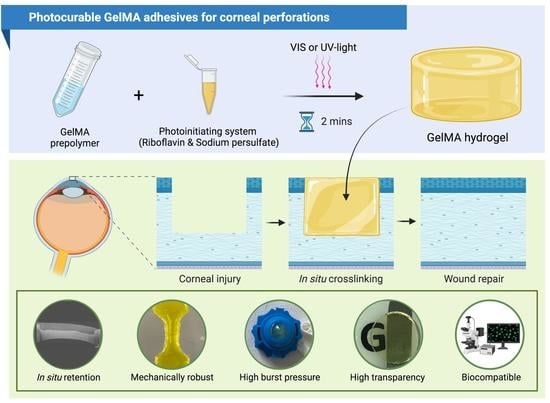
Photocurable GelMA Adhesives for Corneal Perforations
Abstract
1. Introduction
2. Materials and Methods
2.1. GelMA Synthesis
2.2. Degree of Functionalisation
2.3. Preparation of GelMA Bioadhesives
2.4. Adhesive Properties
2.4.1. Ex Vivo Burst Pressure
2.4.2. Lap Shear Strength
2.5. Transparency
2.6. Swelling Properties
2.7. Enzymatic Degradation
2.8. Micro Computed Tomography
2.9. Scanning Electron Microscopy
2.10. Rheological Characterisation
2.11. Mechanical Characterisation
2.11.1. Compressive Modulus
2.11.2. Tensile Modulus
2.12. Ex Vivo Retention Time
2.13. In Vitro Cytocompatibility of GelMA Hydrogels
2.13.1. 2D Cell Seeding on GelMA Gels
2.13.2. Cell Viability
2.13.3. Metabolic Activity
2.13.4. Cell Proliferation
2.14. Statistical Analysis
3. Results
3.1. Synthesis of GelMA Bioadhesives
3.2. Adhesive Properties of GelMA Bioadhesives
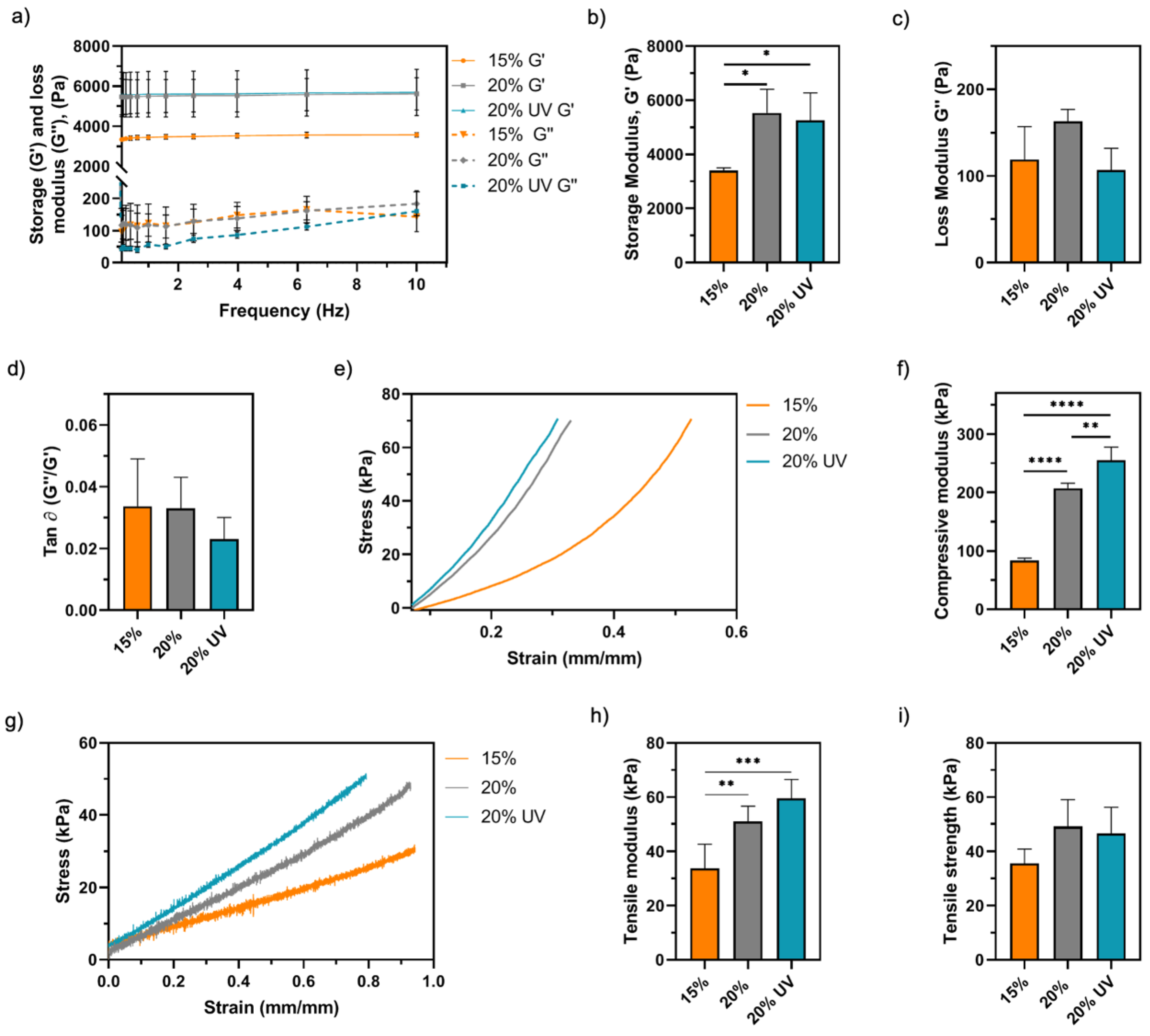
3.3. Physicochemical Characterisation of GelMA Bioadhesives
3.4. Ex Vivo Retention of GelMA Bioadhesives
3.5. In Vitro Assessment of Cytocompatibility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, M.M.; Buznyk, O.; Reddy, J.C.; Pasyechnikova, N.; Alarcon, E.I.; Hayes, S.; Lewis, P.; Fagerholm, P.; He, C.; Iakymenko, S.; et al. Biomaterials-enabled cornea regeneration in patients at high risk for rejection of donor tissue transplantation. Npj Regen. Med. 2018, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K. Biomaterials for Clinical Applications; Springer: New York, NY, USA, 2010; ISBN 9788578110796. [Google Scholar]
- Grinstaff, M.W. Designing hydrogel adhesives for corneal wound repair. Biomaterials 2007, 28, 5205–5214. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.S. Ocular surface sealants and adhesives. Ocul. Surf. 2006, 4, 146–154. [Google Scholar] [CrossRef]
- Tan, J.; Foster, L.J.R.; Watson, S.L. Corneal Sealants in Clinical Use: A Systematic Review. Curr. Eye Res. 2020, 45, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Arthur, S.D.; Chenault, H.K.; Figuly, G.D.; Kodokian, G.K. Polysaccharide-based tissue adhesives for sealing corneal incisions. Curr. Eye Res. 2007, 32, 1045–1050. [Google Scholar] [CrossRef]
