Global Regulator AdpA_1075 Regulates Morphological Differentiation and Ansamitocin Production in Actinosynnema pretiosum subsp. auranticum
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids and Culture Conditions
2.2. Construction of Recombinant Strains
2.3. RNA Isolation, cDNA Synthesis and Quantitative Real-Time PCR (qRT-PCR)
2.4. Determination of AP-3 Production
2.5. Mycelial Morphology Observation
2.6. Scanning Electron Microscope (SEM)
2.7. Heterologous Overexpression of AdpA-1075
2.8. Electrophoretic Mobility Shift Assays (EMSA)
3. Results
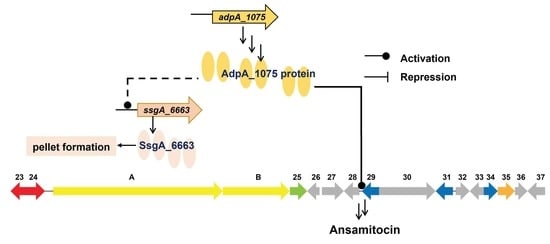
3.1. Identification of ssgA in A. pretiosum subsp. auranticum
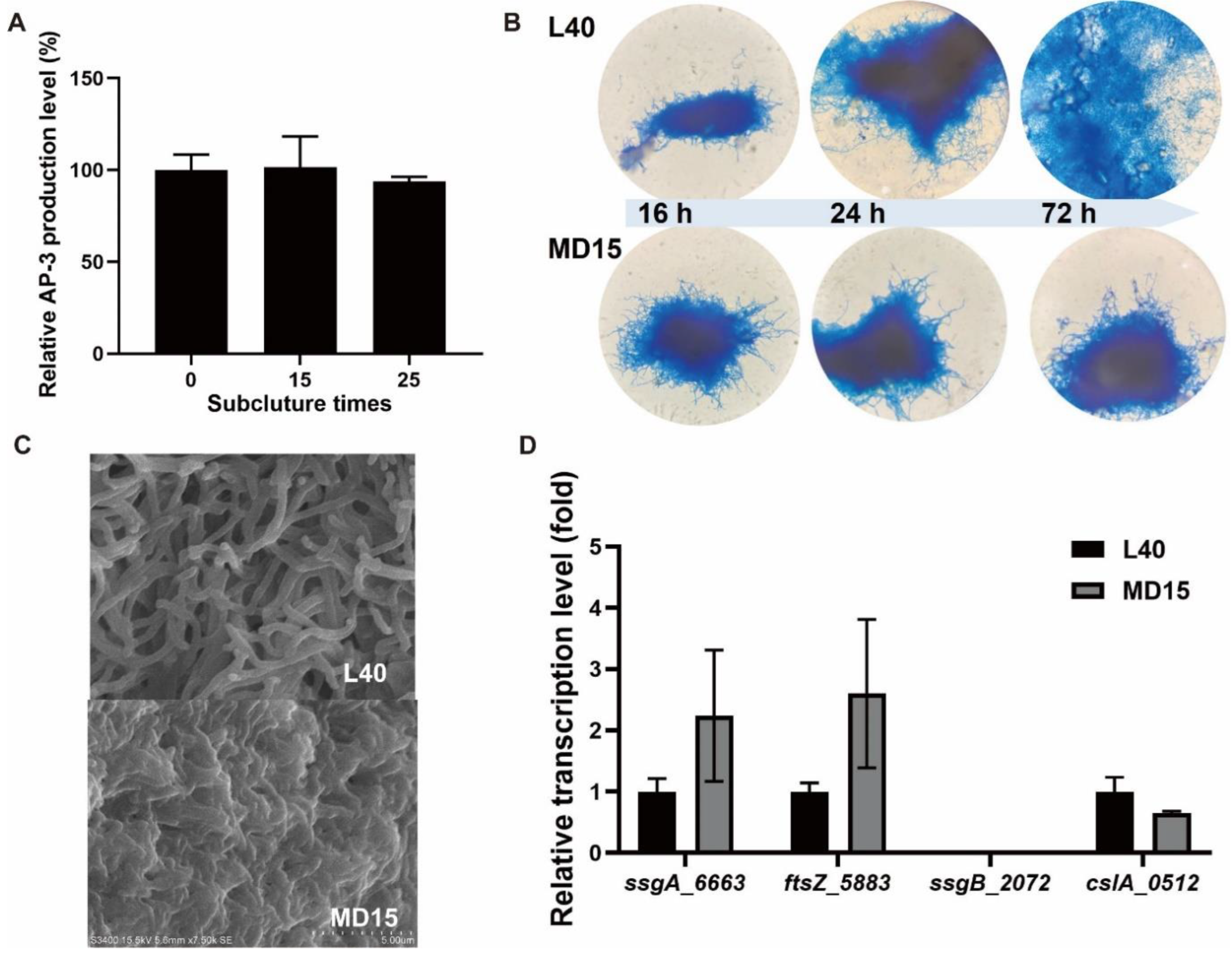
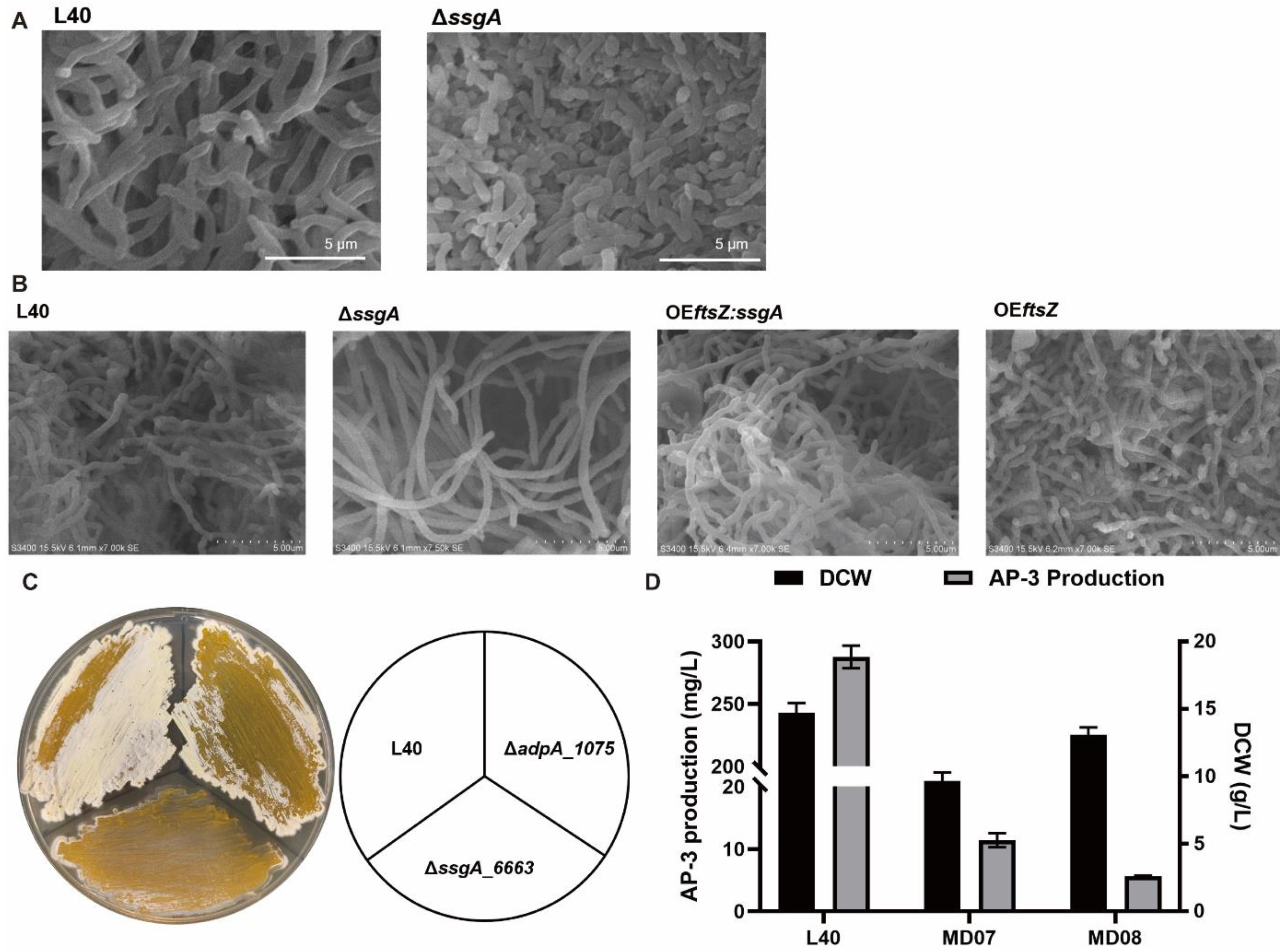
3.2. Deletion of ssgA_6663 Affected the Morphological Differentiation of A. pretiosum
3.3. Overexpression of adpA_1075 Increased the Production of AP-3
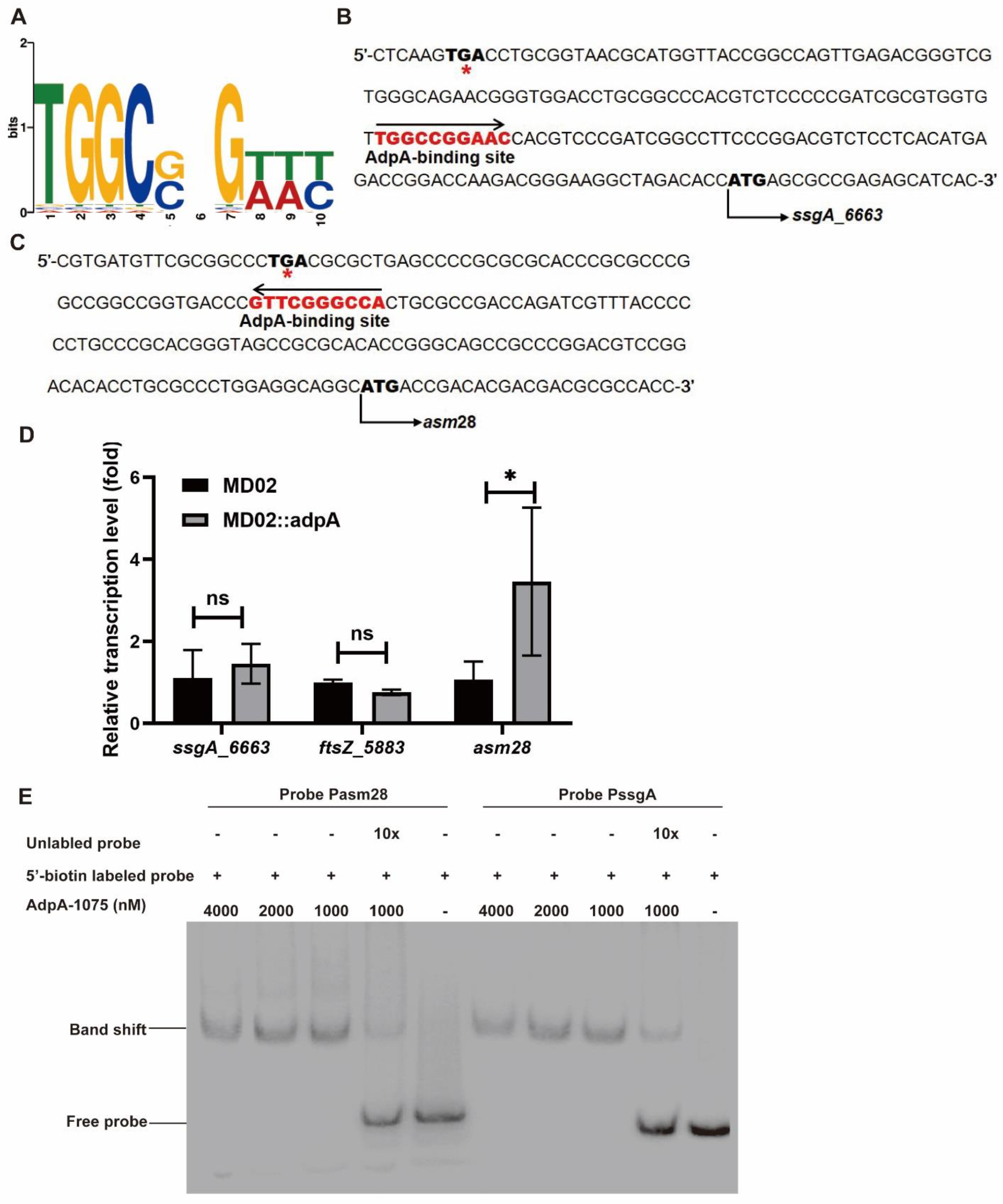
3.4. AdpA_1075 Is Involved in the Regulation of ssgA_6663 Transcription in A. pretiosum
3.5. AdpA_1075 Binds to Promoters of ssgA_6663 and asm28
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prelog, V.; Oppolzer, W. Ansamycins, a novel class of microbial metabolites. Helv. Chim. Acta 1973, 56, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Müller, P.; Schreiner, J.; Prince, S.S.; Lardinois, D.; Heinzelmann-Schwarz, V.A.; Thommen, D.S.; Zippelius, A. The microtubule-depolymerizing agent ansamitocin P3 programs dendritic cells toward enhanced anti-tumor immunity. Cancer Immunol. Immunother 2014, 63, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, A.S.; Fernandez-Rodriguez, L.; Zhao, Y.; Monaco, G.; Trefny, M.P.; Yoshida, N.; Martin, K.; Sharma, A.; Olieric, N.; Shah, P.; et al. GEF-H1 signaling upon microtubule destabilization is required for dendritic cell activation and specific anti-tumor responses. Cell Rep. 2019, 28, 3367–3380.e8. [Google Scholar] [CrossRef] [PubMed]
- Barok, M.; Joensuu, H.; Isola, J. Trastuzumab emtansine: Mechanisms of action and drug resistance. Breast Cancer Res. 2014, 16, 209. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Gao, Y.; Zhou, J.; Wei, L.; Chen, J.; Hua, Q. Process optimization with alternative carbon sources and modulation of secondary metabolism for enhanced ansamitocin P-3 production in Actinosynnema pretiosum. J. Biotechnol. 2014, 192, 1–10. [Google Scholar] [CrossRef]
