Comparison of Osseointegration of Dental Implants Placed in Rabbit Tibia Using Two Dental Laser and Implant Handpiece Systems
Abstract
1. Introduction
2. Materials and Methods
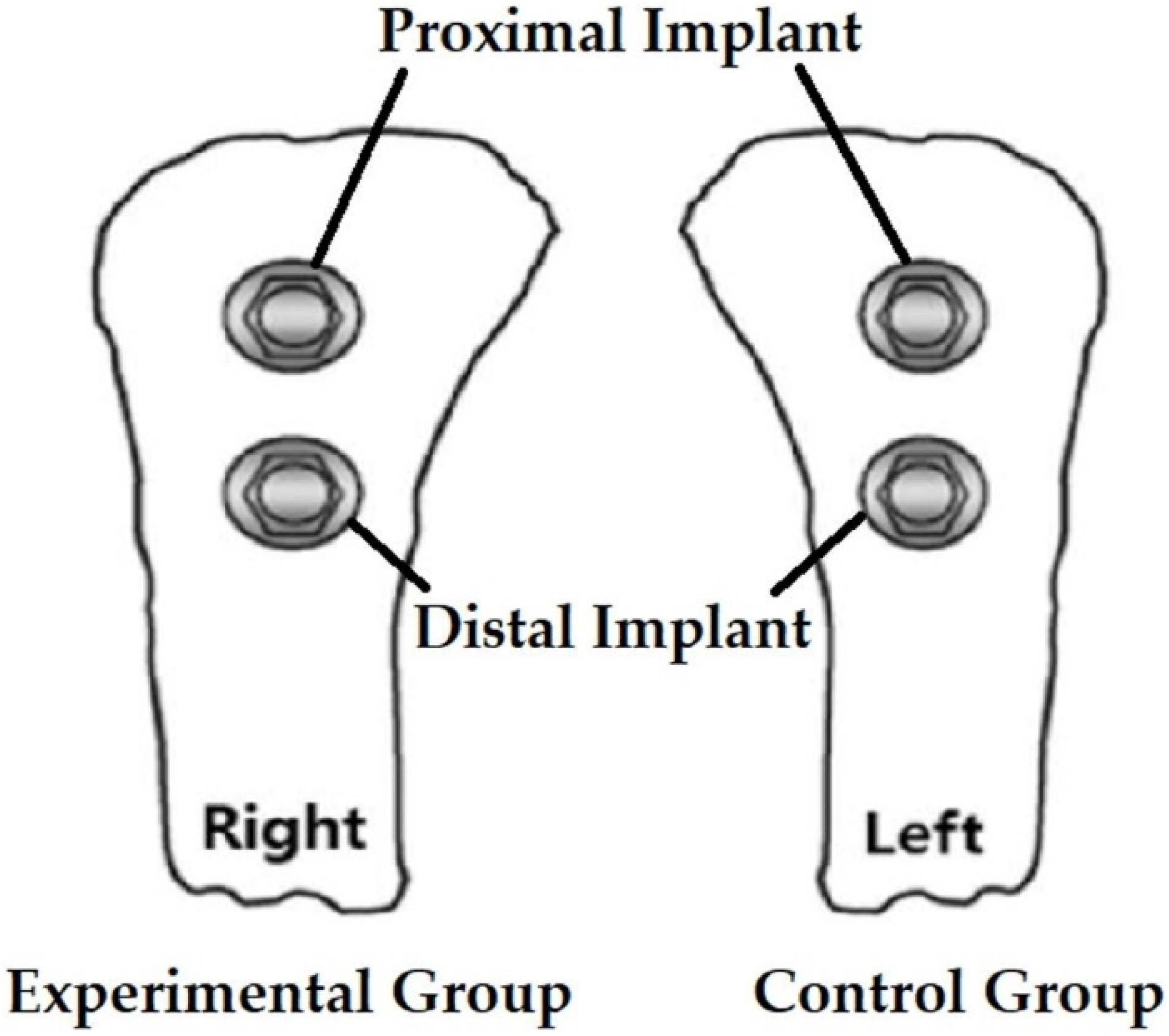
2.1. Experiment Preparation
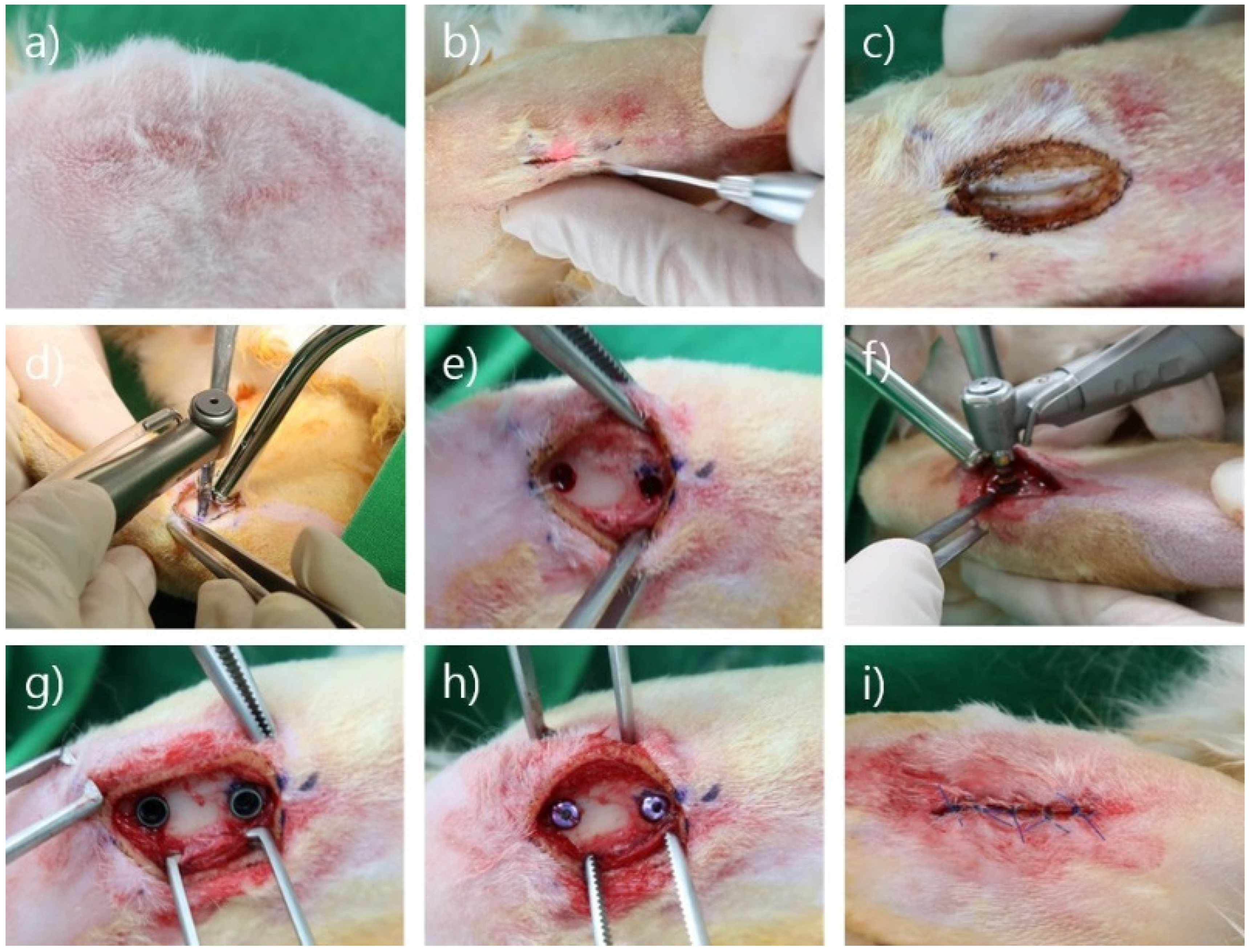
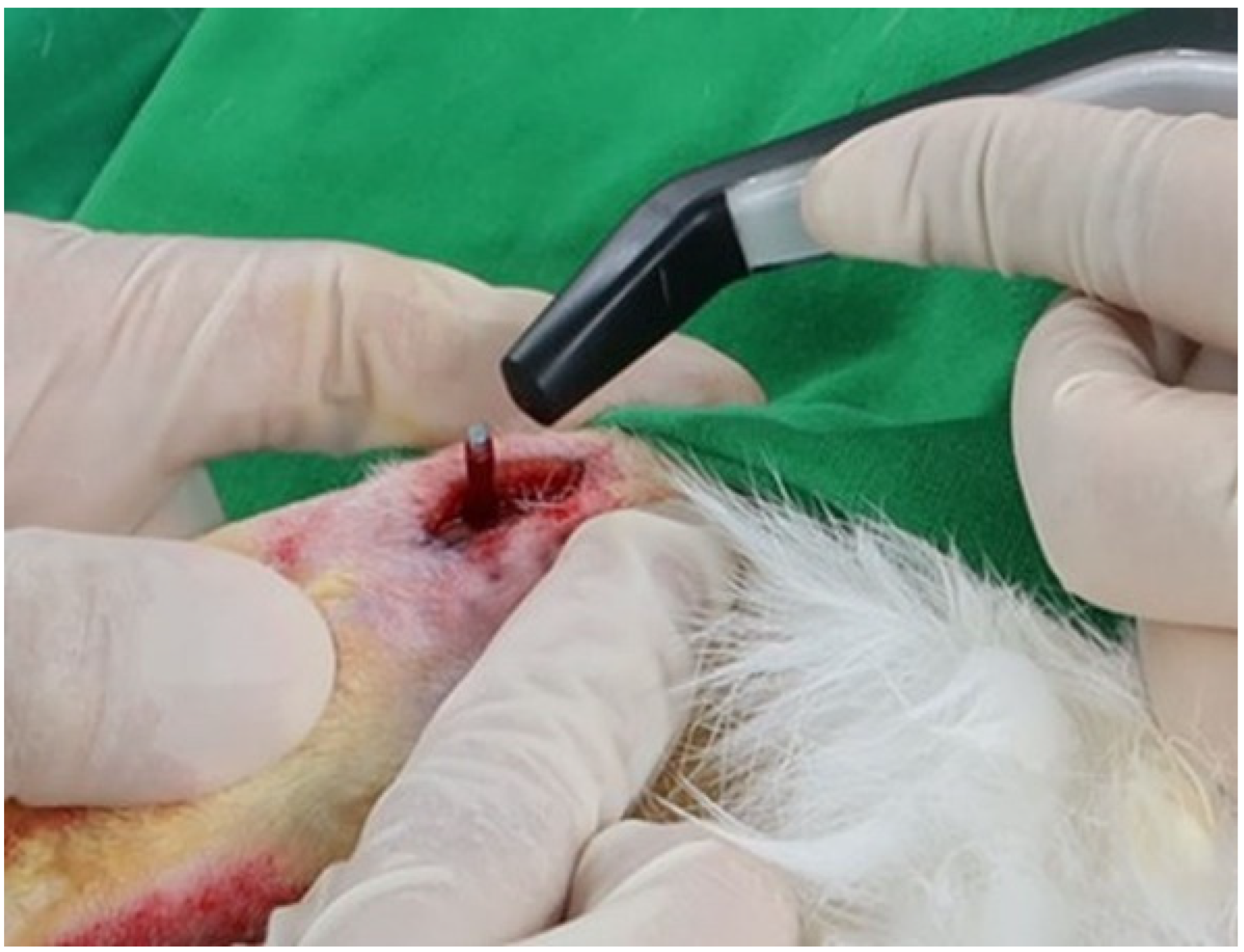
2.2. Surgical Procedure
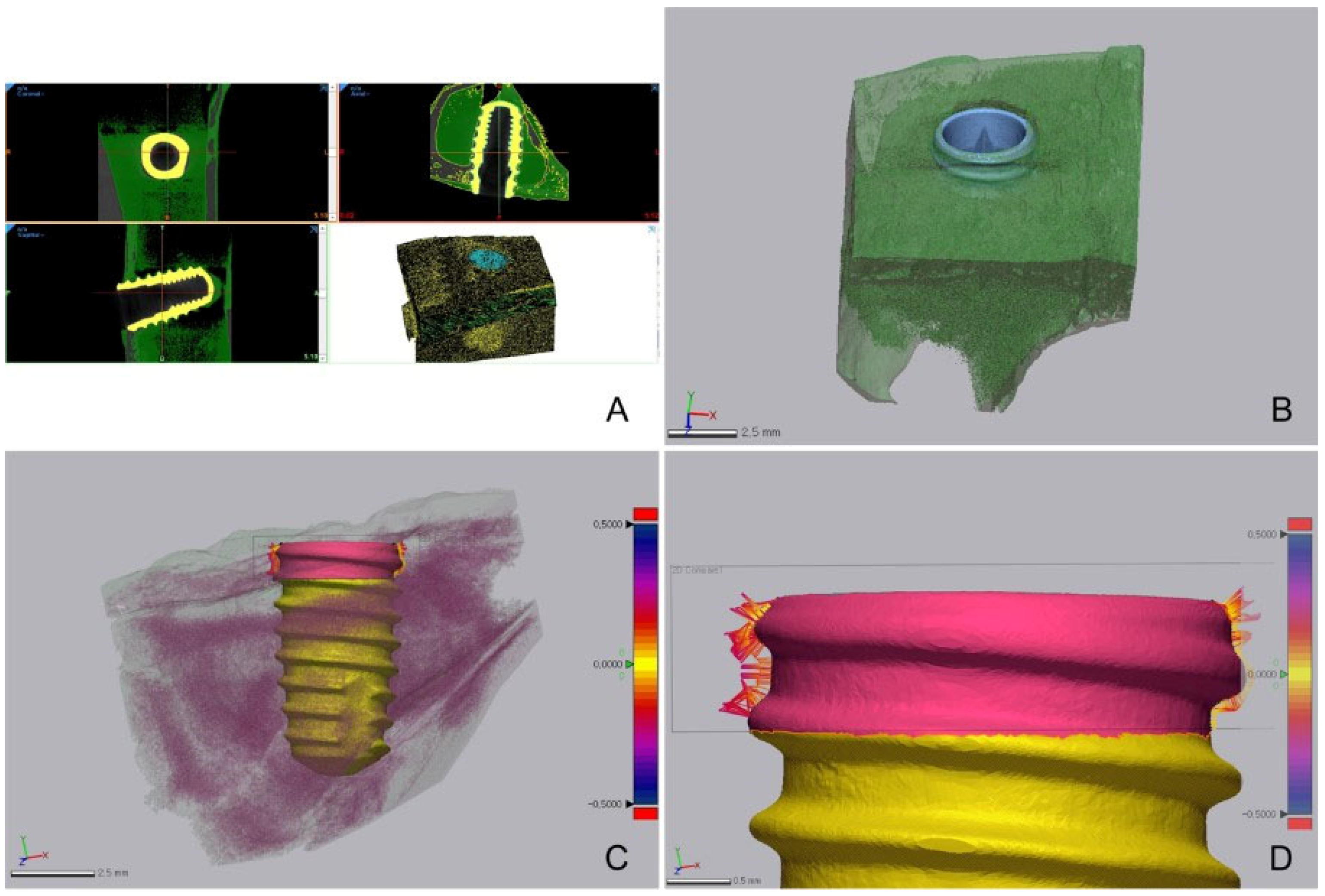
2.3. ISQ, Bone-to-Implant Contact (BIC), and Histological Evaluation
2.4. Statistical Analysis
3. Results
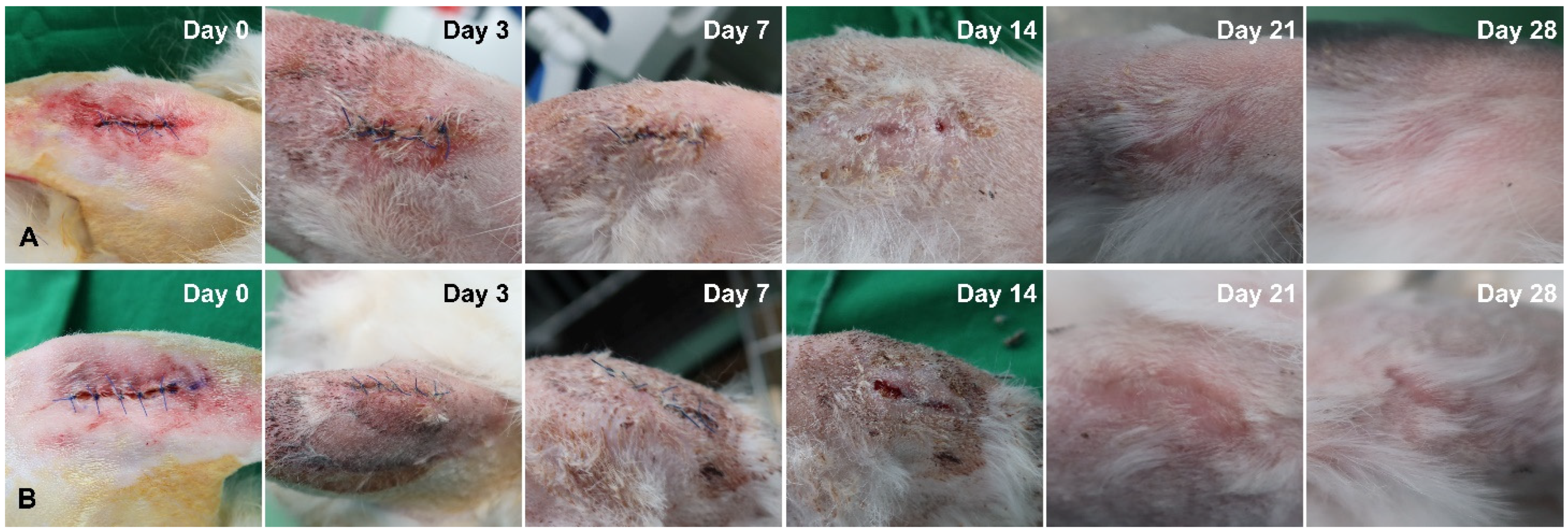
3.1. Comparison of Soft Tissue Healing after Laser Incision
3.2. Comparison of BIC Obtained Using Micro-CT
3.3. Comparison of ISQ Values
3.4. Comparison of Histological Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maiman, T.H. Stimulated optical radiation in ruby. Nature 1960, 187, 493–494. [Google Scholar] [CrossRef]
- Choy, D.S. History of lasers in medicine. Thorac. Cardiovasc. Surg. 1988, 36, 114–117. [Google Scholar] [CrossRef]
- Coluzzi, D.J. An overview of laser wavelengths used in dentistry. Dent. Clin. N. Am. 2000, 44, 753–765. [Google Scholar] [CrossRef]
- Liboon, J.; Funkhouser, W.; Terris, D.J. A comparison of mucosal incisions made by scalpel, CO2 laser, electrocautery, and constant-voltage electrocautery. Otolaryngol. Head Neck Surg. 1997, 116, 379–385. [Google Scholar] [CrossRef]
