Towards a New Concept of Regenerative Endodontics Based on Mesenchymal Stem Cell-Derived Secretomes Products
Abstract
1. Introduction
2. Dental Pulp Tissue Engineering
3. MSC of the Oral Cavity
Postnatal Dental Pulp Stem Cells
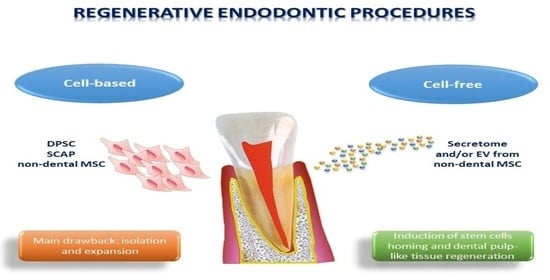
4. Types of Regenerative Endodontics Procedures (REP)
4.1. Cell-Based REP
Limitations of the Cell-Based REP
4.2. Cell-Free REP
4.2.1. Cell-Free REP Based on Blood Derived Products, Bioactive Molecules or Bioingenniering Materials
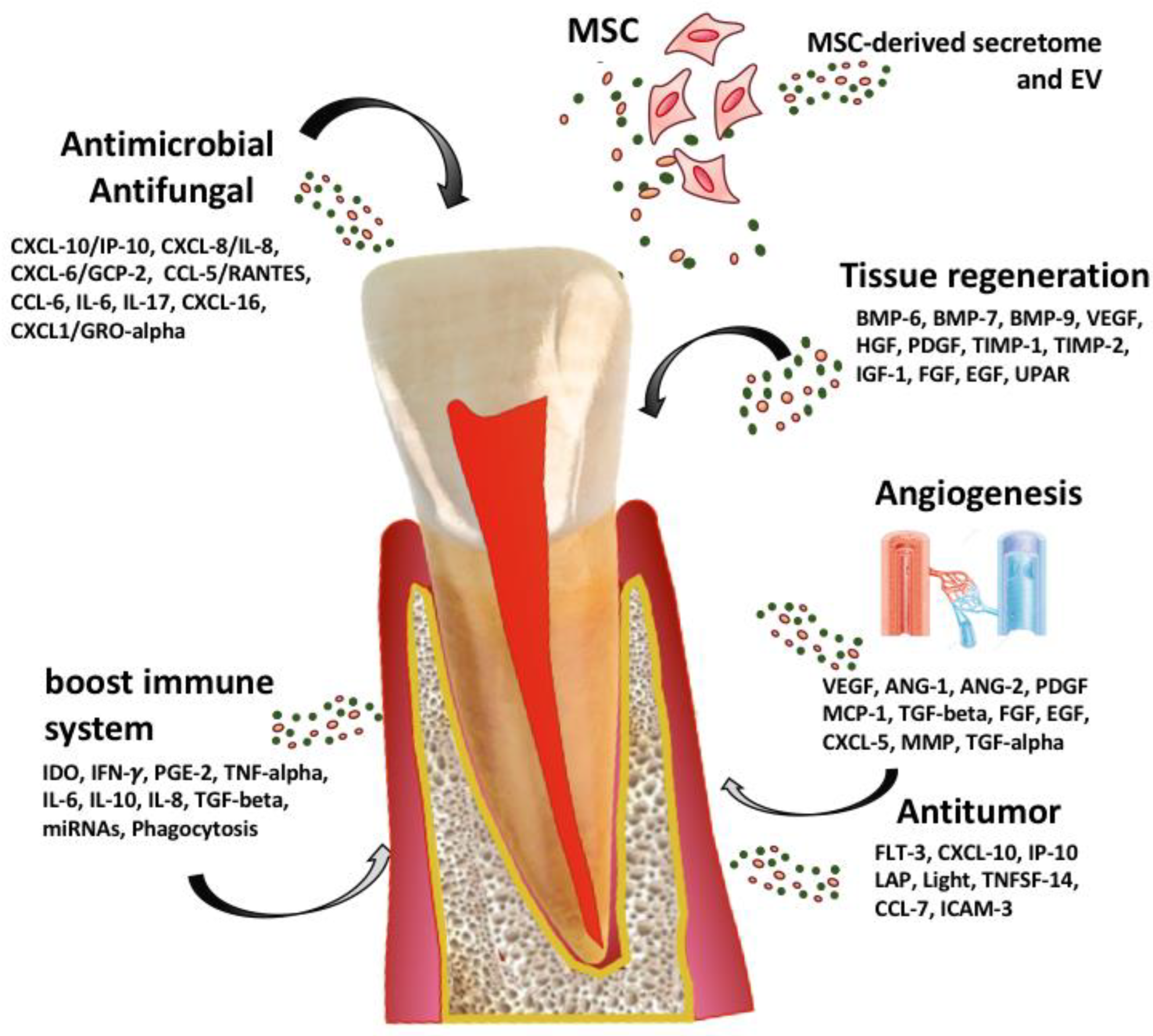
4.2.2. A New Concept of Regenerative Endodontics Based on Secretome-Derived Products from Mesenchymal Stem Cells
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kailembo, A.; Preet, R.; Stewart Williams, J. Common risk factors and edentulism in adults, aged 50 years and over, in China, Ghana, India and South Africa: Results from the WHO Study on global AGEing and adult health (SAGE). BMC Oral Health 2016, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Nitschke, I.; Stark, H.; Micheelis, W.; Jordan, R.A. Epidemiological trends, predictive factors, and projection of tooth loss in Germany 1997–2030: Part II. Edentulism in seniors. Clin. Oral Investig. 2020, 24, 3997–4003. [Google Scholar] [CrossRef] [PubMed]
- Journal of Oral Implantology. AAID: American Academy of Implant Dentistry. Available online: https://www.aaid.com/news_and_publications/Journal_of_Implantology.html (accessed on 10 October 2022).
- Azimi, E.; Song, T.; Yang, C.; Dianat, O. Endodontic Guided Treatment Using Augmented Reality on a Head-Mounted Display System. Healthc. Technol. Lett. 2018, 5, 201–207. [Google Scholar] [CrossRef]
- Schmalz, G.; Widbiller, M.; Galler, K.M. Clinical Perspectives of Pulp Regeneration. J. Endod. 2020, 46, S161–S174. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Oshima, M.; Mizuno, M.; Imamura, A.; Ogawa, M.; Yasukawa, M.; Yamazaki, H.; Morita, R.; Ikeda, E.; Nakao, K.; Takano-Yamamoto, T.; et al. Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS ONE 2011, 6, e21531. [Google Scholar] [CrossRef]
- Hashemi-Beni, B.; Khoroushi, M.; Foroughi, M.R.; Karbasi, S.; Khademi, A.A. Tissue engineering: Dentin-pulp complex regeneration approaches (A review). Tissue Cell 2017, 49, 552–564. [Google Scholar] [CrossRef]
- Galler, K.M.; Brandl, F.P.; Kirchhof, S.; Widbiller, M.; Eidt, A.; Buchalla, W.; Göpferich, A.; Schmalz, G. Suitability of Different Natural and Synthetic Biomaterials for Dental Pulp Tissue Engineering. Tissue Eng. Part A 2018, 24, 234–244. [Google Scholar] [CrossRef]
- Fukushima, K.A.; Marques, M.M.; Tedesco, T.K.; Carvalho, G.L.; Gonçalves, F.; Caballero-Flores, H.; Morimoto, S.; Moreira, M.S. Screening of hydrogel-based scaffolds for dental pulp regeneration—A systematic review. Arch. Oral Biol. 2019, 98, 182–194. [Google Scholar] [CrossRef]
- Bottino, M.C.; Pankajakshan, D.; Nör, J.E. Advanced Scaffolds for Dental Pulp and Periodontal Regeneration. Dent. Clin. N. Am. 2017, 61, 689–711. [Google Scholar] [CrossRef]