- Li, T.; Fan, Y.; Nambou, K.; Hu, F.; Imanaka, T.; Wei, L.; Hua, Q. Improvement of ansamitocin P-3 production by Actinosynnema mirum with fructose as the sole carbon source. Appl. Biochem. Biotechnol. 2015, 175, 2845–2856. [Google Scholar] [CrossRef]
- Fan, Y.; Hu, F.; Wei, L.; Bai, L.; Hua, Q. Effects of modulation of pentose-phosphate pathway on biosynthesis of ansamitocins in Actinosynnema pretiosum. J. Biotechnol. 2016, 230, 3–10. [Google Scholar] [CrossRef]
- Zhao, M.; Fan, Y.; Wei, L.; Hu, F.; Hua, Q. Effects of the methylmalonyl-CoA metabolic pathway on ansamitocin production in Actinosynnema pretiosum. Appl. Biochem. Biotechnol. 2017, 181, 1167–1178. [Google Scholar] [CrossRef]
- Ning, X.; Wang, X.; Wu, Y.; Kang, Q.; Bai, L. Identification and engineering of post-PKS modification bottlenecks for ansamitocin P-3 titer improvement in Actinosynnema pretiosum subsp. pretiosum ATCC 31280. Biotechnol. J. 2017, 12, 1700484. [Google Scholar] [CrossRef]
- Du, Z.Q.; Zhang, Y.; Qian, Z.G.; Xiao, H.; Zhong, J.J. Combination of traditional mutation and metabolic engineering to enhance ansamitocin P-3 production in Actinosynnema pretiosum. Biotechnol. Bioeng. 2017, 114, 2794–2806. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.Q.; Zhong, J.J. Rational approach to improve ansamitocin P-3 production by integrating pathway engineering and substrate feeding in Actinosynnema pretiosum. Biotechnol. Bioeng. 2018, 115, 2456–2466. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, S.; Hua, Q.; Hu, F. Improved AP-3 production through combined ARTP mutagenesis, fermentation optimization, and subsequent genome shuffling. Biotechnol. Lett. 2021, 43, 1143–1154. [Google Scholar] [CrossRef]
- Kumar, P.; Dubey, K.K. Mycelium transformation of Streptomyces toxytricini into pellet: Role of culture conditions and kinetics. Bioresour. Technol. 2017, 228, 339–347. [Google Scholar] [CrossRef]
- Celler, K.; Picioreanu, C.; van Loosdrecht, M.C.M.; van Wezel, G.P. Structured morphological modeling as a framework for rational strain design of Streptomyces species. Antonie Van Leeuwenhoek 2012, 102, 409–423. [Google Scholar] [CrossRef]
