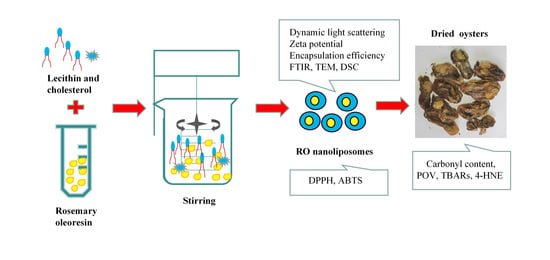
Physicochemical and Antioxidant Properties of Nanoliposomes Loaded with Rosemary Oleoresin and Their Oxidative Stability Application in Dried Oysters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of RO Nanoliposomes and Encapsulation Efficiency
2.3. DLS Measurement
2.4. FTIR Measurement
2.5. Transmission Electron Microscopy (TEM) Measurement
2.6. Differential Scanning Calorimetry (DSC) Analysis
2.7. Temperatures and pH Stability
2.8. Antioxidant Activity of RO
2.9. Samples Preparation of the Dried Oysters
2.10. Determination of Oxidative Stability of Dried Oysters
2.11. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Nanoliposomes and Encapsulation Efficiency
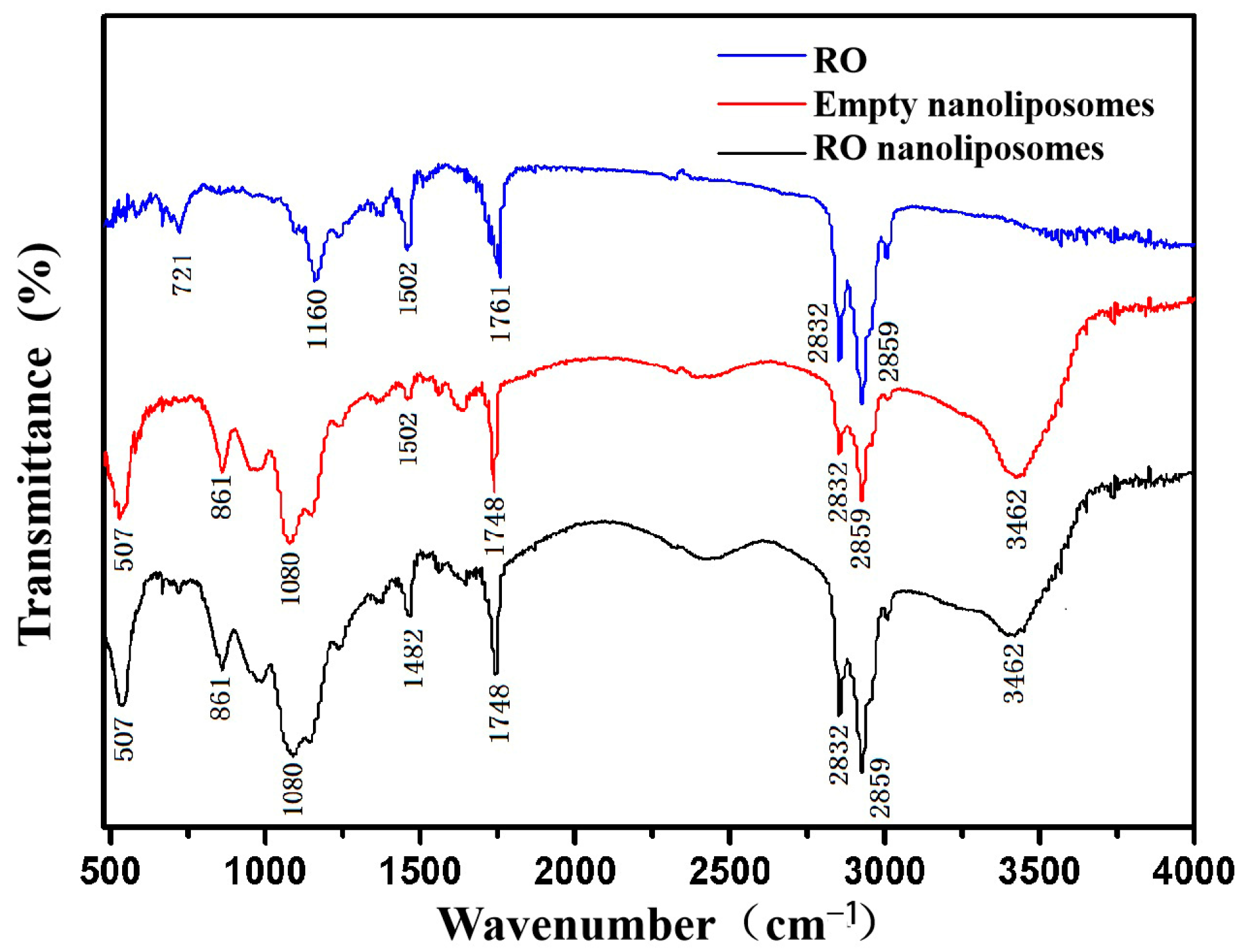
3.2. FTIR Spectroscopy Analysis of Nanoliposomes
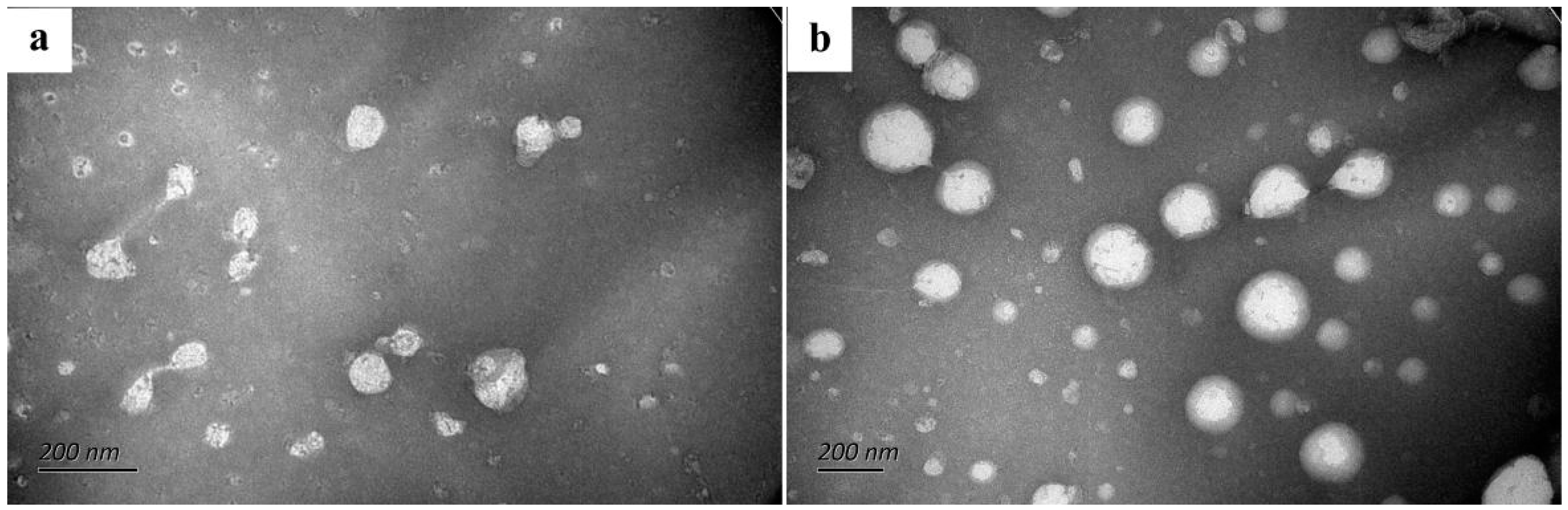
3.3. TEM of Nanoliposomes
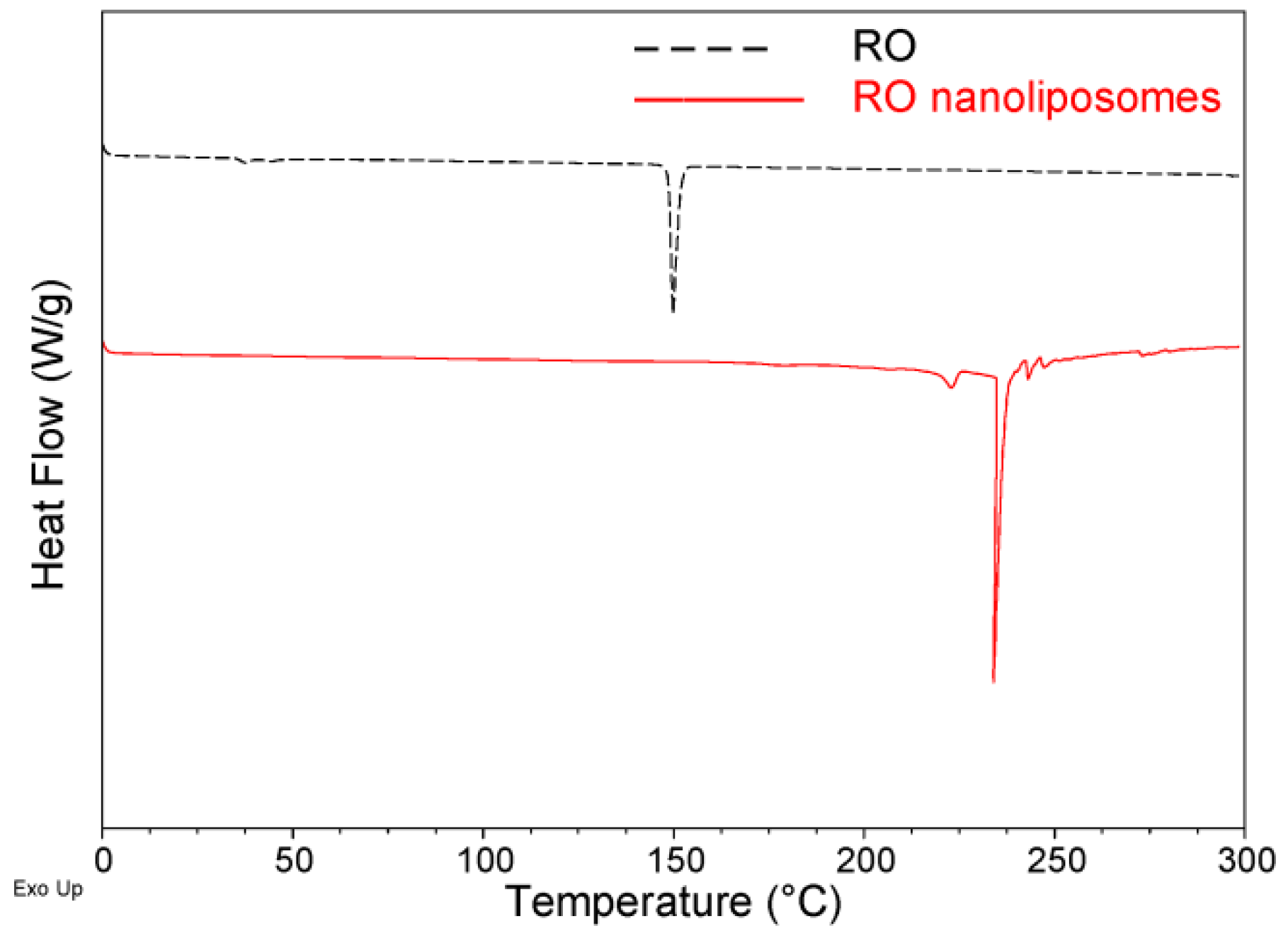
3.4. DSC Analysis of Nanoliposomes
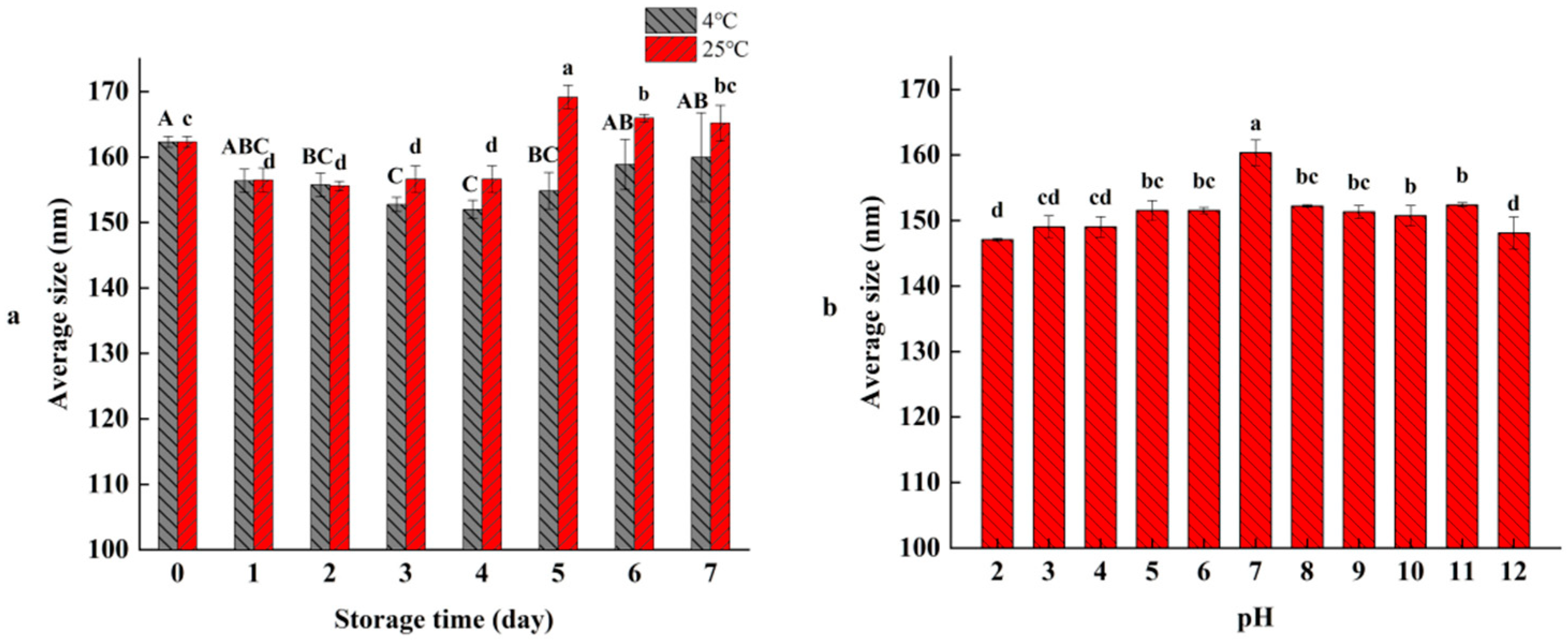
3.5. Temperatures and pH Stability of RO Nanoliposomes
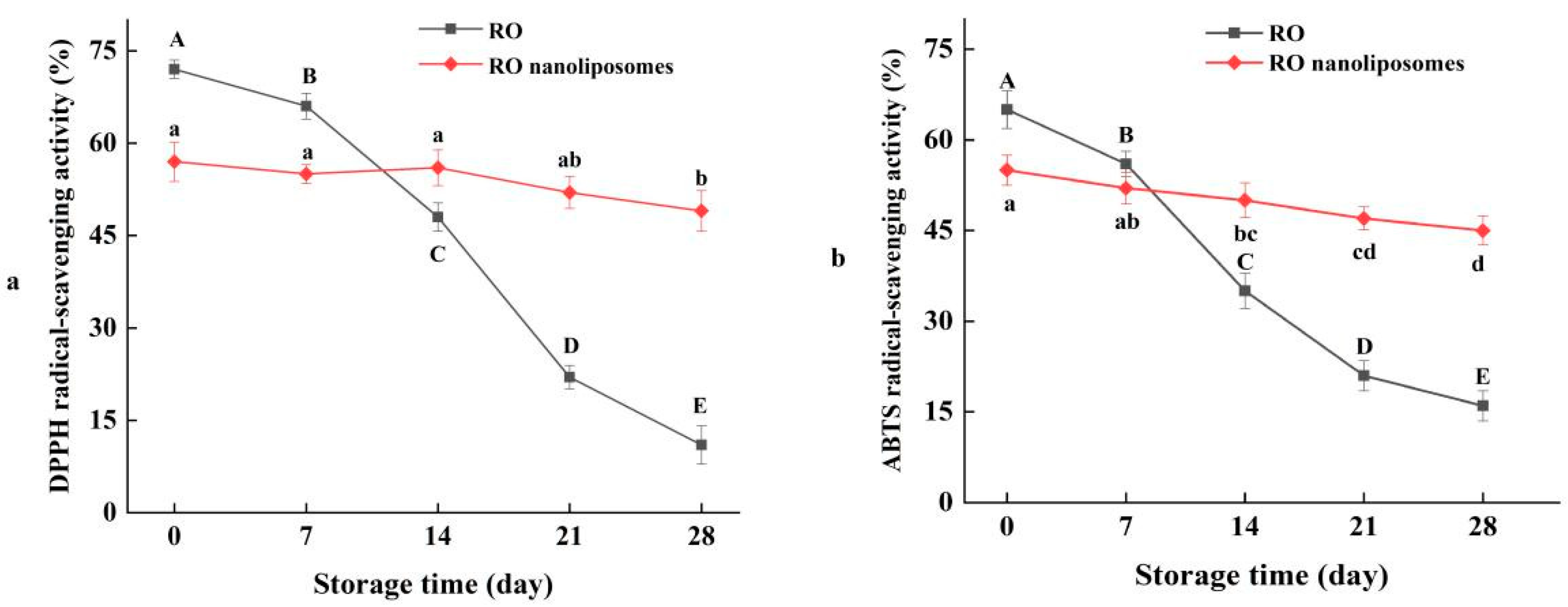
3.6. Radical-Scavenging Activity of RO Nanoliposomes during Storage
3.7. Effect of RO Nanoliposomes on Oxidative Stability of Dried Oysters during Storage
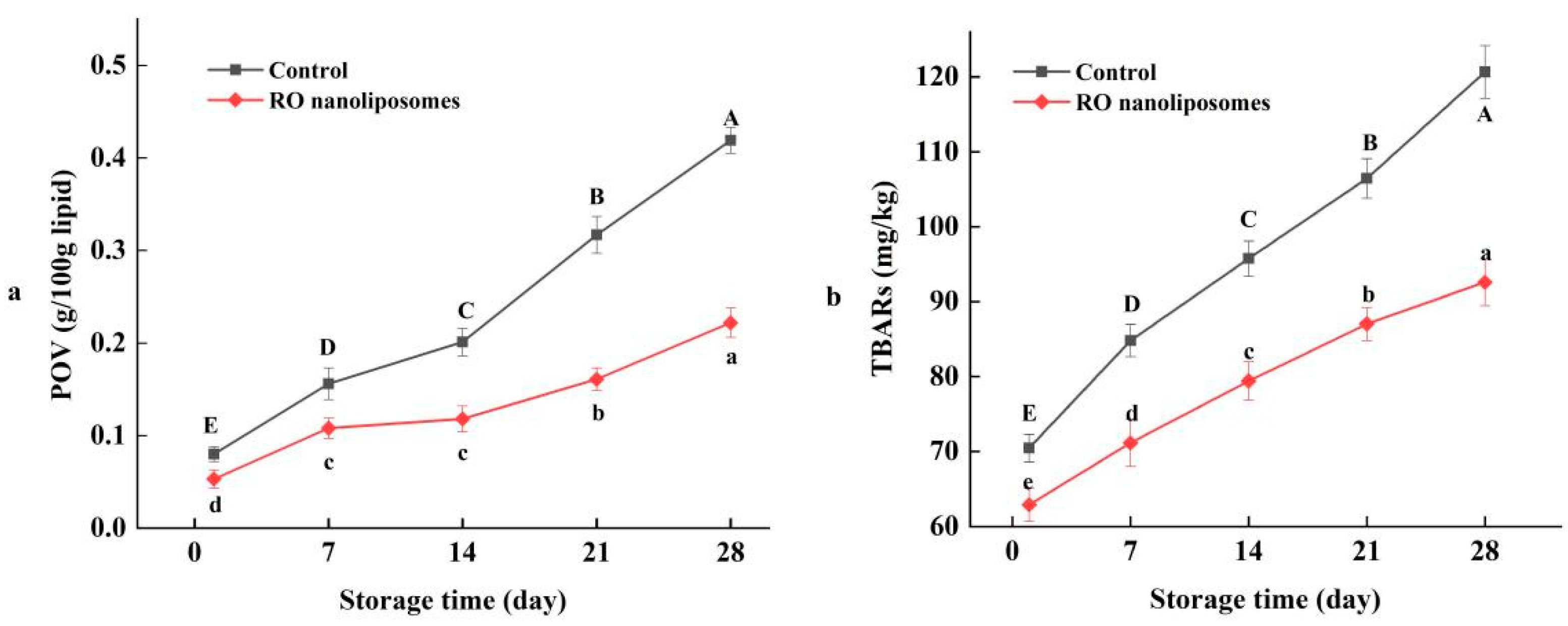
3.7.1. Lipid Oxidation of Dried Oysters
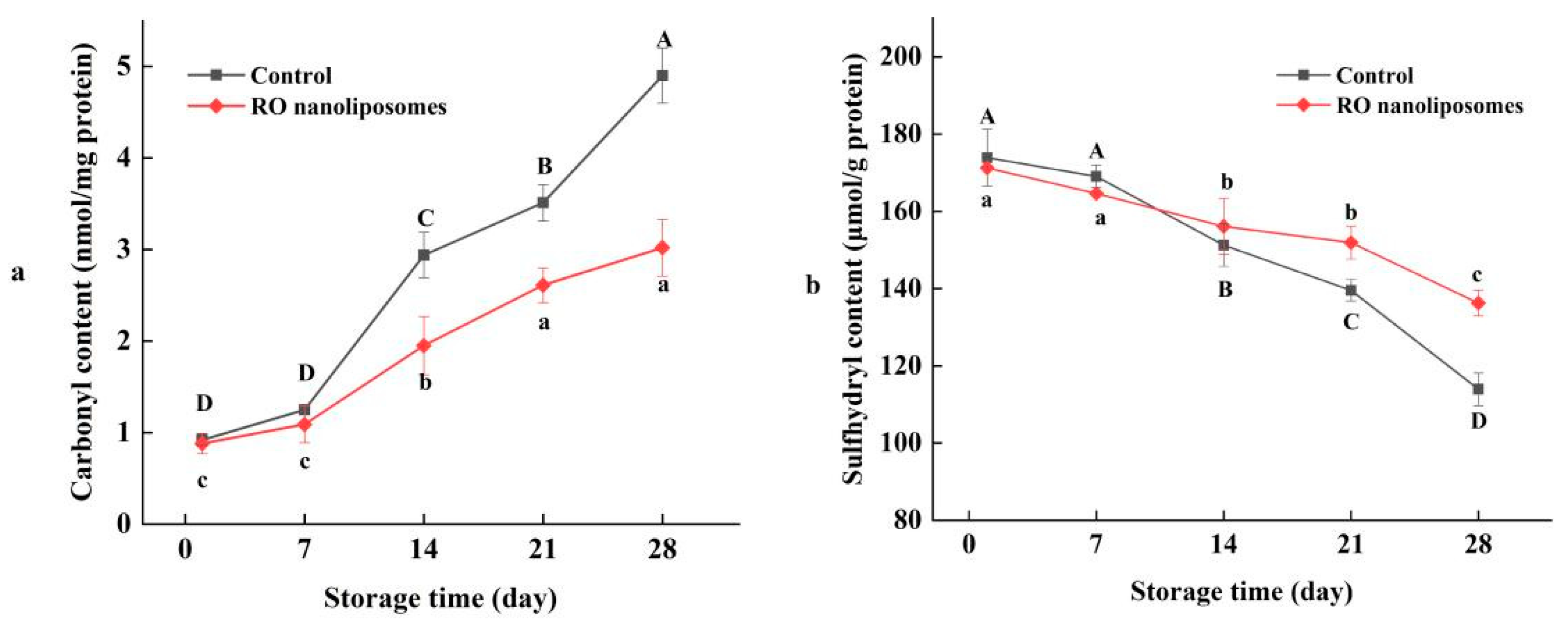
3.7.2. Protein Oxidation of Dried Oysters
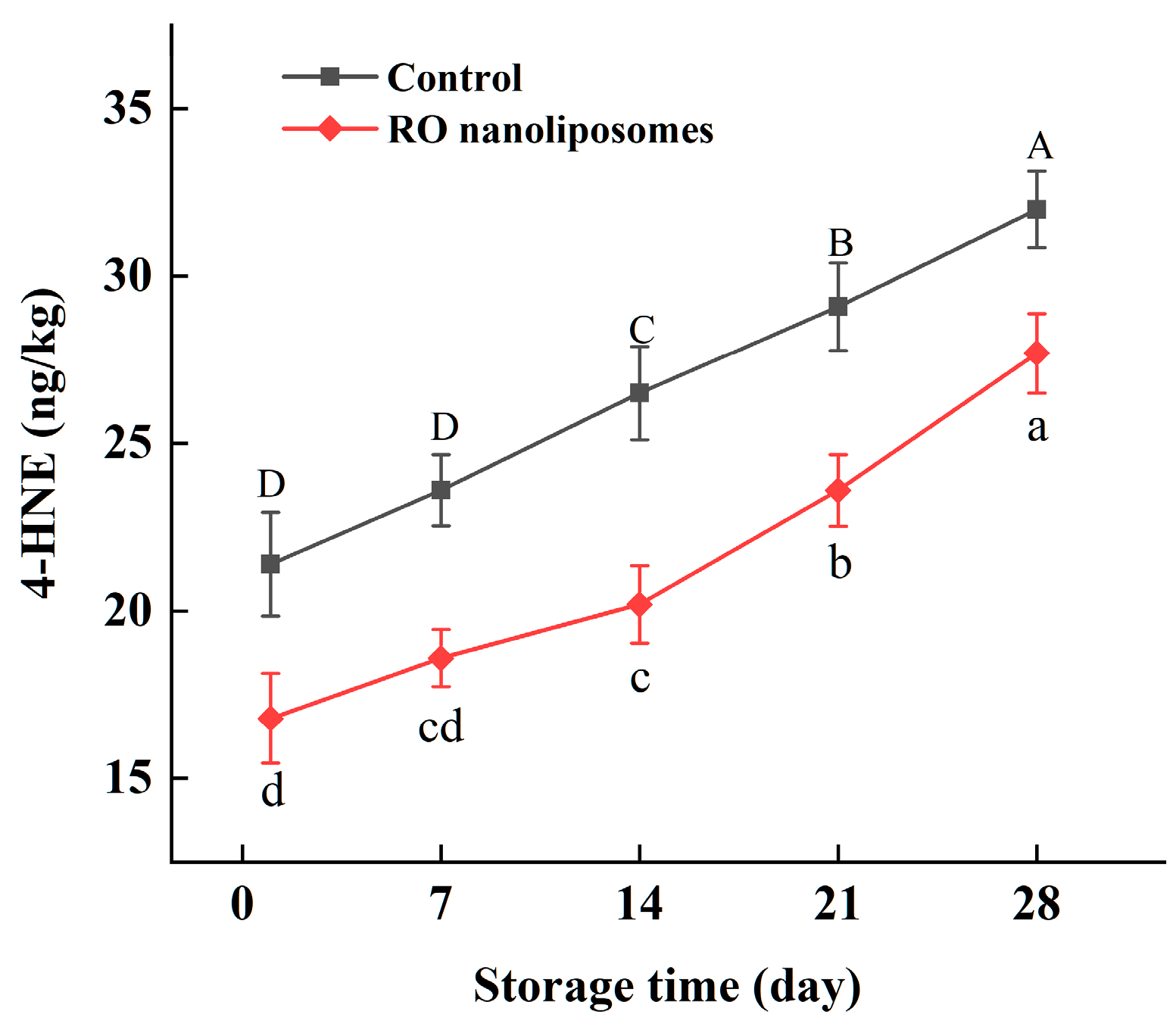
3.7.3. 4-HNE Content Analysis of Dried Oysters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Wang, M.; Lin, X. Optimization and application of spray-drying process on oyster cooking soup byproduct. Food Sci. Technol.-Brazil 2018, 38, 407–412. [Google Scholar] [CrossRef]
- Dietrich, J.P.; Hicks, M.B.R.; Hard, J.J.; Nichols, K.M.; Langdon, C.J.; Divilov, K.; Schoolfield, B.; Arkoosh, M.R. Heritability estimates of disease resistance to Vibrio coralliiyticus in Pacific oyster (Crassostrea gigas) larvae from a selective brood stock program. Aquaculture 2022, 560, 738492. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, Y.; Gu, S.; Zhu, S.; Zhou, X.; Ding, Y. Identification of changes in volatile compounds in dry-cured fish during storage using HS-GC-IMS. Food Res. Int. 2020, 137, 109339. [Google Scholar] [CrossRef]
- Ye, C.; Dai, D.; Hu, W. Antimicrobial and antioxidant activities of the essential oil from onion. Food Control 2013, 30, 48–53. [Google Scholar] [CrossRef]
- Manessis, G.; Kalogianni, A.I.; Lazou, T.; Moschovas, M.; Gelasakis, A.I. Plant-derived natural antioxidants in meat and meat products. Antioxidants 2020, 9, 1215. [Google Scholar] [CrossRef]
- Yuan, J.; Shoeman, D.W.; Csallany, A.S. Formation of 4-Hydroxy-2-Trans-Nonenal, a Toxic Aldehyde, in Thermally Treated Olive and Sunflower Oils. J. Am. Oil Chem. Soc. 2018, 95, 813–823. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Fogliano, V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef]