- Wilder-Smith, P.; Arrastia, A.M.; Liaw, L.H.; Berns, M. Incision properties and thermal effects of three CO2 lasers in soft tissue. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1995, 79, 685–691. [Google Scholar] [CrossRef]
- Judy, M.M.; Matthews, J.L.; Aronoff, B.L.; Hults, D.F. Soft tissue studies with 805 nm diode laser radiation: Thermal effects with contact tips and comparison with effects of 1064 nm Nd:YAG laser radiation. Lasers Surg. Med. 1993, 13, 528–536. [Google Scholar] [CrossRef]
- Renvert, S.; Wikström, M.; Dahlén, G.; Slots, J.; Egelberg, J. Effect of root debridement on the elimination of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis from periodontal pockets. J. Clin. Periodontol. 1990, 17, 345–350. [Google Scholar] [CrossRef]
- Slots, J.; Rosling, B.G. Suppression of the periodontopathic microflora in localized juvenile periodontitis by systemic tetracycline. J Clin Periodontol. 1983, 10, 465–486. [Google Scholar] [CrossRef]
- Carranza, F.A.; Saglie, R.; Newman, M.G.; Valentin, P.L. Scanning and transmission electron microscopic study of tissue-invading microorganisms in localized juvenile periodontitis. J. Periodontol. 1983, 54, 598–617. [Google Scholar] [CrossRef]
- Moritz, A.; Schoop, U.; Goharkhay, K.; Schauer, P.; Doertbudak, O.; Wernisch, J. Treatment of periodontal pockets with a diode laser. Lasers Surg. Med. 1998, 22, 302–311. [Google Scholar] [CrossRef]
- Gold, S.I. Effect of Nd: YAG laser curettage on gingival crevicular tissues. J. Dent. Res. 1992, 71, 299. [Google Scholar]