- Paul, G.C.; Thomas, C.R. Characterisation of mycelial morphology using image analysis. Adv. Biochem. Eng. Biotechnol. 1998, 60, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, G.; Ding, X. Morphology engineering of Streptomyces coelicolor M145 by sub-inhibitory concentrations of antibiotics. Sci. Rep. 2017, 7, 13226. [Google Scholar] [CrossRef] [PubMed]
- Fang, A.; Pierson, D.L.; Mishra, S.K.; Demain, A.L. Growth of Streptomyces hygroscopicus in rotating-wall bioreactor under simulated microgravity inhibits rapamycin production. Appl. Microbiol. Biotechnol. 2000, 54, 33–36. [Google Scholar] [CrossRef]
- Park, Y.; Tamura, S.; Koike, Y.; Toriyama, M.; Okabe, M. Mycelial pellet intrastructure visualization and viability prediction in a culture of Streptomyces fradiae using confocal scanning laser microscopy. J. Ferment. Bioeng. 1997, 84, 483–486. [Google Scholar] [CrossRef]
- Jonsbu, E.; McIntyre, M.; Nielsen, J. The influence of carbon sources and morphology on nystatin production by Streptomyces noursei. J. Biotechnol. 2002, 95, 133–144. [Google Scholar] [CrossRef]
- Vecht-Lifshitz, S.E.; Sasson, Y.; Braun, S. Nikkomycin production in pellets of Streptomyces tendae. J. Appl. Bacteriol. 1992, 72, 195–200. [Google Scholar] [CrossRef]
- Wardell, J.N.; Stocks, S.M.; Thomas, C.R.; Bushell, M.E. Decreasing the hyphal branching rate of Saccharopolyspora erythraea NRRL 2338 leads to increased resistance to breakage and increased antibiotic production. Biotechnol. Bioeng. 2002, 78, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Sarrà, M.; Casas, C.; Poch, M.; Gòdia, F. A simple structured model for continuous production of a hybrid antibiotic by Streptomyces lividans pellets in a fluidized-bed bioreactor. Appl. Biochem. Biotechnol. 1999, 80, 39–50. [Google Scholar] [CrossRef]
- van Dissel, D.; Claessen, D.; van Wezel, G.P. Chapter One-Morphogenesis of Streptomyces in submerged cultures. Adv. Appl. Microbiol. 2014, 89, 1–45. [Google Scholar] [PubMed]
- Noens, E.E.; Mersinias, V.; Willemse, J.; Traag, B.A.; Laing, E.; Chater, K.F.; Smith, C.P.; Koerten, H.K.; Van Wezel, G.P. Loss of the controlled localization of growth stage-specific cell-wall synthesis pleiotropically affects developmental gene expression in an ssgA mutant of Streptomyces coelicolor. Mol. Microbiol. 2007, 64, 1244–1259. [Google Scholar] [CrossRef]