- Bakhtiar, H.; Pezeshki-Modaress, M.; Kiaipour, Z.; Shafiee, M.; Ellini, M.R.; Mazidi, A.; Rajabi, S.; Zamanlui Benisi, S.; Ostad, S.N.; Galler, K.; et al. Pulp ECM-derived macroporous scaffolds for stimulation of dental-pulp regeneration process. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2020, 36, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Piva, E.; Silva, A.F.; Nör, J.E. Functionalized scaffolds to control dental pulp stem cell fate. J. Endod. 2014, 40, S33–S40. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Deng, F.; Wang, W.; Li, A.; Lu, C.; Chen, H.; Wu, G.; Nan, K.; Li, L. Construction of Injectable Self-Healing Macroporous Hydrogels via a Template-Free Method for Tissue Engineering and Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 36721–36732. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, B.N.; Zeitlin, B.D.; Nör, J.E. A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2013, 29, 97–102. [Google Scholar] [CrossRef]
- Katata, C.; Sasaki, J.I.; Li, A.; Abe, G.L.; Nör, J.E.; Hayashi, M.; Imazato, S. Fabrication of Vascularized DPSC Constructs for Efficient Pulp Regeneration. J. Dent. Res. 2021, 100, 1351–1358. [Google Scholar] [CrossRef]
- da Costa Sousa, M.G.; de Almeida, G.C.; Martins Mota, D.C.; da Costa, R.A.; Dias, S.C.; Limberger, S.N.; Ko, F.; Lin, L.T.; Haney, E.F.; Etayash, H.; et al. Antibiofilm and immunomodulatory resorbable nanofibrous filing for dental pulp regenerative procedures. Bioact. Mater. 2022, 16, 173–186. [Google Scholar] [CrossRef]
- Gronthos, S.; Akintoye, S.O.; Wang, C.Y.; Shi, S. Bone marrow stromal stem cells for tissue engineering. Periodontol. 2000 2006, 41, 188–195. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef]
- Morsczeck, C.; Götz, W.; Schierholz, J.; Zeilhofer, F.; Kühn, U.; Möhl, C.; Sippel, C.; Hoffmann, K.H. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. J. Int. Soc. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Mitrano, T.I.; Grob, M.S.; Carrión, F.; Nova-Lamperti, E.; Luz, P.A.; Fierro, F.S.; Quintero, A.; Chaparro, A.; Sanz, A. Culture and characterization of mesenchymal stem cells from human gingival tissue. J. Periodontol. 2010, 81, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Farré-Guasch, E.; Martí-Pagès, C.; Hernández-Alfaro, F.; Klein-Nulend, J.; Casals, N. Buccal Fat Pad, an Oral Access Source of Human Adipose Stem Cells with Potential for Osteochondral Tissue Engineering: An In Vitro Study. Tissue Eng. Part C Methods 2010, 16, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Liu, Y.; Cui, D.; Pan, Y.; Zheng, L.; Wan, M. Dental Tissue-Derived Human Mesenchymal Stem Cells and Their Potential in Therapeutic Application. Stem Cells Int. 2020, 2020, 8864572. [Google Scholar] [CrossRef]
- Shi, S.; Gronthos, S. Perivascular Niche of Postnatal Mesenchymal Stem Cells in Human Bone Marrow and Dental Pulp. J. Bone Miner. Res. 2003, 18, 696–704. [Google Scholar] [CrossRef]
- Feng, J.; Mantesso, A.; Bari, C.D.; Nishiyama, A.; Sharpe, P.T. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc. Natl. Acad. Sci. USA 2011, 108, 6503–6508. [Google Scholar] [CrossRef]