- Yan, W.; Chen, W.; Muhammad, U.; Zhang, J.; Zhuang, H.; Zhou, G. Preparation of α-tocopherol-chitosan nanoparticles/chitosan/montmorillonite film and the antioxidant efficiency on sliced dry-cured ham. Food Control 2019, 104, 132–138. [Google Scholar] [CrossRef]
- Ozdemir, N.; Pola, C.C.; Teixeira, B.N.; Hill, L.E.; Bayrak, A.; Gomes, C.L. Preparation of black pepper oleoresin inclusion complexes based on beta-cyclodextrin for antioxidant and antimicrobial delivery applications using kneading and freeze drying methods: A comparative study. LWT 2018, 91, 439–445. [Google Scholar] [CrossRef]
- Valencia-Cordova, M.G.; Suárez-Jacobo, Á.; del Socorro Cruz-Cansino, N.; Ramírez-Moreno, E.; Zafra-Rojas, Q.Y.; Alberto-Ariza-Ortega, J.; Alanís-García, E. Capsaicin, dihydrocapsaicin content and antioxidants properties of habanero pepper (Capsicum chinense Jacq.) Oleoresin during storage. Emir. J. Food Agric. 2021, 33, 583–588. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.; Pokorný, J. Natural antioxidants from herbs and spices. Eur. J. Lipid Sci. Technol. 2006, 108, 776–793. [Google Scholar] [CrossRef]
- Song, X.C.; Canellas, E.; Wrona, M.; Becerril, R.; Nerin, C. Comparison of two antioxidant packaging based on rosemary oleoresin and green tea extract coated on polyethylene terephthalate for extending the shelf life of minced pork meat. Food Packag. Shelf Life 2020, 26, 100588. [Google Scholar] [CrossRef]
- Senanayake, S.N. Rosemary extract as a natural source of bioactive compounds. J. Food Bioact. 2018, 2, 51–57. [Google Scholar] [CrossRef]
- Attala, O.A.; Assaggaf, H.M.; Alsafi, R.T.; Ahmed, O.B. Efficacy of oleoresins of ginger and rosemary to improve the oxidative stability and sensory attributes in non-irradiated and irradiated minced meat. J. Saudi Soc. Food Nutr. (JSSFN) 2020, 13, 127–137. [Google Scholar]
- Xie, J.; VanAlstyne, P.; Uhlir, A.; Yang, X. A review on rosemary as a natural antioxidation solution. Eur. J. Lipid Sci. Technol. 2017, 119, 1600439. [Google Scholar] [CrossRef]
- Kaur, R.; Gupta, T.B.; Bronlund, J.; Kaur, L. The potential of rosemary as a functional ingredient for meat products—A review. Food Rev. Int. 2021, 1–21. [Google Scholar] [CrossRef]
- Vergara, H.; Cózar, A.; Rubio, N. Lamb meat burgers shelf life: Effect of the addition of different forms of rosemary (Rosmarinus officinalis L.). CyTA-J. Food 2021, 19, 606–613. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Ramezanzade, L.; Mcclements, D.J. Recent advances in nanoencapsulation of hydrophobic marine bioactives: Bioavailability, safety, and sensory attributes of nano-fortified functional foods. Trends Food Sci. Technol. 2021, 109, 322–339. [Google Scholar] [CrossRef]
- Corrêa, A.P.F.; Bertolini, D.; Lopes, N.A.; Veras, F.F.; Gregory, G.; Brandelli, A. Characterization of nanoliposomes containing bioactive peptides obtained from sheep whey hydrolysates. LWT 2019, 101, 107–112. [Google Scholar] [CrossRef]