- Hashemi, H.; Dadgostar, A. Automated lamellar therapeutic keratoplasty with fibrin adhesive in the treatment of anterior corneal opacities. Cornea 2011, 30, 655–659. [Google Scholar] [CrossRef]
- Kaufman, H.E.; Insler, M.S.; Ibrahim-Elzembely, H.A.; Kaufman, S.C. Human Fibrin Tissue Adhesive for Sutureless Lamellar Keratoplasty and Scleral Patch Adhesion: A Pilot Study. Ophthalmology 2003, 110, 2168–2172. [Google Scholar] [CrossRef]
- Annabi, N.; Yue, K.; Tamayol, A.; Khademhosseini, A. Elastic sealants for surgical applications. Eur. J. Pharm. Biopharm. 2015, 95, 27–39. [Google Scholar] [CrossRef]
- Petrie, E.M. Cyanoacrylate adhesives in surgical applications: A critical review. Rev. Adhes. Adhes. 2014, 2, 253–310. [Google Scholar] [CrossRef]
- Assmann, A.; Vegh, A.; Ghasemi-Rad, M.; Bagherifard, S.; Cheng, G.; Sani, E.S.; Ruiz-Esparza, G.U.; Noshadi, I.; Lassaletta, A.D.; Gangadharan, S.; et al. A highly adhesive and naturally derived sealant. Biomaterials 2017, 140, 115–127. [Google Scholar] [CrossRef]
- Shahbazi, J.; Marçal, H.; Watson, S.; Wakefield, D.; Sarris, M.; Foster, L.J.R. Sutureless sealing of penetrating corneal wounds using a laser-activated thin film adhesive. Lasers Surg. Med. 2011, 43, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, C.; Wang, L.; Chen, M.; White, J.; Hao, X.; McLean, K.M.; Chen, H.; Hughes, T.C. Gelatin-Based Photocurable Hydrogels for Corneal Wound Repair. ACS Appl. Mater. Interfaces 2018, 10, 13283–13292. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, M.; Yang, J. Design Strategies and Applications of Tissue Bioadhesives. Macromol. Biosci. 2013, 13, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Kumar, S.; Kumar, A.; Bansal, R.; Bhartiya, S. Fibrin glue in ophthalmology. Indian J. Ophthalmol. 2009, 57, 371. [Google Scholar] [CrossRef]
- Jhanji, V.; Young, A.L.; Mehta, J.S.; Sharma, N.; Agarwal, T.; Vajpayee, R.B. Management of Corneal Perforation. Surv. Ophthalmol. 2011, 56, 522–538. [Google Scholar] [CrossRef]
- Oelker, A.M.; Grinstaff, M.W. Ophthalmic adhesives: A materials chemistry perspective. J. Mater. Chem. 2008, 18, 2521–2536. [Google Scholar] [CrossRef]