- Iohara, K.; Zheng, L.; Ito, M.; Ishizaka, R.; Nakamura, H.; Into, T.; Matsushita, K.; Nakashima, M. Regeneration of dental pulp after pulpotomy by transplantation of CD31-/CD146- side population cells from a canine tooth. Regen. Med. 2009, 4, 377–385. [Google Scholar] [CrossRef]
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: A pilot clinical study. Stem Cell Res. Ther. 2017, 8, 61. [Google Scholar] [CrossRef]
- Vendramini, V.O.; Pouraghaei, S.; Barbosa, R.M.; Aloise, A.C.; Muniz, J.R.F.; Sperandio, M.; Moy, P.K.; Pelegrine, A.A.; Moshaverinia, A. Influence of Dental Pulp Harvesting Method on the Viability and Differentiation Capacity of Adult Dental Pulp-Derived Mesenchymal Stem Cells. Stem Cells Int. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Huang, G.T.J.; Yamaza, T.; Shea, L.D.; Djouad, F.; Kuhn, N.Z.; Tuan, R.S.; Shi, S. Stem/Progenitor Cell–Mediated De Novo Regeneration of Dental Pulp with Newly Deposited Continuous Layer of Dentin in an In Vivo Model. Tissue Eng. Part A 2010, 16, 605–615. [Google Scholar] [CrossRef]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.J.; Yamaza, T.; Song, Y.; Fouad, A.F.; Romberg, E.E.; Shi, S.; Tuan, R.S.; Huang, G.T. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen. Med. 2010, 5, 617–631. [Google Scholar] [CrossRef]
- Yazid, F.B.; Gnanasegaran, N.; Kunasekaran, W.; Govindasamy, V.; Musa, S. Comparison of immunodulatory properties of dental pulp stem cells derived from healthy and inflamed teeth. Clin. Oral Investig. 2014, 18, 2103–2112. [Google Scholar] [CrossRef]
- Iohara, K.; Murakami, M.; Nakata, K.; Nakashima, M. Age-dependent decline in dental pulp regeneration after pulpectomy in dogs. Exp. Gerontol. 2014, 52, 39–45. [Google Scholar] [CrossRef]
- Carvalho, T.S.; Lussi, A. Age-related morphological, histological and functional changes in teeth. J. Oral Rehabil. 2017, 44, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yelick, P.C. Tooth Repair and Regeneration: Potential of Dental Stem Cells. Trends Mol. Med. 2021, 27, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Yamamoto, A.; Matsubara, K.; Nakamura, S.; Naruse, M.; Yamagata, M.; Sakamoto, K.; Tauchi, R.; Wakao, N.; Imagama, S.; et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J. Clin. Investig. 2012, 122, 80–90. [Google Scholar] [CrossRef]
- Kawashima, N. Characterisation of dental pulp stem cells: A new horizon for tissue regeneration? Arch. Oral Biol. 2012, 57, 1439–1458. [Google Scholar] [CrossRef]
- Bhandi, S.; Alkahtani, A.; Mashyakhy, M.; Abumelha, A.S.; Albar, N.H.M.; Renugalakshmi, A.; Alkahtany, M.F.; Robaian, A.; Almeslet, A.S.; Patil, V.R.; et al. Effect of Ascorbic Acid on Differentiation, Secretome and Stemness of Stem Cells from Human Exfoliated Deciduous Tooth (SHEDs). J. Pers. Med. 2021, 11, 589. [Google Scholar] [CrossRef]
- Smith, A.J.; Tobias, R.S.; Cassidy, N.; Bégue-Kirn, C.; Ruch, J.V.; Lesot, H. Influence of Substrate Nature and Immobilization of Implanted Dentin Matrix Components During Induction of Reparative Dentinogenesis. Null 1995, 32, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Achilleos, A.; Trainor, P.A. Neural crest stem cells: Discovery, properties and potential for therapy. Cell Res. 2012, 22, 288–304. [Google Scholar] [CrossRef]