- Goharkhay, K.; Moritz, A.; Wilder-Smith, P.; Schoop, U.; Kluger, W.; Jakolitsch, S. Effects on oral soft tissue produced by a diode laser in vitro. Lasers Surg. Med. 1999, 25, 401–406. [Google Scholar] [CrossRef]
- Moritz, A.; Gutknecht, N.; Doertbudak, O.; Goharkhay, K.; Schoop, U.; Schauer, P. Bacterial reduction in periodontal pockets through irradiation with a diode laser: A pilot study. J. Clin. Laser Med. Surg. 1997, 15, 33–37. [Google Scholar] [CrossRef]
- Mavrogiannis, M.; Thomason, J.M.; Seymour, R.A. Lasers in periodontology. Dent. Update 2004, 31, 535–538. [Google Scholar] [CrossRef]
- White, J.M.; Goodis, H.E.; Rose, C.L. Use of the pulsed Nd:YAG laser for intraoral soft tissue surgery. Lasers Surg. Med. 1991, 11, 455–461. [Google Scholar] [CrossRef]
- Myers, T.D.; Myers, W.D. In vivo caries removal utilizing the YAG laser. J. Mich. Dent. Assoc. 1985, 67, 66–69. [Google Scholar]
- Judy, M.M.; Matthews, J.L.; Goodson, J.R.; Hults, D.F.; Viherkoski, E.; Aronoff, B.L. Thermal effects in tissues from combined simultaneous coaxial CO2 and Nd:YAG laser beams. Lasers Surg. Med. 1992, 12, 222–230. [Google Scholar] [CrossRef]
- Walsh, J.T.; Flotte, T.J.; Deutsch, T.F. Er:YAG laser ablation of tissue: Effect of pulse duration and tissue type on thermal damage. Lasers Surg. Med. 1989, 9, 314–326. [Google Scholar] [CrossRef]
- Walsh, J.T.; Cummings, J.P. Effect of the dynamic optical properties of water on midinfrared laser ablation. Lasers Surg. Med. 1994, 15, 295–305. [Google Scholar] [CrossRef]