- Trujillo-de Santiago, G.; Sharifi, R.; Yue, K.; Sani, E.S.; Kashaf, S.S.; Alvarez, M.M.; Leijten, J.; Khademhosseini, A.; Dana, R.; Annabi, N. Ocular adhesives: Design, chemistry, crosslinking mechanisms, and applications. Biomaterials 2019, 197, 345–367. [Google Scholar] [CrossRef]
- Cavanaugh, T.B.; Gottsch, J.D. Infectious keratitis and cyanoacrylate adhesive. Am. J. Ophthalmol. 1991, 111, 466–472. [Google Scholar] [CrossRef]
- Ferry, A.P.; Barnert, A.H. Granulomatous Keratitis Resulting From use of Cyanoacrylate Adhesive for Closure of Perforated Corneal Ulcer. Am. J. Ophthalmol. 1971, 72, 538–541. [Google Scholar] [CrossRef]
- Markowitz, G.D.; Orlin, S.E.; Frayer, W.C.; Andrews, A.P.; Prince, R.B. Corneal Endothelial Polymerization of Histoacryl Adhesive: A Report of a New Intraocular Complication. Ophthalmic Surg. Lasers Imaging Retin. 1995, 26, 256–258. [Google Scholar] [CrossRef]
- Siegal, J.E.; Zaidman, G.W. Surgical removal of cyanoacrylate adhesive after accidental instillation in the anterior chamber. Ophthalmic Surg. 1989, 20, 179–181. [Google Scholar] [CrossRef]
- Samarawickrama, C.; Samanta, A.; Liszka, A.; Fagerholm, P.; Buznyk, O.; Griffith, M.; Allan, B. Collagen-Based Fillers as Alternatives to Cyanoacrylate Glue for the Sealing of Large Corneal Perforations. Cornea 2018, 37, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Bouten, P.J.M.; Zonjee, M.; Bender, J.; Yauw, S.T.K.; Van Goor, H.; Van Hest, J.C.M.; Hoogenboom, R. The chemistry of tissue adhesive materials. Prog. Polym. Sci. 2014, 39, 1375–1405. [Google Scholar] [CrossRef]
- Natour, E.; Suedkamp, M.; Dapunt, O.E. Assessment of the effect on blood loss and transfusion requirements when adding a polyethylene glycol sealant to the anastomotic closure of aortic procedures: A case-control analysis of 102 patients undergoing Bentall procedures. J. Cardiothorac. Surg. 2012, 7, 1. [Google Scholar] [CrossRef]
- Covidien. DuraSeal Package Insert; Covidien: Mansfield, MA, USA, 2005. [Google Scholar]
- CoSeal Package Insert; Baxter: Hong Kong, China, 2006.