- Nothaft, H.; Dresel, D.; Willimek, A.; Mahr, K.; Niederweis, M.; Titgemeyer, F. The phosphotransferase system of Streptomyces coelicolor is biased for N-acetylglucosamine metabolism. J. Bacteriol. 2003, 185, 7019–7023. [Google Scholar] [CrossRef]
- Jiang, H.; Kendrick, K.E. Characterization of ssfR and ssgA, two genes involved in sporulation of Streptomyces griseus. J. Bacteriol. 2000, 182, 5521–5529. [Google Scholar] [CrossRef] [PubMed]
- van Wezel, G.P.; van der Meulen, J.; Kawamoto, S.; Luiten, R.G.M.; Koerten, H.K.; Kraal, B. SsgA Is Essential for Sporulation of Streptomyces Coelicolor A3(2) and Affects Hyphal Development by Stimulating Septum Formation. J. Bacteriol. 2000, 182, 5653–5662. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Ohnishi, Y.; Horinouchi, S. Transcriptional Switch on of SsgA by A-Factor, Which Is Essential for Spore Septum Formation in Streptomyces Griseus. J. Bacteriol. 2003, 185, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Bi, E.; Lutkenhaus, J. FtsZ Ring Structure Associated with Division in Escherichia Coli. Nature 1991, 354, 161–164. [Google Scholar] [CrossRef]
- Xu, W.; Huang, J.; Lin, R.; Shi, J.; Cohen, S.N. Regulation of Morphological Differentiation in S. Coelicolor by RNase III (AbsB) Cleavage of MRNA Encoding the AdpA Transcription Factor: AbsB Regulates AdpA in S. Coelicolor. Mol. Microbiol. 2010, 75, 781–791. [Google Scholar] [CrossRef]
- Xiao, X.; Willemse, J.; Voskamp, P.; Li, X.; Prota, A.E.; Lamers, M.; Pannu, N.; Abrahams, J.P.; van Wezel, G.P. Ectopic Positioning of the Cell Division Plane Is Associated with Single Amino Acid Substitutions in the FtsZ-Recruiting SsgB in Streptomyces. Open. Biol. 2021, 11, 200409. [Google Scholar] [CrossRef] [PubMed]
- Van Wezel, G.P.; Krabben, P.; Traag, B.A.; Keijser, B.J.; Kerste, R.; Vijgenboom, E.; Heijnen, J.J.; Kraal, B. Unlocking Streptomyces spp. for use as sustainable industrial production platforms by morphological engineering. Appl. Environ. Microbiol. 2006, 72, 5283–5288. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Pham, V.T.T.; Nguyen, C.T.; Pokhrel, A.R.; Kim, T.-S.; Kim, D.; Na, K.; Yamaguchi, T.; Sohng, J.K. Exploration of cryptic organic photosensitive compound as zincphyrin IV in Streptomyces venezuelae ATCC 15439. Appl. Microbiol. Biotechnol. 2020, 104, 713–724. [Google Scholar] [CrossRef]