- Oskoueian, E.; Karimi, E.; Noura, R.; Ebrahimi, M.; Shafaei, N.; Karimi, E. Nanoliposomes encapsulation of enriched phenolic fraction from pistachio hulls and its antioxidant, anti-inflammatory, and anti-melanogenic activities. J. Microencapsul. 2020, 37, 1–13. [Google Scholar] [CrossRef]
- Zhang, M.; Hagan, C.T., IV; Foley, H.; Tian, X.; Yang, F.; Au, K.M.; Mi, Y.; Medik, Y.; Roche, K.; Wagner, K.; et al. Co-delivery of etoposide and cisplatin in dual-drug loaded nanoparticles synergistically improves chemoradiotherapy in non-small cell lung cancer models. Acta Biomater. 2021, 124, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Ma, G.; Yuan, Z.; Qian, H.; Xu, L.; Sidransky, E.; Chen, S. Development of zwitterionic polypeptide nanoformulation with high doxorubicin loading content for targeted drug delivery. Langmuir 2018, 35, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Sapper, M.; Wilcaso, P.; Santamarina, M.P.; Roselló, J.; Chiralt, A. Antifungal and functional properties of starch-gellan films containing thyme (Thymus zygis) essential oil. Food Control 2018, 92, 505–515. [Google Scholar] [CrossRef]
- Pabast, M.; Shariatifar, N.; Beikzadeh, S.; Jahed, G. Effects of chitosan coatings incorporating with free or nano-encapsulated Satureja plant essential oil on quality characteristics of lamb meat. Food Control 2018, 91, 185–192. [Google Scholar] [CrossRef]
- Cui, H.; Yuan, L.; Li, W.; Li, L. Edible film incorporated with chitosan and artemisia annua oil nanoliposomes for inactivation ofescherichia colio157:h7 on cherry tomato. Int. J. Food Sci. Technol. 2016, 52, 687–698. [Google Scholar] [CrossRef]
- Sangsuwan, J.; Sutthasupa, S. Effect of chitosan and alginate beads incorporated with lavender, clove essential oils, and vanillin against botrytis cinerea and their application in fresh table grapes packaging system. Packag. Technol. Sci. 2019, 32, 595–605. [Google Scholar] [CrossRef]
- Seyedabadi, M.M.; Rostami, H.; Jafari, S.M.; Fathi, M. Development and characterization of chitosan-coated nanoliposomes for encapsulation of caffeine. Food Biosci. 2021, 40, 100857. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M. Effect of chitosan coating on the properties of nanoliposomes loaded with flaxseed-peptide fractions: Stability during spray-drying. Food Chem. 2020, 310, 125951. [Google Scholar] [CrossRef]
- Al-Samydai, A.; Alshaer, W.; Al-Dujaili, E.A.; Azzam, H.; Aburjai, T. Preparation, characterization, and anticancer effects of capsaicin-loaded nanoliposomes. Nutrients 2021, 13, 3995. [Google Scholar] [CrossRef]
- Ahmad, S.; Truran, S.; Karamanova, N.; Kindelin, A.; Lozoya, M.; Weissig, V.; Emerson, H.; Griffiths, D.R.; Vail, T.; Lifshitz, J.; et al. Nanoliposomes Reduce Stroke Injury Following Middle Cerebral Artery Occlusion in Mice. Stroke 2022, 53, e37–e41. [Google Scholar] [CrossRef]