- Pick, R.M.; Pecaro, B.C.; Silberman, C.J. The laser gingivectomy. The use of the CO2 laser for the removal of phenytoin hyperplasia. J. Periodontol. 1985, 56, 492–496. [Google Scholar] [CrossRef]
- Pick, R.M.; Pecaro, B.C. Use of the CO2 laser in soft tissue dental surgery. Lasers Surg. Med. 1987, 7, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Ishii, J.; Fujita, K.; Komori, T. Laser surgery as a treatment for oral leukoplakia. Oral Oncol. 2003, 39, 759–769. [Google Scholar] [CrossRef]
- Klim, J.D.; Fox, D.B.; Coluzzi, D.J.; Neckel, C.P.; Swick, M.D. The diode laser in dentistry. Rev. Wavelengths 2000, 8, 13–16. [Google Scholar]
- Rowson, J.E. The contact diode laser-A useful instrument for excising oral lesions. Br. J. Oral Maxillofac. Surg. 1995, 33, 123. [Google Scholar]
- Kim, H.R.; Son, K.; Son, Y.T.; Kim, Y.G.; Lee, K.B.; Lee, S.C.; Suh, J.Y.; Lee, J.M. A Comparative Immunohistochemical Study of Wound Healing after Dental Diode Laser Treatment in the Rat Oral Mucosa. Bioengineering 2022, 9, 466. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Chen, C.J.; Singh, M.; Weber, H.P.; Gallucci, G.O. Success criteria in implant dentistry: A systematic review. J. Dent. Res. 2012, 91, 242–248. [Google Scholar] [CrossRef]
- Lang, N.P.; Lindhe, J. Clinical Periodontology and Implant Dentistry; John Wiley & Sons: Hoboken, NJ, USA, 2015; Volume 2. [Google Scholar]
