Advances in Cardiac Tissue Engineering
Abstract
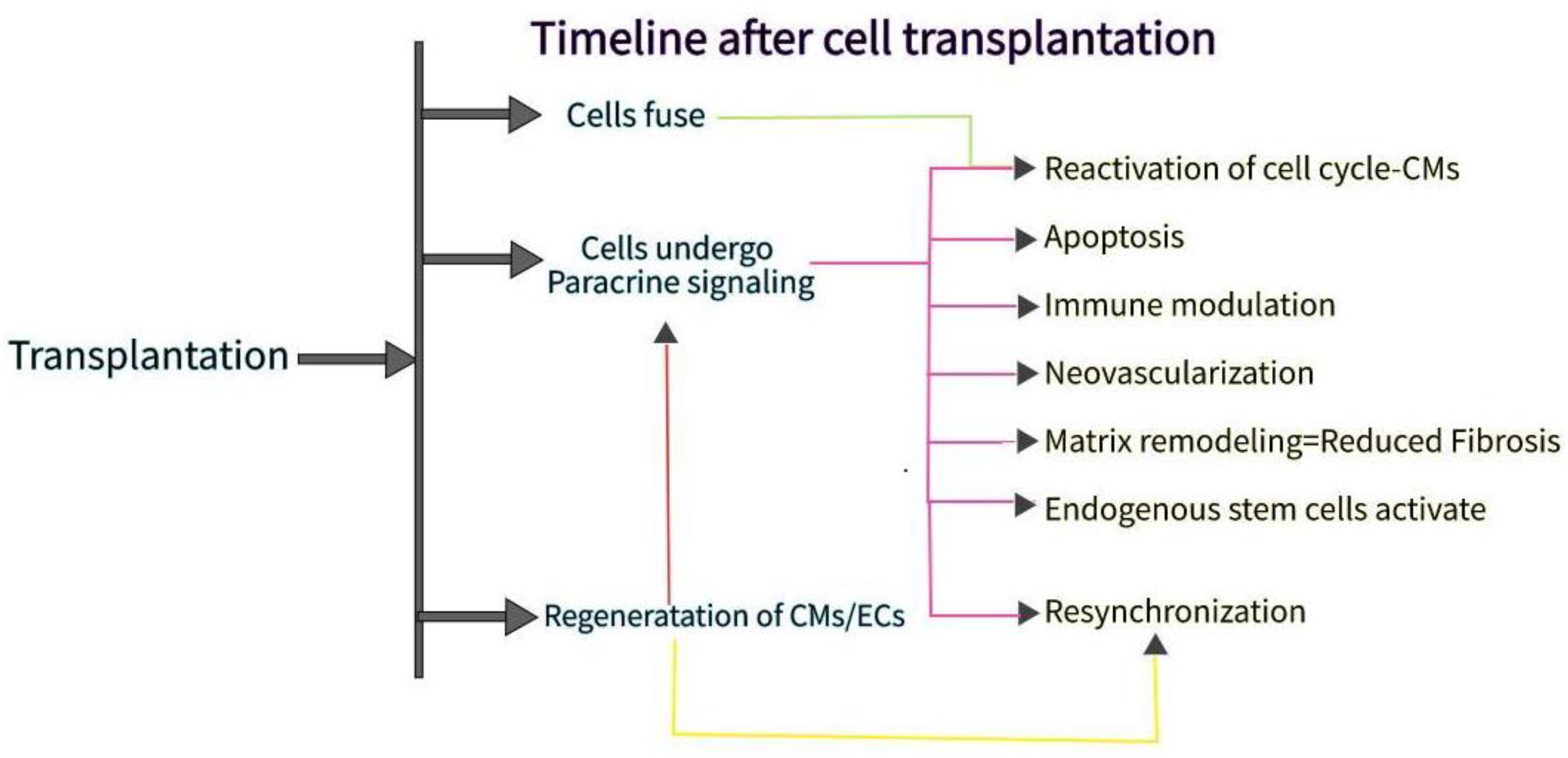
1. Introduction
2. Cell Types and Sources for Artificial hCMP Fabrication
2.1. Skeletal Myoblasts (SMs)
2.2. MSCs
2.3. Cardiomyocytes (CMs) (Fetal Myocardium and iPSCs)
2.4. Supporting Cells: Vascular Endothelial Cells, Fibroblasts, and SMCs
| Cell Sources | Comments | Ref. |
|---|---|---|
| Skeletal myoblasts (SMs) | Source of progenitor cells, to repair in the event of MI. Activated in response to muscle damage, then expresses Myf-5 or MyoD, myogenin, and MRF4. Ability to expand in vitro, resist ischemia, and have myogenic differentiation. Surrounds the sarcolemma. Advantages include a reduced likelihood of an immune response reaction, resistance to hypoxic conditions, production of angionenic factors, and a contractle phenotype. On the other hand, arrhythmias could occur, whilst having a low survival rate and a high chance of rejection. | [4,34,35] |
| Mesenchymal stem cells (MSCs) | Located in the blood vessel wall, and difficult to distinguish due to lack of unique markers. Ability to release anti-apoptopic and pro-angiogenic factors, as well as inflammatory agents, in order to inhibit inflammatory reactions. Although MSCs have advantages including an immunosuppressive potential and easy harvesting, the lack of evidence for safety, as well as its profibrogenic potential holds these cells back. | [36,37,38,39] |
| Cardiomyocytes | Derived from some sources, although rarely available: neonatal animals, Sca-1 (+) and C-kit (+) cardiac progenitor stem cells (CPCs) from adult murine hearts, ESC/iPSC-derived pure cardiomyocytes. Release extracellular vesicles for regenerative ability, contributing to cell contraction and relaxation. Although cardiomyocytes do show promise, they are hard to culture ethically, so similar alternatives have to be considered. | [6,40,41] |
| Bone marrow cells | Ability to produce bioactive molecules while interacting with the immune system. Overall, increase the ability of compromised tissue to regenerate. Can also be used to create disease models. Advantages include an immunopriveleged profile, paracrine/proangiogenic effects, and reliability, for it has been tested in several clinical studies. | [42,43] |
| Adipocytes, adipose-derived stem cells | Different characteristics and density arise if harvested from different areas and cells. Several reports have been made on adipose-derived stem cells’ ability to differentiate into several lineages; endodermal, ectodermal, and mesodermal. Can secrete multiple growth factors and cytokines, for regenerative capabilities. Moreover, it is easy to obtain large numbers of them, by using liposuction. | [42,44,45] |
| Supporting cells: vascular endothelial cells, fibroblasts, and SMCs | Fibroblasts with the optimal combination of cell types and ratios will produce an improved scaffold; moreover, SMCs can secrete various factors and induce differentiation from IPSC. |
3. How to Create Sheets
3.1. Cell Sheet Approach to Producing hCMP
3.2. 3D Printing; Spheroids, Contractile Forces, and Tubular EHTs
4. Common Problems for All
4.1. Increased Thickness of hCMP
Designed Vascular Network