- Jung, J.; Kim, J.W.; Moon, H.J.; Hong, J.Y.; Hyun, J.K. Characterization of Neurogenic Potential of Dental Pulp Stem Cells Cultured in Xeno/Serum-Free Condition: In Vitro and In Vivo Assessment. Stem Cells Int. 2016, 2016, 6921097. [Google Scholar] [CrossRef] [PubMed]
- Kawase-Koga, Y.; Fujii, Y.; Yamakawa, D.; Sato, M.; Chikazu, D. Identification of neurospheres generated from human dental pulp stem cells in xeno-/serum-free conditions. Regen. Ther. 2020, 14, 128–135. [Google Scholar] [CrossRef]
- Bhandi, S.; Alkahtani, A.; Reda, R.; Mashyakhy, M.; Boreak, N.; Maganur, P.C.; Vishwanathaiah, S.; Mehta, D.; Vyas, N.; Patil, V.; et al. Parathyroid Hormone Secretion and Receptor Expression Determine the Age-Related Degree of Osteogenic Differentiation in Dental Pulp Stem Cells. J. Pers. Med. 2021, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Morad, G.; Kheiri, L.; Khojasteh, A. Dental pulp stem cells for in vivo bone regeneration: A systematic review of literature. Arch. Oral Biol. 2013, 58, 1818–1827. [Google Scholar] [CrossRef]
- Yamaza, T.; Sonoda, S.; Tomoda, E.; Tanaka, Y. Properties and Possibilities of Human Dental Pulp-Derived Stem Cells. Arch. Stem Cell Res. 2015, 2, 1012. [Google Scholar]
- Yildirim, S.; Zibandeh, N.; Genc, D.; Ozcan, E.M.; Goker, K.; Akkoc, T. The Comparison of the Immunologic Properties of Stem Cells Isolated from Human Exfoliated Deciduous Teeth, Dental Pulp, and Dental Follicles. Stem Cells Int. 2016, 2016, 4682875. [Google Scholar] [CrossRef]
- Özdemir, A.T.; Özgül Özdemir, R.B.; Kırmaz, C.; Sarıboyacı, A.E.; Ünal Halbutoğlları, Z.S.; Özel, C.; Karaöz, E. The paracrine immunomodulatory interactions between the human dental pulp derived mesenchymal stem cells and CD4 T cell subsets. Cell Immunol. 2016, 310, 108–115. [Google Scholar] [CrossRef]
- Wang, X.; Sha, X.-J.; Li, G.-H.; Yang, F.-S.; Ji, K.; Wen, L.-Y.; Liu, S.-Y.; Chen, L.; Ding, Y.; Xuan, K. Comparative characterization of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. Arch. Oral Biol. 2012, 57, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, C.; Meza, G.; Urrejola, D.; Quezada, M.A.; Concha, G.; Ramírez, V.; Angelopoulos, I.; Cadiz, M.I.; Tapia-Limonchi, R.; Khoury, M. Cell-Based Regenerative Endodontics for Treatment of Periapical Lesions: A Randomized, Controlled Phase I/II Clinical Trial. J. Dent. Res. 2020, 99, 523–529. [Google Scholar] [CrossRef]
- Rosa, V.; Sriram, G. A critical analysis of research methods and biological experimental models to study pulp regeneration. Int. Endod. J. 2022, 55 (Suppl. S2), 446–455. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Codispoti, B. Strategic Tools in Regenerative and Translational Dentistry. Int. J. Mol. Sci. 2019, 20, 1879. [Google Scholar] [CrossRef] [PubMed]
- Aimetti, M.; Ferrarotti, F.; Gamba, M.N.; Giraudi, M.; Romano, F. Regenerative Treatment of Periodontal Intrabony Defects Using Autologous Dental Pulp Stem Cells: A 1-Year Follow-Up Case Series. Int. J. Periodontics Restor. Dent. 2018, 38, 51–58. [Google Scholar] [CrossRef]