- Bilal, M.; Qindeel, M.; Raza, A.; Mehmood, S.; Rahdar, A. Stimuli-responsive nanoliposomes as prospective nanocarriers for targeted drug delivery. J. Drug Deliv. Sci. Technol. 2021, 66, 102916. [Google Scholar] [CrossRef]
- Hamadou, A.H.; Huang, W.C.; Xue, C.; Mao, X. Formulation of vitamin C encapsulation in marine phospholipids nanoliposomes: Characterization and stability evaluation during long term storage. LWT 2020, 127, 109439. [Google Scholar] [CrossRef]
- Zhao, Y.; Sedighi, R.; Wang, P.; Chen, H.; Zhu, Y.; Sang, S. Carnosic acid as a major bioactive component in rosemary extract ameliorates high-fat-diet-induced obesity and metabolic syndrome in mice. J. Agric. Food Chem. 2015, 63, 4843–4852. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Liu, T.; Ma, C.; Lei, Y.; Zu, Y.; Lin, Z.; Wang, H. Microwave irradiation to pretreat rosemary (Rosmarinus officinalis L.) for maintaining antioxidant content during storage and to extract essential oil simultaneously. Food Chem. 2012, 131, 1399–1405. [Google Scholar] [CrossRef]
- Sarabandi, K.; Mahoonak, A.; Hamishehkar, H.; Ghorbani, M.; Jafari, S. Protection of casein hydrolysates within nanoliposomes: Antioxidant and stability characterization. J. Food Eng. 2019, 251, 19–28. [Google Scholar] [CrossRef]
- Shishir, M.; Karim, N.; Gowd, V.; Xie, J.; Zheng, X.; Chen, W. Pectin-chitosan conjugated nanoliposome as a promising delivery system for neohesperidin: Characterization, release behavior, cellular uptake, and antioxidant property. Food Hydrocolloid 2019, 95, 432–444. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Decker, E.A.; Robe, G.H.; Moody, W.G. Gelation of crude myofifibrillar protein isolated from beef heart under antioxidative conditions. J. Food Sci. 1993, 58, 1241–1244. [Google Scholar] [CrossRef]
- Cao, J.; Zhou, C.; Wang, Y.; Sun, Y.; Pan, D. The effect of oxidation on the structure of G-actin and its binding ability with aroma compounds in carp grass skeletal muscle. Food Chem. 2018, 240, 346–353. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Q.; Li, S.; Chang, Y.; Zheng, X.; Cao, H.; Zheng, Y. Usage of nanocrystalline cellulose as a novel cryoprotective substance for the Nemipterus virgatus surimi during frozen storage. Food Chem. X 2022, 16, 100506. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Zhou, D.Y.; Zhou, X.; Yin, F.W.; Zhao, Q.; Xie, H.K.; Li, D.Y.; Zhu, B.W.; Wang, T.; Shahidi, F. Effect of Various Hot-Air Drying Processes on Clam Ruditapesphilippinarum Lipids: Composition Changes and Oxidation Development. J. Food Sci. 2018, 83, 2976–2982. [Google Scholar] [CrossRef]
- Cui, H.Y.; Zhao, C.T.; Lin, L. The specific antibacterial activity of liposome encapsulated Clove oil and its application in tofu. Food Control 2015, 56, 128–134. [Google Scholar] [CrossRef]
- Huang, C.M.; Chen, C.H.; Pornpattananangkul, D.; Zhang, L.; Chan, M.; Hsieh, M.F.; Zhang, L. Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials 2011, 32, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Mousavipour, N.; Babaei, S.; Moghimipour, E.; Moosavi-Nasab, M.; Ceylan, Z. A novel perspective with characterized nanoliposomes: Limitation of lipid oxidation in fish oil. LWT 2021, 152, 112387. [Google Scholar] [CrossRef]