- Deppe, H.; Horch, H.H. Laser applications in oral surgery and implant dentistry. Lasers Med. Sci. 2007, 22, 217–221. [Google Scholar] [CrossRef]
- De Ávila Kfouri, F.; Duailibi, M.T.; Bretos, J.L.G.; Carvalho, A.B.; Pallos, D.; Duailibi, S.E. Piezoelectric osteotomy for the placement of titanium implants in rabbits: Histomorphometry study. Clin. Oral Implants Res. 2014, 25, 1182–1188. [Google Scholar] [CrossRef]
- Barak, S.; Neuman, M.; Iezzi, G.; Piattelli, A.; Perrotti, V.; Gabet, Y. A new device for improving dental implants anchorage: A histological and micro-computed tomography study in the rabbit. Clin. Oral Implants Res. 2016, 27, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahalawy, H.; Marei, H.F.; Abuohashish, H.; Alhawaj, H.; Alrefaee, M.; Al-Jandan, B. Effects of cisplatin chemotherapy on the osseointegration of titanium implants. J. Cranio-Maxillofac. Surg. 2016, 44, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.S.; Han, J.S.; Yang, J.H. Biomechanical and histomorphometric study of dental implants with different surface characteristics. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008, 87, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Carrasco, B.; Lemos, B.F.; Herrero-Climent, M.; Gil Mur, F.J.; Ríos-Santos, J.V. Effect of the acid-etching on grit-blasted dental implants to improve osseointegration: Histomorphometric analysis of the bone-implant contact in the rabbit tibia model. Coatings 2021, 11, 1426. [Google Scholar] [CrossRef]