- Hernández-Monjaraz, B.; Santiago-Osorio, E.; Ledesma-Martínez, E.; Alcauter-Zavala, A.; Mendoza-Núñez, V.M. Retrieval of a periodontally compromised tooth by allogeneic grafting of mesenchymal stem cells from dental pulp: A case report. J. Int. Med. Res. 2018, 46, 2983–2993. [Google Scholar] [CrossRef]
- Anitua, E.; Troya, M.; Zalduendo, M. Progress in the use of dental pulp stem cells in regenerative medicine. Cytotherapy 2018, 20, 479–498. [Google Scholar] [CrossRef]
- da Silva, G.S.; Moreira, M.S.; Fukushima, K.A.; Raggio, D.P.; V Mello-Moura, A.C.; Lara, J.S.; Gimenez, T.; Junior, S.A.; Morimoto, S.; Tedesco, T.K. Current evidence of tissue engineering for dentine regeneration in animal models: A systematic review. Regen. Med. 2020, 15, 1345–1360. [Google Scholar] [CrossRef]
- Iohara, K.; Imabayashi, K.; Ishizaka, R.; Watanabe, A.; Nabekura, J.; Ito, M.; Matsushita, K.; Nakamura, H.; Nakashima, M. Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng. Part A 2011, 17, 1911–1920. [Google Scholar] [CrossRef]
- Rosa, V.; Zhang, Z.; Grande, R.H.; Nör, J.E. Dental pulp tissue engineering in full-length human root canals. J. Dent. Res. 2013, 92, 970–975. [Google Scholar] [CrossRef]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.; Iohara, K.; Watanabe, H.; Ishikawa, M.; Tominaga, M.; Nakashima, M. Characterization of stable hypoxia-preconditioned dental pulp stem cells compared with mobilized dental pulp stem cells for application for pulp regenerative therapy. Stem Cell Res. Ther. 2021, 12, 302. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Nakamura-Yamada, S.; Konoki, R.; Baba, S. Promising advances in clinical trials of dental tissue-derived cell-based regenerative medicine. Stem Cell Res. Ther. 2020, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, W.; Li, Y.; Ren, L.; Deng, H.; Yin, X.; Gao, X.; Pan, S.; Niu, Y. Human Umbilical Cord Mesenchymal Stem Cell Differentiation Into Odontoblast-Like Cells and Endothelial Cells: A Potential Cell Source for Dental Pulp Tissue Engineering. Front. Physiol. 2020, 11, 593. [Google Scholar] [CrossRef] [PubMed]
- Graziano, A.; d’Aquino, R.; Laino, G.; Papaccio, G. Dental pulp stem cells: A promising tool for bone regeneration. Stem Cell Rev. 2008, 4, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Tang, L.; Jin, F.; Liu, X.H.; Yu, J.H.; Wu, J.J.; Yang, Z.H.; Wang, Y.X.; Duan, Y.Z.; Jin, Y. The apical region of developing tooth root constitutes a complex and maintains the ability to generate root and periodontium-like tissues. J. Periodontal. Res. 2009, 44, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kidwai, F.K.; Movahednia, M.M.; Iqbal, K.; Jokhun, D.S.; Cao, T.; Fawzy, A.S. Human embryonic stem cell differentiation into odontoblastic lineage: An in vitro study. Int. Endod. J. 2014, 47, 346–355. [Google Scholar] [CrossRef]
- Xie, H.; Dubey, N.; Shim, W.; Ramachandra, C.J.A.; Min, K.S.; Cao, T.; Rosa, V. Functional Odontoblastic-Like Cells Derived from Human iPSCs. J. Dent. Res. 2018, 97, 77–83. [Google Scholar] [CrossRef]
