|
| GAPDH | CCACAGTCCATGCCATCACT TAGGAACACGGAAGGCCATG | NM_017008.4 | 183 |
| Scleraxis | ACAGAAAGACGGCGATTCGA GGCCTGGGTACAAGTGTTCA | NM_001130508 | 249 |
| Tenomodulin | TGCTGGATGAGAGAGGTTACTG TAGACTCTCCCAAGCATGCG | NM_022290 | 181 |
| Tenascin-C | ACGGTTTCTGTCTGTCCTGG TCGTACTCAGTGGCCTCTCT | NM_053861 | 160 |
| Wnt 16 | CAAGAGGAAGATGCGCAGGA ACGTACGGTTGCACTCTCTG | NM_001109223 | 152 |
| Input | Justification/Explanation |
|---|---|
| Device must apply physiological strain levels between 1–10% (accurate to 0.1%) both statically and cyclically at a frequency of 0.2–0.5 Hz (accurate to 0.01 Hz). | Physiological strain levels and those investigated for MSC differentiation cover the range specified [38]. Similarly, cycle frequencies used for cell culture systems on in the range indicated. The accuracy targets limit experimental variation. |
| Device must adjust to and maintain physiological temperature range of 25–42 °C (accurate within 1 °C of set point). | The specified range covers room temperature to heat shock conditions allowing for unique environmental conditions. The accuracy target limits experimental variation. |
Device must measure loads up to:
| Specified load primary and secondary targets required to tension grafts to maximum physiological strain levels based on small animal (primary) and human (secondary) ACL. Adapted from [39] for activities of daily living. Target load defines load cell specification. |
| Device must allow for graft placement with minimal user manipulation and without disrupting construct-grip connection. | This requirement ensures that grip-scaffold manipulations are performed in a controlled biological safety cabinet, minimizing potential contamination. It also supports modularity to allow transfer to a mechanical testing frame for subsequent testing without disrupting the grip-scaffold connection. |
| Device must be sized to accommodate scaffold dimensions up to 52 mm in length and 11 mm in diameter. | Specifications are based on insertion requirements for large animal reconstruction models [40]. |
| All tissue culture-contacting surfaces must be biocompatible and sterilizable. | This requirement is necessary for long-term cell culture. |
| Strain regimen and culture environmental condition setpoints must be user-specified inputs. | Specifications include environmental temperature and CO2 setpoints, strain cycle parameters, and cycle and culture duration parameters. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delakowski, A.J.; Posselt, J.D.; Wagner, C.T. Modular Bioreactor Design for Directed Tendon/Ligament Tissue Engineering. Bioengineering 2022, 9, 127. https://doi.org/10.3390/bioengineering9030127
Delakowski AJ, Posselt JD, Wagner CT. Modular Bioreactor Design for Directed Tendon/Ligament Tissue Engineering. Bioengineering. 2022; 9(3):127. https://doi.org/10.3390/bioengineering9030127
Chicago/Turabian StyleDelakowski, Axel J., Jared D. Posselt, and Christopher T. Wagner. 2022. "Modular Bioreactor Design for Directed Tendon/Ligament Tissue Engineering" Bioengineering 9, no. 3: 127. https://doi.org/10.3390/bioengineering9030127
APA StyleDelakowski, A. J., Posselt, J. D., & Wagner, C. T. (2022). Modular Bioreactor Design for Directed Tendon/Ligament Tissue Engineering. Bioengineering, 9(3), 127. https://doi.org/10.3390/bioengineering9030127