4.2. hCMP Constructs
4.2.1. hCMP Delivery Method
4.2.2. Animal Models for Testing hCMP
4.2.3. Addressing Obstacles of hCMP
4.3. Safety Concerns
5. Summary—Current Challenges and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinhauser, M.L.; Lee, R.T. Regeneration of the heart. EMBO Mol. Med. 2011, 3, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Kitsuka, T.; Shiraki, A.; Oyama, J.I.; Nakagami, H.; Tanaka, A.; Node, K. A novel soluble epoxide hydrolase vaccine protects murine cardiac muscle against myocardial infarction. Sci. Rep. 2022, 12, 6923. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, S. Chronic kidney disease after liver, cardiac, lung, heart-lung, and hematopoietic stem cell transplant. Pediatr. Nephrol. 2008, 23, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Westerdahl, D.E.; Kobashigawa, J.A. Heart Transplantation for Advanced Heart Failure. Card. Intensive Care 2019, 504–524.e2. [Google Scholar] [CrossRef]
- Kim, W.; Kim, E.J. Heart Failure as a Risk Factor for Stroke. J. Stroke 2018, 20, 33–45. [Google Scholar] [CrossRef]
- Liu, Y.W.; Chen, B.; Yang, X.; Fugate, J.A.; Kalucki, F.A.; Futakuchi-Tsuchida, A.; Couture, L.; Vogel, K.W.; Astley, C.A.; Baldessari, A.; et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018, 36, 597–605. [Google Scholar] [CrossRef]
- Wang, L.; Serpooshan, V.; Zhang, J. Engineering Human Cardiac Muscle Patch Constructs for Prevention of Post-infarction LV Remodeling. Front. Cardiovasc. Med. 2021, 8, 621781. [Google Scholar] [CrossRef]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef]
- Tadevosyan, K.; Iglesias-García, O.; Mazo, M.M.; Prósper, F.; Raya, A. Engineering and Assessing Cardiac Tissue Complexity. Int. J. Mol. Sci. 2021, 22, 1479. [Google Scholar] [CrossRef]
- Guo, R.; Morimatsu, M.; Feng, T.; Lan, F.; Chang, D.; Wan, F.; Ling, Y. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res. Ther. 2020, 11, 19. [Google Scholar] [CrossRef]
- Shudo, Y.; Miyagawa, S.; Ohkura, H.; Fukushima, S.; Saito, A.; Shiozaki, M.; Kawaguchi, N.; Matsuura, N.; Shimizu, T.; Okano, T.; et al. Addition of mesenchymal stem cells enhances the therapeutic effects of skeletal myoblast cell-sheet transplantation in a rat ischemic cardiomyopathy model. Tissue Eng. Part A 2014, 20, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Mahar, M.A.A.; Lakkisto, P.; Tikkanen, I.; Vento, A.; Patila, T.; Harjula, A. Heat shock attenuates VEGF expression in three-dimensional myoblast sheets deteriorating therapeutic efficacy in heart failure. Med. Sci. Monit. 2011, 17, 345–353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uchinaka, A.; Tasaka, K.; Mizuno, Y.; Maeno, Y.; Ban, T.; Mori, S.; Hamada, Y.; Miyagawa, S.; Saito, A.; Sawa, A.; et al. Laminin α2-secreting fibroblasts enhance the therapeutic effect of skeletal myoblast sheets. Eur. J. Cardio-Thorac. Surg. 2017, 51, 457–464. [Google Scholar]
- Uchinaka, A.; Kawaguchi, N.; Hamada, Y.; Mori, S.; Miyagawa, S.; Saito, A.; Sawa, Y.; Matsuura, N. Transplantation of myoblast sheets that secrete the novel peptide SVVYGLR improves cardiac function in failing hearts. Cardiovasc. Res. 2013, 99, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Endler, A.; Shibasaki, F. Hypoxia and angiogenesis: Regulation of hypoxia-inducible factors via novel binding factors. Exp. Mol. Med. 2009, 41, 849–857. [Google Scholar] [CrossRef]