- Ozeki, N.; Mogi, M.; Kawai, R.; Yamaguchi, H.; Hiyama, T.; Nakata, K.; Nakamura, H. Mouse-induced pluripotent stem cells differentiate into odontoblast-like cells with induction of altered adhesive and migratory phenotype of integrin. PLoS ONE 2013, 8, e80026. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Hirono, H.; Nakahara, T.; Ishikawa, H. Dental pulp cell bank as a possible future source of individual hepatocytes. World J. Hepatol. 2018, 10, 702–707. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed]
- Müller-Ehmsen, J.; Whittaker, P.; Kloner, R.A.; Dow, J.S.; Sakoda, T.; Long, T.I.; Laird, P.W.; Kedes, L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J. Mol. Cell Cardiol. 2002, 34, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.; Wagner, W.R.; Bowry, S.; Schwartz, A.; Villanueva, F. Fate Of Culture-Expanded Mesenchymal Stem Cells in The Microvasculature. Circ. Res. 2009, 104, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Ide, C.; Nakai, Y.; Nakano, N.; Seo, T.-B.; Yamada, Y.; Endo, K.; Noda, T.; Saito, F.; Suzuki, Y.; Fukushima, M.; et al. Bone marrow stromal cell transplantation for treatment of sub-acute spinal cord injury in the rat. Brain Res. 2010, 1332, 32–47. [Google Scholar] [CrossRef]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs Improve Myocardial Infarction in Mice because Cells Embolized in Lung Are Activated to Secrete the Anti-inflammatory Protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef]
- Gao, X.; Shen, Z.; Guan, M.; Huang, Q.; Chen, L.; Qin, W.; Ge, X.; Chen, H.; Xiao, Y.; Lin, Z. Immunomodulatory Role of Stem Cells from Human Exfoliated Deciduous Teeth on Periodontal Regeneration. Tissue Eng. Part A 2018, 24, 1341–1353. [Google Scholar] [CrossRef]
- Prockop, D.J. The Exciting Prospects of New Therapies with Mesenchymal Stromal Cells. Cytotherapy 2017, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ostby, B.N. The role of the blood clot in endodontic therapy. An experimental histologic study. Acta Odontol. Scand. 1961, 19, 324–353. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, T.W.; Henry, M.A.; Hargreaves, K.M.; Diogenes, A. Evaluation of the Delivery of Mesenchymal Stem Cells into the Root Canal Space of Necrotic Immature Teeth after Clinical Regenerative Endodontic Procedure. J. Endod. 2011, 37, 133–138. [Google Scholar] [CrossRef]
- Torabinejad, M.; Turman, M. Revitalization of Tooth with Necrotic Pulp and Open Apex by Using Platelet-rich Plasma: A Case Report. J. Endod. 2011, 37, 265–268. [Google Scholar] [CrossRef]
- Shimizu, E.; Ricucci, D.; Albert, J.; Alobaid, A.S.; Gibbs, J.L.; Huang, G.T.J.; Lin, L.M. Clinical, Radiographic, and Histological Observation of a Human Immature Permanent Tooth with Chronic Apical Abscess after Revitalization Treatment. J. Endod. 2013, 39, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Sedgley, C.M.; Cherkas, P.; Chogle, S.M.A.; Geisler, T.M.; Hargreaves, K.M.; Paranjpe, A.K.; Yamagishi, V.T.-K. Endodontics: Colleagues for Excellence Newsletter. Available online: https://www.aae.org/specialty/newsletter/regenerative-endodontics/ (accessed on 1 November 2022).