- Traag, B.A.; Kelemen, G.H.; Van Wezel, G.P. Transcription of the sporulation gene ssgA is activated by the IclR-type regulator SsgR in a whi-independent manner in Streptomyces coelicolor A3(2). Mol. Microbiol. 2004, 53, 985–1000. [Google Scholar] [CrossRef]
- Horinouchi, S.; Beppu, T. Hormonal control by A-factor of morphological development and secondary metabolism in Streptomyces. Proc. Jpn. Acad. Ser. B 2007, 83, 277–295. [Google Scholar] [CrossRef]
- Ohnishi, Y.; Kameyama, S.; Onaka, H.; Horinouchi, S. The A-factor regulatory cascade leading to streptomycin biosynthesis in Streptomyces griseus: Identification of a target gene of the A-factor receptor. Mol. Microbiol. 1999, 34, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Yamazaki, H.; Kato, J.; Tomono, A.; Horinouchi, S. AdpA, a central transcriptional regulator in the A-factor regulatory cascade that leads to morphological development and secondary metabolism in Streptomyces griseus. Biosci. Biotechnol. Biochem. 2005, 69, 431–439. [Google Scholar] [CrossRef]
- Akanuma, G.; Hara, H.; Ohnishi, Y.; Horinouchi, S. Dynamic changes in the extracellular proteome caused by absence of a pleiotropic regulator AdpA in Streptomyces griseus. Mol. Microbiol. 2009, 73, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Bush, M.J.; Tschowri, N.; Schlimpert, S.; Flärdh, K.; Buttner, M.J. c-di-GMP signalling and the regulation of developmental transitions in streptomycetes. Nat. Rev. Microbiol. 2015, 13, 749–760. [Google Scholar] [CrossRef]
- Bu, X.-L.; Weng, J.-Y.; He, B.-B.; Xu, M.-J.; Xu, J. A novel AdpA homologue negatively regulates morphological differentiation in Streptomyces xiamenensis 318. Appl. Environ. Microbiol. 2019, 85, e03107-18. [Google Scholar] [CrossRef]
- Romero-Rodríguez, A.; Robledo-Casados, I.; Sánchez, S. An overview on transcriptional regulators in Streptomyces. Biochim. Biophys. Acta 2015, 1849, 1017–1039. [Google Scholar] [CrossRef] [PubMed]
- Higo, A.; Hara, H.; Horinouchi, S.; Ohnishi, Y. Genome-wide distribution of AdpA, a global regulator for secondary metabolism and morphological differentiation in Streptomyces, revealed the extent and complexity of the AdpA regulatory network. DNA Res. 2012, 19, 259–273. [Google Scholar] [CrossRef]