- Da Silva, B.D.; do Rosário, D.K.A.; Weitz, D.A.; Conte-Junior, C.A. Essential oil nanoemulsions: Properties, development, and application in meat and meat products. Trends Food Sci. Technol. 2022, 121, 1–13. [Google Scholar] [CrossRef]
- Shetti, P.; Jalalpure, S.S. A single robust stability-indicating RP-HPLC analytical tool for apigenin quantification in bulk powder and in nanoliposomes: A novel approach. Future J. Pharm. Sci. 2021, 7, 122. [Google Scholar] [CrossRef]
- Ramezanzade, L.; Hosseini, S.; Nikkhah, M. Biopolymer-coated nanoliposomes as carriers of rainbow trout skin-derived antioxidant peptides. Food Chem. 2017, 234, 220. [Google Scholar] [CrossRef]
- Heckler, C.; Silva, C.M.M.; Cacciatore, F.A.; Daroit, D.J.; da Silva Malheiros, P. Thymol and carvacrol in nanoliposomes: Characterization and a comparison with free counterparts against planktonic and glass-adhered Salmonella. LWT 2020, 127, 109382. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M.; Mohammadi, M.; Akbarbaglu, Z.; Pezeshki, A.; Heshmati, M.K. Production of reconstitutablenanoliposomes loaded with flaxseed protein hydrolysates: Stability and characterization. Food Hydrocoll. 2019, 96, 442–450. [Google Scholar] [CrossRef]
- Ajun, W.; Yan, S.; Li, G.; Huili, L. Preparation of aspirin and probucol in combination loaded chitosan nanoparticles and in vitro release study. Carbohydr. Polym. 2009, 75, 566–574. [Google Scholar] [CrossRef]
- Yoksan, R.; Jirawutthiwongchai, J.; Arpo, K. Encapsulation of ascorbyl palmitate in chitosan nanoparticles by oil-in-water emulsion and ionic gelation processes. Colloids Surf. B 2010, 76, 292–297. [Google Scholar] [CrossRef]
- Lin, L.; Chen, W.; Li, C.; Cui, H. Enhancing stability of eucalyptus citriodora essential oil by solid nanoliposomes encapsulation. Ind. Crops Prod. 2019, 140, 111615. [Google Scholar] [CrossRef]
- Hosseini, S.; Rezaei, M.; Zandi, M.; Ghavi, F. Preparation and functional properties of fish gelatin-chitosan blend edible films. Food Chem. 2013, 136, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Balyklova, K.S.; Gegechkori, V.; Morton, D.W. HPTLC and ATR/FTIR Characterization of Antioxidants in Different Rosemary Extracts. Molecules 2021, 26, 6064. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer effects of rosemary (Rosmarinus officinalis L.) extract and rosemary extract polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, S.; Benjakul, S. Characteristics and storage stability of nanoliposomes loaded with shrimp oil as affected by ultrasonication and microfluidization. Food Chem. 2019, 310, 125916. [Google Scholar] [CrossRef]
- Nagahama, H.; Maeda, H.; Kashiki, T.; Jayakumar, R.; Furuike, T.; Tamura, H. Preparation and characterization of novel chitosan/gelatin membranes using chitosan hydrogel. Carbohyd. Polym. 2009, 76, 255–260. [Google Scholar] [CrossRef]