- Jawad, M.M.; AbdulQader, S.T.; Alam, M.K.; Al-Azzawi, L.M.; Husein, A.; Mahmood, A.S. Clinical Healing Response of Soft Tissue Incisions Made by Diode Laser on Rabbits Skin. Int. Med. J. 2013, 20, 499–501. [Google Scholar]
- Jawad, M.M.; Alam, M.K.; AbdulQader, S.T.; Al-Azzawi, L.M.; Husein, A.; Mahmood, A.S. Histological healing response of soft tissue incisions made by diode laser on rabbits skin. Int. Med. J. 2013, 20, 502–504. [Google Scholar]
- Meredith, N.; Alleyne, D.; Cawley, P. Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin. Oral Implants Res. 1996, 7, 261–267. [Google Scholar] [CrossRef]
- Gedrange, T.; Hietschold, V.; Mai, R.; Wolf, P.; Nicklisch, M.; Harzer, W. An evaluation of resonance frequency analysis for the determination of the primary stability of orthodontic palatal implants. A study in human cadavers. Clin. Oral Implants Res. 2005, 16, 425–431. [Google Scholar] [CrossRef]
- Park, I.P.; Kim, S.K.; Lee, S.J.; Lee, J.H. The relationship between initial implant stability quotient values and bone-to-implant contact ratio in the rabbit tibia. J. Adv. Prosthodont. 2011, 3, 76–80. [Google Scholar] [CrossRef]
- Atsumi, M.; Park, S.H.; Wang, H.L. Methods used to assess implant stability: Current status. Int. J. Oral Maxillofac. Implants 2007, 22, 743–754. [Google Scholar]
- El-Kholey, K.E. Efficacy and safety of a diode laser in second-stage implant surgery: A comparative study. Int. J. Oral Maxillofac. Surg. 2014, 43, 633–638. [Google Scholar] [CrossRef] [PubMed]