- Lv, B.; Hua, T.; Li, F.; Han, J.; Fang, J.; Xu, L.; Sun, C.; Zhang, Z.; Feng, Z.; Jiang, X. Hypoxia-inducible factor 1 α protects mesenchymal stem cells against oxygen-glucose deprivation-induced injury via autophagy induction and PI3K/AKT/mTOR signaling pathway. Am. J. Transl. Res. 2017, 9, 2492–2499. [Google Scholar]
- Dabrowska, S.; Andrzejewska, A.; Janowski, M.; Lukomska, B. Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cells and Extracellular Vesicles: Therapeutic Outlook for Inflammatory and Degenerative Diseases. Front. Immunol. 2020, 11, 591065. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.F.; Engler, A.J.; Woo, Y.J.; Pirolli, T.J.; Bish, L.T.; Jayasankar, V.; Morine, K.J.; Gardner, T.J.; Discher, D.E.; Sweeney, H.L. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2196–H2203. [Google Scholar] [CrossRef]
- Kitsuka, T.; Hama, R.; Ulziibayar, A.; Matsuzaki, Y.; Kelly, J.; Shinoka, T. Clinical Application for Tissue Engineering Focused on Materials. Biomedicines 2022, 10, 1439. [Google Scholar] [CrossRef]
- Romito, A.; Cobellis, G. Pluripotent Stem Cells: Current Understanding and Future Directions. Stem Cells Int. 2016, 2016, 9451492. [Google Scholar] [CrossRef]
- Ciuffi, S.; Zonefrati, R.; Brandi, M.L. Adipose stem cells for bone tissue repair. Clin. Cases Miner. Bone Metab. 2017, 14, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, J.K.; Takano, M.; Hiraoka-Kanie, M.; Shimazu, C.; Peishi, Y.; Yanagi, K.; Nakano, A.; Inoue, E.; Kita, F.; Nishikawa, S. Prospective identification of cardiac progenitors by a novel single cell-based cardiomyocyte induction. FASEB J. 2005, 19, 1534–1536. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.L.; Tulloch, N.L.; Razumova, M.V.; Saiget, M.; Muskheli, V.; Pabon, L.; Reinecke, H.; Regnier, M.; Murry, C.E. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation 2016, 134, 1557–1567. [Google Scholar] [CrossRef]
- Bikkina, M.; Larson, M.G.; Levy, D. Asymptomatic ventricular arrhythmias and mortality risk in subjects with left ventricular hypertrophy. J. Am. Coll. Cardiol. 1993, 22, 1111–1116. [Google Scholar] [CrossRef]
- Curran, M.E.; Splawski, I.; Timothy, K.W.; Vincent, G.M.; Green, E.D.; Keating, M.T. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell 1995, 80, 795–803. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Brigham, M.D.; Naik, S.R.; Karajanagi, S.S.; Levy, O.; Jin, H.; Parker, K.K.; Langer, R.; Kohane, D.S. Nanowired three-dimensional cardiac patches. Nat. Nanotechnol. 2011, 6, 720–725. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, J.; Sun, H.; Qiu, X.; Mou, Y.; Liu, Z.; Zhao, Y.; Li, X.; Han, Y.; Duan, C.; et al. Engineering the heart: Evaluation of conductive nanomaterials for improving implant integration and cardiac function. Sci. Rep. 2014, 4, 3733. [Google Scholar] [CrossRef]
- You, J.O.; Rafat, M.; Ye, G.J.; Auguste, D.T. Nanoengineering the heart: Conductive scaffolds enhance connexin 43 expression. Nano Lett. 2011, 11, 3643–3648. [Google Scholar] [CrossRef]