- Liang, Y.; Li, Z.; Huang, Y.; Yu, R.; Guo, B. Dual-Dynamic-Bond Cross-Linked Antibacterial Adhesive Hydrogel Sealants with On-Demand Removability for Post-Wound-Closure and Infected Wound Healing. ACS Nano 2021, 15, 7078–7093. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Sarrion, P.; Pouraghaei, S.; Chau, Y.; Ansari, S.; Li, S.; Aghaloo, T.; Moshaverinia, A. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Zhou, L.; Dai, C.; Fan, L.; Jiang, Y.; Liu, C.; Zhou, Z.; Guan, P.; Tian, Y.; Xing, J.; Li, X.; et al. Injectable Self-Healing Natural Biopolymer-Based Hydrogel Adhesive with Thermoresponsive Reversible Adhesion for Minimally Invasive Surgery. Adv. Funct. Mater. 2021, 31, 2007457. [Google Scholar] [CrossRef]
- Han, W.; Zhou, B.; Yang, K.; Xiong, X.; Luan, S.; Wang, Y.; Xu, Z.; Lei, P.; Luo, Z.; Gao, J.; et al. Biofilm-inspired adhesive and antibacterial hydrogel with tough tissue integration performance for sealing hemostasis and wound healing. Bioact. Mater. 2020, 5, 768–778. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Payanam, U.; Laurent, A.; Wassef, M.; Jayakrishnan, A. Efficacy evaluation of an in situ forming tissue adhesive hydrogel as sealant for lung and vascular injury. Biomed. Mater. 2021, 16, 044106. [Google Scholar] [CrossRef]
- Sani, E.S.; Lassaletta, A.D.; Wang, X.; Assmann, A.; Zhang, Y.-N.; Weiss, A.S.; Annabi, N.; Cheng, G.; Vegh, A.; Khademhosseini, A.; et al. Engineering a highly elastic human protein–based sealant for surgical applications. Sci. Transl. Med. 2017, 9, eaai7466. [Google Scholar]
- Elvin, C.M.; Vuocolo, T.; Brownlee, A.G.; Sando, L.; Huson, M.G.; Liyou, N.E.; Stockwell, P.R.; Lyons, R.E.; Kim, M.; Edwards, G.A.; et al. A highly elastic tissue sealant based on photopolymerised gelatin. Biomaterials 2010, 31, 8323–8331. [Google Scholar] [CrossRef]
- Kilic Bektas, C.; Burcu, A.; Gedikoglu, G.; Telek, H.H.; Ornek, F.; Hasirci, V. Methacrylated gelatin hydrogels as corneal stroma substitutes: In vivo study. J. Biomater. Sci. Polym. Ed. 2019, 30, 1803–1821. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, F.; Hua, Y.; Zhang, X.; Ni, C.; Pan, D.; Jiang, D.; Yang, L.; Lin, Q.; Zou, Y.; et al. A strongly adhesive haemostatic hydrogel for the repair of arterial and heart bleeds. Nat. Commun. 2019, 10, 2060. [Google Scholar] [CrossRef]
- Sharifi, S.; Islam, M.M.; Sharifi, H.; Islam, R.; Koza, D.; Reyes-Ortega, F.; Alba-Molina, D.; Nilsson, P.H.; Dohlman, C.H.; Mollnes, T.E.; et al. Tuning gelatin-based hydrogel towards bioadhesive ocular tissue engineering applications. Bioact. Mater. 2021, 6, 3947–3961. [Google Scholar] [CrossRef]
- Anthis, A.H.C.; Hu, X.; Matter, M.T.; Neuer, A.L.; Wei, K.; Schlegel, A.A.; Starsich, F.H.L.; Herrmann, I.K. Chemically Stable, Strongly Adhesive Sealant Patch for Intestinal Anastomotic Leakage Prevention. Adv. Funct. Mater. 2021, 31, 2007099. [Google Scholar] [CrossRef]
- Bayat, N.; Zhang, Y.; Falabella, P.; Menefee, R.; Whalen, J.J.; Humayun, M.S.; Thompson, M.E. A reversible thermoresponsive sealant for temporary closure of ocular trauma. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Fernandes-Cunha, G.M.; Chen, K.M.; Chen, F.; Le, P.; Han, J.H.; Mahajan, L.A.; Lee, H.J.; Na, K.S.; Myung, D. In situ-forming collagen hydrogel crosslinked via multi-functional PEG as a matrix therapy for corneal defects. Sci. Rep. 2020, 10, 16671. [Google Scholar] [CrossRef]