| Site | BV/TV Ratio (%) | t | p * | |||
|---|---|---|---|---|---|---|
| Control Group | Experimental Group | |||||
| Mean | SD | Mean | SD | |||
| Proximal | 2.85 | 0.34 | 3.14 | 0.16 | −1.766 | 0.115 |
| Distal | 2.85 | 0.40 | 2.95 | 0.49 | −0.351 | 0.734 |
| Total | 2.85 | 0.35 | 3.05 | 0.36 | −1.243 | 0.230 |
| Site | BIC Ratio (%) | t | p * | |||
|---|---|---|---|---|---|---|
| Control Group | Experimental Group | |||||
| Mean | SD | Mean | SD | |||
| Proximal | 52.34 | 8.33 | 51.32 | 6.54 | 0.214 | 0.836 |
| Distal | 45.22 | 5.39 | 38.05 | 8.13 | 1.644 | 0.144 |
| Total | 48.78 | 7.61 | 44.69 | 9.87 | 1.039 | 0.312 |
| Site (Side of Rabbit Tibia) | BIC Distance (µm) | t | p * | |||
|---|---|---|---|---|---|---|
| Control Group | Experimental Group | |||||
| Mean | SD | Mean | SD | |||
| Proximal | 94.54 | 16.26 | 99.28 | 7.09 | −0.598 | 0.574 |
| Distal | 110.28 | 10.03 | 117.68 | 11.59 | −1.08 | 0.312 |
| Total | 102.41 | 15.20 | 108.48 | 13.27 | −0.951 | 0.354 |
| Group | ISQ Value (Mean ± SD) | t | p * | |
|---|---|---|---|---|
| Immediately after Surgery | 1 Month after Surgery | |||
| Experimental group | 70.1 ± 10.4 | 79.9 ± 4.9 | −9.527 | <0.001 * |
| Control group | 65.2 ± 11.6 | 79.0 ± 4.5 | −7.018 | <0.001 * |
| t | −2.458 | −1.018 | ||
| p * | 0.015 ** | 0.311 ** | ||
| Site (Side of Rabbit Tibia) | BIC Ratio (%) | t | p * | |||
|---|---|---|---|---|---|---|