- Sharma, P.; Gentile, C. Cardiac Spheroids as in vitro Bioengineered Heart Tissues to Study Human Heart Pathophysiology. J. Vis. Exp. 2021, e61962. [Google Scholar] [CrossRef]
- Zhang, J.; Tao, R.; Campbell, K.F.; Carvalho, J.L.; Ruiz, E.C.; Kim, G.C.; Schmuck, E.G.; Raval, A.N.; da Rocha, A.M.; Herron, T.J.; et al. Functional cardiac fibroblasts derived from human pluripotent stem cells via second heart field progenitors. Nat. Commun. 2019, 10, 2238. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Becker, M.W.; Davis, M.G.; Dorn, G.W., 2nd. Dissociation of vasoconstrictor-stimulated basic fibroblast growth factor expression from hypertrophic growth in cultured vascular smooth muscle cells. Relevant roles Protein of protein kinase C. Circ. Res. 1994, 75, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Kim, H.S.; Kim, H.W.; Leong, K.W. Application of induced pluripotent stem cells to model smooth muscle cell function in vascular diseases. Curr. Opin. Biomed. Eng. 2017, 1, 38–44. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Menasché, P. Cell Therapy with Human ESC-Derived Cardiac Cells: Clinical Perspectives. Front. Bioeng. Biotechnol. 2020, 8, 601560. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Tchao, J.; Tobita, K. Concise review: Skeletal muscle stem cells and cardiac lineage: Potential for heart repair. Stem Cells Transl. Med. 2014, 3, 183–193. [Google Scholar] [CrossRef]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef]
- Pardue, E.L.; Ibrahim, S.; Ramamurthi, A. Role of hyaluronan in angiogenesis and its utility to angiogenic tissue engineering. Organogenesis 2008, 4, 203–214. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624. [Google Scholar] [CrossRef]
- Lee, S.H. The advantages and limitations of mesenchymal stem cells in clinical application for treating human diseases. Osteoporos. Sarcopenia 2018, 4, 150. [Google Scholar] [CrossRef]
- Barreto, S.; Hamel, L.; Schiatti, T.; Yang, Y.; George, V. Cardiac Progenitor Cells from Stem Cells: Learning from Genetics and Biomaterials. Cells 2019, 8, 11536. [Google Scholar] [CrossRef]
- Menasché, P. Skeletal myoblasts and cardiac repair. J. Mol. Cell. Cardiol. 2008, 45, 545–553. [Google Scholar] [PubMed]
- Polymeri, A.; Giannobile, W.V.; Kaigler, D. Bone Marrow Stromal Stem Cells in Tissue Engineering and Regenerative Medicine. Horm. Metab. Res. 2016, 48, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Xu, H.; Wang, L.; Kryczek, I.; Wu, K.; Hu, Y.; Wang, G.; Zou, W. Bone marrow and the control of immunity. Cell. Mol. Immunol. 2012, 9, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, W.; Rubin, J.P.; Marra, K.G. Adipose-derived stem cells: Implications in tissue regeneration. World J. Stem Cells 2014, 6, 312–321. [Google Scholar] [CrossRef]
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.R.; Soria, B.; Capilla-Gonzalez, V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 43. [Google Scholar] [CrossRef]