- Xiang, L.; Cui, W. Biomedical application of photo-crosslinked gelatin hydrogels. J. Leather Sci. Eng. 2021, 3, 3. [Google Scholar] [CrossRef]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef]
- Hutson, C.B.; Nichol, J.W.; Aubin, H.; Bae, H.; Yamanlar, S.; Al-Haque, S.; Koshy, S.T.; Khademhosseini, A. Synthesis and Characterization of Tunable Poly(Ethylene Glycol): Gelatin Methacrylate Composite Hydrogels. Tissue Eng. Part A 2011, 17, 1713–1723. [Google Scholar] [CrossRef]
- Goto, R.; Nishida, E.; Kobayashi, S.; Aino, M.; Ohno, T.; Iwamura, Y. Gelatin Methacryloyl—Riboflavin (GelMA—RF) Hydrogels for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 1635. [Google Scholar] [CrossRef] [PubMed]
- Sani, E.S.; Kheirkhah, A.; Rana, D.; Sun, Z.; Foulsham, W.; Sheikhi, A.; Khademhosseini, A.; Dana, R.; Annabi, N. Sutureless repair of corneal injuries using naturally derived bioadhesive hydrogels. Sci. Adv. 2019, 5, eaav1281. [Google Scholar] [CrossRef] [PubMed]
- Tavafoghi, M.; Sheikhi, A.; Tutar, R.; Jahangiry, J.; Baidya, A.; Haghniaz, R.; Khademhosseini, A. Engineering Tough, Injectable, Naturally Derived, Bioadhesive Composite Hydrogels. Adv. Healthc. Mater. 2020, 9, 1901722. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Rana, D.; Shirzaei Sani, E.; Portillo-Lara, R.; Gifford, J.L.; Fares, M.M.; Mithieux, S.M.; Weiss, A.S. Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials 2017, 139, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.A.; Saleh, B.; Ibrahim, D.M.; Jumelle, C.; Yung, A.; Dana, R.; Annabi, N. Ciprofloxacin-loaded Bioadhesive Hydrogels for Ocular Applications. Biomater. Sci. 2020, 8, 5196–5209. [Google Scholar] [CrossRef] [PubMed]
- Randleman, J.B.; Khandelwal, S.S.; Hafezi, F. Corneal cross-linking. Surv. Ophthalmol. 2015, 60, 509–523. [Google Scholar] [CrossRef]
- Jeng, B.H. Advances in Medical and Surgical Cornea; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9783662448878. [Google Scholar]
- Lee, H.J.; Fernandes-Cunha, G.M.; Myung, D. In situ-forming hyaluronic acid hydrogel through visible light-induced thiol-ene reaction. React. Funct. Polym. 2018, 131, 29–35. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, Z.; Menard, F.; Kim, K. Comparative study of gelatin methacrylate hydrogels from different sources for biofabrication applications. Biofabrication 2017, 9, 044101. [Google Scholar] [CrossRef]
- Cui, X.; Soliman, B.G.; Alcala-Orozco, C.R.; Li, J.; Vis, M.A.M.; Santos, M.; Wise, S.G.; Levato, R.; Malda, J.; Woodfield, T.B.F.; et al. Rapid Photocrosslinking of Silk Hydrogels with High Cell Density and Enhanced Shape Fidelity. Adv. Healthc. Mater. 2020, 9, 1901667. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Fernández-Pérez, J.; Ahearne, M. Potential for combined delivery of riboflavin and all-trans retinoic acid, from silk fibroin for corneal bioengineering. Mater. Sci. Eng. C 2019, 105, 110093. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.J.; Lee, B.H. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Lum, N.; Seow, L.Y.; Lim, P.Q.; Tan, L.P. Synthesis and characterization of types A and B gelatin methacryloyl for bioink applications. Materials 2016, 9, 797. [Google Scholar] [CrossRef] [PubMed]