| Control Group | Experimental Group | |||||
| Mean | SD | Mean | SD | |||
| Proximal | 53.90 | 0.00 | ||||
| Distal | 61.64 | 14.33 | 55.52 | 8.33 | −0.55 | 0.656 |
| Total | 59.06 | 11.07 | 55.52 | 8.33 | −0.44 | 0.682 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-H.; Son, K.; Son, Y.-T.; Kim, Y.-G.; Suh, J.-Y.; Lee, K.-B.; Lee, J.-M. Comparison of Osseointegration of Dental Implants Placed in Rabbit Tibia Using Two Dental Laser and Implant Handpiece Systems. Bioengineering 2022, 9, 681. https://doi.org/10.3390/bioengineering9110681
Park J-H, Son K, Son Y-T, Kim Y-G, Suh J-Y, Lee K-B, Lee J-M. Comparison of Osseointegration of Dental Implants Placed in Rabbit Tibia Using Two Dental Laser and Implant Handpiece Systems. Bioengineering. 2022; 9(11):681. https://doi.org/10.3390/bioengineering9110681
Chicago/Turabian StylePark, Jin-Han, Keunbada Son, Young-Tak Son, Yong-Gun Kim, Jo-Young Suh, Kyu-Bok Lee, and Jae-Mok Lee. 2022. "Comparison of Osseointegration of Dental Implants Placed in Rabbit Tibia Using Two Dental Laser and Implant Handpiece Systems" Bioengineering 9, no. 11: 681. https://doi.org/10.3390/bioengineering9110681
APA StylePark, J.-H., Son, K., Son, Y.-T., Kim, Y.-G., Suh, J.-Y., Lee, K.-B., & Lee, J.-M. (2022). Comparison of Osseointegration of Dental Implants Placed in Rabbit Tibia Using Two Dental Laser and Implant Handpiece Systems. Bioengineering, 9(11), 681. https://doi.org/10.3390/bioengineering9110681