- Rabyk, M.; Yushchuk, O.; Rokytskyy, I.; Anisimova, M.; Ostash, B. Genomic insights into evolution of AdpA family master regulators of morphological differentiation and secondary metabolism in Streptomyces. J. Mol. Evol. 2018, 86, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Kang, Q.; Zhang, L.-L.; Bai, L. Subtilisin-involved morphology engineering for improved antibiotic production in actinomycetes. Biomolecules 2020, 10, 851. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, R.; Kang, Q.; Bai, L. The antitumor agent ansamitocin P-3 binds to cell division protein FtsZ in Actinosynnema pretiosum. Biomolecules 2020, 10, 699. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, K.; Liu, Y.; Fu, J.; Zong, G.; Ma, X.; Cao, G. Deletion of the response regulator PhoP accelerates the formation of aerial mycelium and spores in Actinosynnema pretiosum. Front. Microbiol. 2022, 13, 845620. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Sun, X.; Li, R.; Zhang, T.; Hu, F.; Liu, F.; Hua, Q. Two strategies to improve the supply of pks extender units for ansamitocin P-3 biosynthesis by CRISPR–Cas9. Bioresour. Bioprocess. 2022, 9, 90. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Xu, H.; Chater, K.F.; Deng, Z.; Tao, M. A cellulose synthase-like protein involved in hyphal tip growth and morphological differentiation in Streptomyces. J. Bacteriol. 2008, 190, 4971–4978. [Google Scholar] [CrossRef]
- Kawamoto, S.; Watanabe, H.; Hesketh, A.; Ensign, J.C.; Ochi, K. Expression analysis of the ssgA gene product, associated with sporulation and cell division in Streptomyces griseus. Microbiology 1997, 143, 1077–1086. [Google Scholar] [CrossRef][Green Version]
- McCormick, J.R.; Su, E.P.; Driks, A.; Losick, R. Growth and viability of Streptomyces coelicolor mutant for the cell division gene ftsZ. Mol. Microbiol. 1994, 14, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Santos-Beneit, F.; Roberts, D.M.; Cantlay, S.; McCormick, J.R.; Errington, J. A mechanism for FtsZ-independent proliferation in Streptomyces. Nat. Commun. 2017, 8, 1378. [Google Scholar] [CrossRef] [PubMed]
- Yushchuk, O.; Ostash, I.; Vlasiuk, I.; Gren, T.; Luzhetskyy, A.; Kalinowski, J.; Fedorenko, V.; Ostash, B. Heterologous AdpA transcription factors enhance landomycin production in Streptomyces cyanogenus S136 under a broad range of growth conditions. Appl. Microbiol. Biotechnol. 2018, 102, 8419–8428. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Wang, Y.; Hou, B.; Wang, R.; Ye, J.; Zhu, X.; Wu, H.; Zhang, H. AdpAlin, a pleiotropic transcriptional regulator, is involved in the cascade regulation of lincomycin biosynthesis in Streptomyces lincolnensis. Front. Microbiol. 2019, 10, 2428. [Google Scholar] [CrossRef] [PubMed]