- Pahoff, S.; Meinert, C.; Bas, O.; Nguyen, L.; Klein, T.J.; Hutmacher, D.W. Effect of gelatin source and photoinitiator type on chondrocyte redifferentiation in gelatin methacryloyl-based tissue-engineered cartilage constructs. J. Mater. Chem. B 2019, 7, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Barroso, I.A.; Man, K.; Villapun, V.M.; Cox, S.S.; Ghag, A.K. Methacrylated Silk Fibroin Hydrogels: pH as a Tool to Control Functionality. ACS Biomater. Sci. Eng. 2021, 7, 4779–4791. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Good, G.W. Light and Eye Damage. Am. Optom. Assoc. 2014, 1–13. [Google Scholar]
- Piluso, S.; Flores Gomez, D.; Dokter, I.; Moreira Texeira, L.; Li, Y.; Leijten, J.; Van Weeren, R.; Vermonden, T.; Karperien, M.; Malda, J. Rapid and cytocompatible cell-laden silk hydrogel formation: Via riboflavin-mediated crosslinking. J. Mater. Chem. B 2020, 8, 9566–9575. [Google Scholar] [CrossRef]
- Fancy, D.A.; Kodadek, T. Chemistry for the analysis of protein-protein interactions: Rapid and efficient cross-linking triggered by long wavelength light. Proc. Natl. Acad. Sci. USA 1999, 96, 6020–6024. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xu, L.; Wei, W.B.; Jonas, J.B. Intraocular pressure and its normal range adjusted for ocular and systemic parameters. The Beijing eye study 2011. PLoS ONE 2018, 13, e0196926. [Google Scholar] [CrossRef]
- Rafat, M.; Li, F.; Fagerholm, P.; Lagali, N.S.; Watsky, M.A.; Munger, R.; Matsuura, T.; Griffith, M. PEG-stabilized carbodiimide crosslinked collagen-chitosan hydrogels for corneal tissue engineering. Biomaterials 2008, 29, 3960–3972. [Google Scholar] [CrossRef]
- Freegard, T.J. The physical basis of transparency of the normal cornea. Eye 1997, 11 Pt 4, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Gorth, D.; Webster, T.J. Matrices for tissue engineering and regenerative medicine. In Biomaterials for Artificial Organs; Woodhead Publishing: Sawston, UK, 2010; pp. 270–286. [Google Scholar] [CrossRef]
- Hatami-Marbini, H. Viscoelastic shear properties of the corneal stroma. J. Biomech. 2014, 47, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.I.; et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 2018, 9, 1620. [Google Scholar] [CrossRef] [PubMed]
- BD. BD Ocuseal Liquid Bandage Leaflet; BD: Le Pont de Claix, France, 2009. [Google Scholar]
- Ocular Therapeutix, Inc. ReSure Sealant Instructions for Use. Ocular Therapeutix: Bedford, MA, USA, 2014. [Google Scholar]
- Dell, S.J.; Hovanesian, J.A.; Raizman, M.B.; Crandall, A.S.; Doane, J.; Snyder, M.; Masket, S.; Lane, S.; Fram, N. Randomized comparison of postoperative use of hydrogel ocular bandage and collagen corneal shield for wound protection and patient tolerability after cataract surgery. J. Cataract Refract. Surg. 2011, 37, 113–121. [Google Scholar] [CrossRef]
- FDA. ReSure Summary of Safety and Effectiveness Data; FDA: Bedford, MA, USA, 2014.