- Pinilla, C.; Brandelli, A. Antimicrobial activity of nanoliposomes co-encapsulating nisin and garlic extract against gram-positive and gram-negative bacteria in milk. Innov. Food Sci. Emerg. 2016, 36, 287–293. [Google Scholar] [CrossRef]
- Mohammadi, M.; Ghanbarzadeh, B.; Hamishehkar, H. Formulation of nanoliposomal vitamin D3 for potential application in beverage fortification. Adv. Pharm. Bull. 2014, 4 (Suppl. 2), 569. [Google Scholar] [CrossRef]
- Gülseren, I.; Corredig, M. Storage stability and physical characteristics of tea-polyphenol-bearing nanoliposomes prepared with milk fat globule membrane phospholipids. J. Agric. Food Chem. 2013, 61, 3242–3251. [Google Scholar] [CrossRef]
- Cheng, C.; Wu, Z.; McClements, D.J.; Zou, L.; Peng, S.; Zhou, W.; Liu, W. Improvement on stability, loading capacity and sustained release of rhamnolipids modified curcumin liposomes. Colloids Surf. B Biointerfaces 2019, 183, 110460. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical profiling of flavonoids, phenolic acids, terpenoids, and volatile fraction of a rosemary (Rosmarinus officinalis L.) extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef] [PubMed]
- Ademosun, M.T.; Omoba, O.S.; Olagunju, A.I. Antioxidant properties, glycemic indices, and carbohydrate hydrolyzing enzymes activities of formulated ginger-based fruit drinks. J. Food Biochem. 2021, 45, e13324. [Google Scholar] [CrossRef] [PubMed]
- Nahr, F.; Ghanbarzadeh, B.; Hamishehkar, H.; Kafil, H.; Hoseini, M.; Moghadam, B. Investigation of physicochemical properties of essential oil loaded nanoliposome for enrichment purposes. LWT 2019, 105, 282–289. [Google Scholar] [CrossRef]
- Mazloomi, S.N.; Mahoonak, A.S.; Ghorbani, M.; Houshmand, G. Physicochemical properties of chitosan-coated nanoliposome loaded with orange seed protein hydrolysate. J. Food Eng. 2020, 280, 109976. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, O.O.; Benjakul, S. Nonthermal processes for shelf-life extension of seafoods: A revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 892–904. [Google Scholar] [CrossRef]
- Hematyar, N.; Rustad, T.; Sampels, S.; KastrupDalsgaard, T. Relationship between lipid and protein oxidation in fish. Aquac. Res. 2019, 50, 1393–1403. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, G.; Yin, F.; Liu, Z.; Wang, J.; Qin, L.; Zhou, D.; Shahidi, F.; Zhu, B. Effects of roasting temperature and time on aldehyde formation derived from lipid oxidation in scallop (Patinopecten yessoensis) and the deterrent effect by antioxidants of bamboo leaves. Food Chem. 2022, 369, 130936. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guan, R.; Cao, G.; Liu, Z.; Wang, Z.; Shen, H.; Xia, Q. Antioxidant and antimicrobial effects of catechin liposomes on Chinese dried pork. J. Food Prot. 2018, 81, 827–834. [Google Scholar] [CrossRef]