- Higo, A.; Horinouchi, S.; Ohnishi, Y. Strict regulation of morphological differentiation and secondary metabolism by a positive feedback loop between two global regulators AdpA and BldA in Streptomyces griseus. Mol. Microbiol. 2011, 81, 1607–1622. [Google Scholar] [CrossRef] [PubMed]
- Guyet, A.; Benaroudj, N.; Proux, C.; Gominet, M.; Coppée, J.-Y.; Mazodier, P. Identified members of the Streptomyces lividans AdpA regulon involved in differentiation and secondary metabolism. BMC Microbiol. 2014, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Tomono, A.; Ohnishi, Y.; Horinouchi, S. DNA-binding specificity of AdpA, a transcriptional activator in the A-factor regulatory cascade in Streptomyces griseus: DNA-binding specificity of AdpA. Mol. Microbiol. 2004, 53, 555–572. [Google Scholar] [CrossRef]
- Watanabe, K.; Okuda, T.; Yokose, K.; Furumai, T.; Maruyama, H. Actinosynnema mirum, a new producer of nocardicin antibiotics. J. Antibiot. 1983, 36, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Keijser, B.J.F.; Noens, E.E.E.; Kraal, B.; Koerten, H.K.; van Wezel, G.P. The Streptomyces coelicolor ssgB gene is required for early stages of sporulation. FEMS Microbiol. Lett. 2003, 225, 59–67. [Google Scholar] [CrossRef]
- Xu, Q.; Traag, B.A.; Willemse, J.; McMullan, D.; Miller, M.D.; Elsliger, M.-A.; Abdubek, P.; Astakhova, T.; Axelrod, H.L.; Bakolitsa, C.; et al. Structural and functional characterizations of SsgB, a conserved activator of developmental cell division in morphologically complex actinomycetes. J. Biol. Chem. 2009, 284, 25268–25279. [Google Scholar] [CrossRef] [PubMed]
- Makitrynskyy, R.; Ostash, B.; Tsypik, O.; Rebets, Y.; Doud, E.; Meredith, T.; Luzhetskyy, A.; Bechthold, A.; Walker, S.; Fedorenko, V. Pleiotropic regulatory genes bldA, adpA and absB are implicated in production of phosphoglycolipid antibiotic moenomycin. Open Biol. 2013, 3, 130121. [Google Scholar] [CrossRef] [PubMed]