- Potvin, R.; Makari, S. Cataract surgery and methods of wound closure: A review. Clin. Ophthalmol. 2015, 9, 921–928. [Google Scholar] [CrossRef]
- Mah, F.S. Effect on gel formation time of adding topical ophthalmic medications to resure sealant, an in situ hydrogel. J. Ocul. Pharmacol. Ther. 2016, 32, 396–399. [Google Scholar] [CrossRef]
- Uy, H.S.; Kenyon, K.R. Surgical outcomes after application of a liquid adhesive ocular bandage to clear corneal incisions during cataract surgery. J. Cataract Refract. Surg. 2013, 39, 1668–1674. [Google Scholar] [CrossRef]
- Lerit, S.J.T.; Abano, J.M.R. Comparison of Tensile Strength of Fibrin Glue, 2-Octyl Cyanoacrylate, Liquid Ocular Bandage, and Conventional Nylon 10-0 Sutures in Corneal Laceration Repair in an Animal Model. Philipp. Acad. Opthamlmol. 2012, 37, 52–58. [Google Scholar]
- Liang, Y.; Liu, W.; Han, B.; Yang, C.; Ma, Q.; Zhao, W.; Rong, M.; Li, H. Fabrication and characters of a corneal endothelial cells scaffold based on chitosan. J. Mater. Sci. Mater. Med. 2011, 22, 175–183. [Google Scholar] [CrossRef]
- Taylor, Z.D.; Garritano, J.; Sung, S.; Bajwa, N.; Bennett, D.B.; Nowroozi, B.; Tewari, P.; Sayre, J.W.; Hubschman, J.-P.; Deng, S.X.; et al. THz and mm-Wave Sensing of Corneal Tissue Water Content: Electromagnetic Modeling and Analysis. Physiol. Behav. 2016, 176, 139–148. [Google Scholar] [CrossRef]
- Pircher, M.; Götzinger, E.; Leitgeb, R.; Fercher, A.F.; Hitzenberger, C.K. Measurement and imaging of water concentration in human cornea with differential absorption optical coherence tomography. Opt. Express 2010, 11, 2190. [Google Scholar] [CrossRef] [PubMed]
- Brightbill, F.S.; McDonnell, P.J.; McGhee, C.N.; Farjo, A.A.; Serdarevic, O. Corneal Surgery: Theory Technique and Tissue; Elsevier Health Sciences: Amsterdam, The Netherlands, 2009; ISBN 9780323048835. [Google Scholar]
- Oie, Y.; Nishida, K. Corneal regenerative medicine. Regen. Ther. 2016, 5, 40–45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, Y.X. Corneal tissue engineering. Biointegr. Med. Implant Mater. Sci. Des. 2010, 86–115. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barroso, I.A.; Man, K.; Robinson, T.E.; Cox, S.C.; Ghag, A.K. Photocurable GelMA Adhesives for Corneal Perforations. Bioengineering 2022, 9, 53. https://doi.org/10.3390/bioengineering9020053
Barroso IA, Man K, Robinson TE, Cox SC, Ghag AK. Photocurable GelMA Adhesives for Corneal Perforations. Bioengineering. 2022; 9(2):53. https://doi.org/10.3390/bioengineering9020053
Chicago/Turabian StyleBarroso, Inês A., Kenny Man, Thomas E. Robinson, Sophie C. Cox, and Anita K. Ghag. 2022. "Photocurable GelMA Adhesives for Corneal Perforations" Bioengineering 9, no. 2: 53. https://doi.org/10.3390/bioengineering9020053
APA StyleBarroso, I. A., Man, K., Robinson, T. E., Cox, S. C., & Ghag, A. K. (2022). Photocurable GelMA Adhesives for Corneal Perforations. Bioengineering, 9(2), 53. https://doi.org/10.3390/bioengineering9020053