- Cruz-Romero, M.C.; Kerry, J.P.; Kelly, A.L. Fatty acids, volatile compounds and colour changes in high-pressure-treated oysters (Crassostrea gigas). Innov. Food Sci. Emerg. Technol. 2008, 9, 54–61. [Google Scholar] [CrossRef]
- Ojeda-Sana, A.M.; van Baren, C.M.; Elechosa, M.A.; Juárez, M.A.; Moreno, S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control 2013, 31, 189–195. [Google Scholar] [CrossRef]
- Ozogul, Y.; Ayas, D.; Yazgan, H.; Ozogul, F.; Boga, E.K.; Ozyurt, G. The capability of rosemary extract in preventing oxidation of fish lipid. Int. J. Food Sci. Technol. 2010, 45, 1717–1723. [Google Scholar] [CrossRef]
- Cadun, A.; Kışla, D.; Çaklı, Ş. Marination of deep-water pink shrimp with rosemary extract and the determination of its shelf-life. Food Chem. 2008, 109, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control 2018, 84, 312–320. [Google Scholar] [CrossRef]
- Shi, J.; Lei, Y.; Shen, H.; Hong, H.; Yu, X.; Zhu, B.; Luo, Y. Effect of glazing and rosemary (Rosmarinus officinalis) extract on preservation of mud shrimp (Solenoceramelantho) during frozen storage. Food Chem. 2019, 272, 604–612. [Google Scholar] [CrossRef]
- Zhang, X.; Lan, W.; Xie, J. Combined citric acid and rosemary extract to maintain the quality of chilled Pacific white shrimp (Litopenaeus vannamei). J. Food Process. Preserv. 2021, 45, e15614. [Google Scholar] [CrossRef]
- Cui, H.; Yang, M.; Shi, C.; Li, C.; Lin, L. Application of Xanthan-Gum-Based Edible Coating Incorporated with Litseacubeba Essential Oil Nanoliposomes in Salmon Preservation. Foods 2022, 11, 1535. [Google Scholar] [CrossRef]
- Lund, M.N.; Lametsch, R.; Hviid, M.S.; Jensen, O.N.; Skibsted, L.H. High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Sci. 2007, 77, 295–303. [Google Scholar] [CrossRef]
- Chen, X.; Lv, M.; Gan, H.; Zeng, D.; Yang, C.; Ma, H. Impact of chitosan-based coatings on myofibrillar protein denaturation, muscle microstructure and lipid oxidation of oyster (Crassostrea hongkongensis) during 0 °C storage. J. Aquat. Food Prod. Technol. 2020, 29, 1001–1012. [Google Scholar] [CrossRef]
- Li, P.; Yang, H.; Zhu, Y.; Wang, Y.; Bai, D.; Dai, R.; Ren, X.; Yang, H.; Ma, L. Influence of washing and cold storage on lipid and protein oxidation in catfish (Clarias lazera) surimi. J. Aquat. Food Prod. Technol. 2016, 25, 790–801. [Google Scholar] [CrossRef]
- Liao, H.; Zhu, M.; Chen, Y. 4-Hydroxy-2-nonenal in food products: A review of the toxicity, occurrence, mitigation strategies and analysis methods. Trends Food Sci. Technol. 2020, 96, 188–198. [Google Scholar] [CrossRef]