- Shimizu, T.; Yamato, M.; Kikuchi, A.; Okano, T. Two-dimensional manipulation of cardiac myocyte sheets utilizing temperature-responsive culture dishes augments the pulsatile amplitude. Tissue Eng. 2001, 7, 141–151. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Li, T.; Liu, S.; Guo, B.; Huang, W.; Wu, Y. 3D bioprinting in cardiac tissue engineering. Theranostics 2021, 11, 7948–7969. [Google Scholar] [CrossRef]
- Kitsuka, T.; Itoh, M.; Amamoto, S.; Arai, K.I.; Oyama, J.; Node, K.; Toda, S.; Morita, S.; Nishida, T.; Nakayama, K. 2-Cl-C.OXT-A stimulates contractio n through the suppression of phosphodiesterase activity in human induced pluripotent stem cell-derived cardiac organoids. PLoS ONE 2019, 14, e0213114. [Google Scholar] [CrossRef]
- Ban, K.; Bae, S.; Yoon, Y.S. Current Strategies and Challenges for Purification of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Theranostics 2017, 7, 2067–2077. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Breckwoldt, K.; Letuffe-Brenière, D.; Mannhardt, I.; Schulze, T.; Ulmer, B.; Werner, T.; Benzin, A.; Klampe, B.; Reinsch, M.C.; Laufer, S.; et al. Differentiation of cardiomyocytes and generation of human engineered heart tissue. Nat. Protoc. 2017, 12, 1177–1197. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, K.; Takahashi, H.; Tobe, Y.; Sasaki, D.; Matsuura, K.; Iwasaki, K.; Shimizu, T.; Umezu, M. Measuring the Contractile Force of Multilayered Human Cardiac Cell Sheets. Tissue Eng. Part C Methods 2020, 26, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Murata, D.; Takao, S.; Nakamura, A.; Itoh, M.; Kitsuka, T.; Nakayama, K. Drug response analysis for scaffold-free cardiac constructs fabricated using bio-3D printer. Sci. Rep. 2020, 10, 8972. [Google Scholar] [CrossRef] [PubMed]
- Kawai, Y.; Tohyama, S.; Arai, K.; Tamura, T.; Soma, Y.; Fukuda, K.; Shimizu, H.; Nakayama, K.; Kobayashi, E. Scaffold-Free Tubular Engineered Heart Tissue from Human Induced Pluripotent Stem Cells Using Bio-3D Printing Technology in vivo. Front. Cardiovasc. Med. 2021, 8, 806215. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J. Layer-By-Layer Fabrication of Thicker and Larger Human Cardiac Muscle Patches for Cardiac Repair in Mice. Front. Cardiovasc. Med. 2021, 8, 800667. [Google Scholar] [CrossRef]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef]
- Shin, S.R.; Aghaei-Ghareh-Bolagh, B.; Gao, X.; Nikkhah, M.; Jung, S.M.; Dolatshahi-Pirouz, A.; Kim, S.B.; Kim, S.M.; Dokmeci, M.R.; Tang, X.; et al. Layer-by-layer assembly of 3D tissue constructs with functionalized graphene. Adv. Funct. Mater. 2014, 24, 6136–6144. [Google Scholar]
- Hasan, A.; Paul, A.; Vrana, N.E.; Zhao, X.; Memic, A.; Hwang, Y.S.; Dokmeci, M.R.; Khademhosseini, A. Microfluidic techniques for development of 3D vascularized tissue. Biomaterials 2014, 35, 7308–7325. [Google Scholar] [CrossRef]
- Mastrolia, I.; Foppiani, E.M.; Murgia, A.; Candini, O.; Samarelli, A.V.; Grisendi, G.; Veronesi, E.; Horwitz, E.M.; Dominici, M. Challenges in Clinical Development of Mesenchymal Stromal/Stem Cells: Concise Review. Stem Cells Transl. Med. 2019, 8, 1135–1148. [Google Scholar] [CrossRef]
- Arjmand, B.; Abedi, M.; Arabi, M.; Alavi-Moghadam, S.; Rezaei-Tavirani, M.; Hadavandkhani, M.; Tayanloo-Beik, A.; Kordi, R.; Roudsari, P.P.; Larijani, B. Regenerative Medicine for the Treatment of Ischemic Heart Disease; Status and Future Perspectives. Front. Cell Dev. Biol. 2021, 9, 704903. [Google Scholar] [CrossRef]