- Bandi, S.; Kim, Y.; Chang, Y.K.; Shang, G.; Yu, T.W.; Floss, H.G. Construction of asm2 deletion mutant of Actinosynnema pretiosum and medium optimization for ansamitocin P-3 production using statistical approach. J. Microbiol. Biotechnol. 2006, 16, 1338–1346. [Google Scholar]
- Ng, D.; Chin, H.K.; Wong, V.V.T. Constitutive overexpression of asm2 and asm39 increases AP-3 production in the actinomycete Actinosynnema pretiosum. J. Ind. Microbiol. Biotechnol. 2009, 36, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Kang, Q.; Wang, L.; Bai, L.; Deng, Z. Asm8, a specific LAL-type activator of 3-amino-5-hydroxybenzoate biosynthesis in ansamitocin production. Sci. China Life Sci. 2013, 56, 601–608. [Google Scholar] [CrossRef]
- Li, S.; Lu, C.; Chang, X.; Shen, Y. Constitutive overexpression of asm18 increases the production and diversity of maytansinoids in Actinosynnema pretiosum. Appl. Microbiol. Biotechnol. 2016, 100, 2641–2649. [Google Scholar] [CrossRef]
- Hackl, S.; Bechthold, A. The gene bldA, a regulator of morphological differentiation and antibiotic production in Streptomyces. Arch. Pharm. 2015, 348, 455–462. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Leng, T.; Sun, X.; Zheng, J.; Li, R.; Chen, J.; Hu, F.; Liu, F.; Hua, Q. Global Regulator AdpA_1075 Regulates Morphological Differentiation and Ansamitocin Production in Actinosynnema pretiosum subsp. auranticum. Bioengineering 2022, 9, 719. https://doi.org/10.3390/bioengineering9110719
Guo S, Leng T, Sun X, Zheng J, Li R, Chen J, Hu F, Liu F, Hua Q. Global Regulator AdpA_1075 Regulates Morphological Differentiation and Ansamitocin Production in Actinosynnema pretiosum subsp. auranticum. Bioengineering. 2022; 9(11):719. https://doi.org/10.3390/bioengineering9110719
Chicago/Turabian StyleGuo, Siyu, Tingting Leng, Xueyuan Sun, Jiawei Zheng, Ruihua Li, Jun Chen, Fengxian Hu, Feng Liu, and Qiang Hua. 2022. "Global Regulator AdpA_1075 Regulates Morphological Differentiation and Ansamitocin Production in Actinosynnema pretiosum subsp. auranticum" Bioengineering 9, no. 11: 719. https://doi.org/10.3390/bioengineering9110719
APA StyleGuo, S., Leng, T., Sun, X., Zheng, J., Li, R., Chen, J., Hu, F., Liu, F., & Hua, Q. (2022). Global Regulator AdpA_1075 Regulates Morphological Differentiation and Ansamitocin Production in Actinosynnema pretiosum subsp. auranticum. Bioengineering, 9(11), 719. https://doi.org/10.3390/bioengineering9110719