- Zarkovic, N. 4-Hydroxynonenal as a bioactive marker of pathophysiological processes. Mol. Aspects Med. 2003, 24, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Alghazeer, R.; Howell, N.K. Formation of 4-hydroxynonenal (4-HNE) in frozen mackerel (Scomber scombrus) in the presence and absence of green tea. J. Sci. Food Agric. 2008, 88, 1128–1134. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Zhou, D.Y.; Li, A.; Zhao, M.T.; Hu, Y.Y.; Li, D.Y.; Xie, H.K.; Zhao, Q.; Hu, X.P.; Zhang, J.H.; et al. Effects of temperature and heating time on the formation of aldehydes during the frying process of clam assessed by an HPLC-MS/MS method. Food Chem. 2019, 308, 125650. [Google Scholar] [CrossRef]
- Saka, I.T.; Yamauchi, K.; Kuwazuru, S.; Gotoh, N. Relationships between 4-hydroxy-2-nonenal, 2-thiobarbituric acid reactive substances and n-6 polyunsaturated fatty acids in refrigerated and frozen pork. Biosci. Biotechnol. Biochem 1998, 62, 2028–2029. [Google Scholar] [CrossRef][Green Version]
- Asha, K.K.; Anandan, R.; Mathew, S.; Lakshmanan, P.T. Biochemical profile of oyster Crassostrea madrasensis and its nutritional attributes. Egypt. J. Aquat. Res. 2014, 40, 35–41. [Google Scholar] [CrossRef]
- Nadkarni, D.V. Structural definition of early lysine and histidine adduction chemistry of 4-hydroxynonenal. Chem. Res. Toxicol. 1995, 8, 284–291. [Google Scholar] [CrossRef]
| Treatment | Average Size (nm) | PDI | Zeta Potential (mV) | Encapsulation Efficiency (%) |
|---|---|---|---|---|
| Empty nanoliposome | 95.31 ± 1.02 a | 0.266 ± 0.005 a | −14.27 ± 0.32 a | - |
| Coated 13 mg RO | 160.48 ± 0.95 b | 0.281 ± 0.005 a | −15.37 ± 0.21 b | 59.57 ± 1.02 a |
| Coated 26 mg RO | 162.32 ± 0.86 b | 0.283 ± 0.006 a | −16.03 ± 0.28 c | 68.25 ± 2.38 b |
| Coated 39 mg RO | 162.65 ± 0.96 b | 0.269 ± 0.008 a | −16.07 ± 0.33 c | 55.69 ± 1.96 c |
| Coated 52 mg RO | 162.25 ± 1.21 b | 0.266 ± 0.007 a | −16.30 ± 0.12 c | 52.77 ± 2.89 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Zang, M.; Wang, S.; Zhao, X.; Zhai, G.; Wang, L.; Li, X.; Zhao, Y.; Yue, Y. Physicochemical and Antioxidant Properties of Nanoliposomes Loaded with Rosemary Oleoresin and Their Oxidative Stability Application in Dried Oysters. Bioengineering 2022, 9, 818. https://doi.org/10.3390/bioengineering9120818
Cheng X, Zang M, Wang S, Zhao X, Zhai G, Wang L, Li X, Zhao Y, Yue Y. Physicochemical and Antioxidant Properties of Nanoliposomes Loaded with Rosemary Oleoresin and Their Oxidative Stability Application in Dried Oysters. Bioengineering. 2022; 9(12):818. https://doi.org/10.3390/bioengineering9120818
Chicago/Turabian StyleCheng, Xiaoyu, Mingwu Zang, Shouwei Wang, Xin Zhao, Guozhen Zhai, Le Wang, Xiang Li, Yan Zhao, and Yijing Yue. 2022. "Physicochemical and Antioxidant Properties of Nanoliposomes Loaded with Rosemary Oleoresin and Their Oxidative Stability Application in Dried Oysters" Bioengineering 9, no. 12: 818. https://doi.org/10.3390/bioengineering9120818
APA StyleCheng, X., Zang, M., Wang, S., Zhao, X., Zhai, G., Wang, L., Li, X., Zhao, Y., & Yue, Y. (2022). Physicochemical and Antioxidant Properties of Nanoliposomes Loaded with Rosemary Oleoresin and Their Oxidative Stability Application in Dried Oysters. Bioengineering, 9(12), 818. https://doi.org/10.3390/bioengineering9120818