- Zhu, D.; Li, Z.; Huang, K.; Caranasos, T.G.; Rossi, J.S.; Cheng, K. Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nat. Commun. 2021, 12, 1412. [Google Scholar] [CrossRef] [PubMed]
- Sack, K.L.; Aliotta, E.; Choy, J.S.; Ennis, D.B.; Davies, N.H.; Franz, T.; Kassab, G.S.; Guccione, J.M. Intra-myocardial alginate hydrogel injection acts as a left ventricular mid-wall constraint in swine. Acta Biomater. 2020, 111, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Piktel, J.S.; Wilson, L.D. Translational Models of Arrhythmia Mechanisms and Susceptibility: Success and Challenges of Modeling Human Disease. Front. Cardiovasc. Med. 2019, 6, 135. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.M.; Skibsbye, L.; Linz, D.; Lubberding, A.F.; Tfelt-Hansen, J.; Jespersen, T. Ventricular Arrhythmias in First Acute Myocardial Infarction: Epidemiology, Mechanisms, and Interventions in Large Animal Models. Front. Cardiovasc. Med. 2019, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Romagnuolo, R.; Masoudpour, H.; Porta-Sánchez, A.; Qiang, B.; Barry, J.; Laskary, A.; Qi, X.; Massé, S.; Magtibay, K.; Kawajiri, H.; et al. Human Embryonic Stem Cell-Derived Cardiomyocytes Regenerate the Infarcted Pig Heart but Induce Ventricular Tachyarrhythmias. Stem Cell Rep. 2019, 12, 967–981. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukherjee, U. A comprehensive review of immunosuppression used for liver transplantation. J. Transplant. 2009, 2009, 701464. [Google Scholar] [CrossRef]
- Deuse, T.; Hu, X.; Gravina, A.; Wang, D.; Tediashvili, G.; De, C.; Thayer, W.O.; Angela Wahl, J.V.G.; Reichenspurner, H.; Davis, M.M.; et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 2019, 37, 252–258. [Google Scholar] [CrossRef]
- Kainuma, S.; Miyagawa, S.; Toda, K.; Yoshikawa, Y.; Hata, H.; Yoshioka, D.; Kawamura, T.; Kawamura, A.; Kashiyama, N.; Ito, Y.; et al. Long-term outcomes of autologous skeletal myoblast cell-sheet transplantation for end-stage ischemic cardiomyopathy. Mol. Ther. J. Am. Soc. Gene Ther. 2021, 29, 1425–1438. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitsuka, T.; Takahashi, F.; Reinhardt, J.; Watanabe, T.; Ulziibayar, A.; Yimit, A.; Kelly, J.; Shinoka, T. Advances in Cardiac Tissue Engineering. Bioengineering 2022, 9, 696. https://doi.org/10.3390/bioengineering9110696
Kitsuka T, Takahashi F, Reinhardt J, Watanabe T, Ulziibayar A, Yimit A, Kelly J, Shinoka T. Advances in Cardiac Tissue Engineering. Bioengineering. 2022; 9(11):696. https://doi.org/10.3390/bioengineering9110696
Chicago/Turabian StyleKitsuka, Takahiro, Fuga Takahashi, James Reinhardt, Tatsuya Watanabe, Anudari Ulziibayar, Asigul Yimit, John Kelly, and Toshiharu Shinoka. 2022. "Advances in Cardiac Tissue Engineering" Bioengineering 9, no. 11: 696. https://doi.org/10.3390/bioengineering9110696
APA StyleKitsuka, T., Takahashi, F., Reinhardt, J., Watanabe, T., Ulziibayar, A., Yimit, A., Kelly, J., & Shinoka, T. (2022). Advances in Cardiac Tissue Engineering. Bioengineering, 9(11), 696. https://doi.org/10.3390/bioengineering9110696