- Banchs, F.; Trope, M. Revascularization of immature permanent teeth with apical periodontitis: New treatment protocol? J. Endod. 2004, 30, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative endodontics: A review of current status and a call for action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhong, J.; Gong, Q.; Cheng, B.; Kim, S.G.; Ling, J.; Mao, J.J. Regenerative Endodontics by Cell Homing. Dent. Clin. N. Am. 2017, 61, 143–159. [Google Scholar] [CrossRef]
- Yin, Y.; Li, X.; He, X.T.; Wu, R.X.; Sun, H.H.; Chen, F.M. Leveraging Stem Cell Homing for Therapeutic Regeneration. J. Dent. Res. 2017, 96, 601–609. [Google Scholar] [CrossRef]
- Kim, J.Y.; Xin, X.; Moioli, E.K.; Chung, J.; Lee, C.H.; Chen, M.; Fu, S.Y.; Koch, P.D.; Mao, J.J. Regeneration of Dental-Pulp-like Tissue by Chemotaxis-Induced Cell Homing. Tissue Eng. Part A 2010, 16, 3023–3031. [Google Scholar] [CrossRef]
- Nosrat, A.; Kolahdouzan, A.; Hosseini, F.; Mehrizi, E.A.; Verma, P.; Torabinejad, M. Histologic Outcomes of Uninfected Human Immature Teeth Treated with Regenerative Endodontics: 2 Case Reports. J. Endod. 2015, 41, 1725–1729. [Google Scholar] [CrossRef]
- Becerra, P.; Ricucci, D.; Loghin, S.; Gibbs, J.L.; Lin, L.M. Histologic Study of a Human Immature Permanent Premolar with Chronic Apical Abscess after Revascularization/Revitalization. J. Endod. 2014, 40, 133–139. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review—Part III: Clinical Applications, Drawbacks, and Mechanism of Action. J. Endod. 2010, 36, 400–413. [Google Scholar] [CrossRef]
- Xie, H.; Chua, M.; Islam, I.; Bentini, R.; Cao, T.; Viana-Gomes, J.C.; Castro Neto, A.H.; Rosa, V. CVD-grown monolayer graphene induces osteogenic but not odontoblastic differentiation of dental pulp stem cells. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2017, 33, e13–e21. [Google Scholar] [CrossRef]
- Herford, A.S.; Boyne, P.J. Reconstruction of mandibular continuity defects with bone morphogenetic protein-2 (rhBMP-2). J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2008, 66, 616–624. [Google Scholar] [CrossRef]
- Casagrande, L.; Demarco, F.F.; Zhang, Z.; Araujo, F.B.; Shi, S.; Nör, J.E. Dentin-derived BMP-2 and odontoblast differentiation. J. Dent. Res. 2010, 89, 603–608. [Google Scholar] [CrossRef]
- Woo, E.J. Adverse events reported after the use of recombinant human bone morphogenetic protein 2. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2012, 70, 765–767. [Google Scholar] [CrossRef]
- Perri, B.; Cooper, M.; Lauryssen, C.; Anand, N. Adverse swelling associated with use of rh-BMP-2 in anterior cervical discectomy and fusion: A case study. Spine J. Off. J. N. Am. Spine Soc. 2007, 7, 235–239. [Google Scholar] [CrossRef]
- Vaidya, R.; Carp, J.; Sethi, A.; Bartol, S.; Craig, J.; Les, C.M. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur. Spine J. 2007, 16, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Aihara, M.; Honmo, J.; Sakurai, S.; Fujimaki, Y.; Sakamoto, K.; Fujimaki, E.; Wozney, J.M.; Yamaguchi, A. Effects of recombinant human bone morphogenetic protein-2 on differentiation of cells isolated from human bone, muscle, and skin. Bone 1998, 23, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, A.T.; Turedi, I.; Cimen, M.; Cehreli, Z.C. Evaluation of Blood Clot, Platelet-rich Plasma, Platelet-rich Fibrin, and Platelet Pellet as Scaffolds in Regenerative Endodontic Treatment: A Prospective Randomized Trial. J. Endod. 2019, 45, 560–566. [Google Scholar] [CrossRef] [PubMed]
- De Jong, O.G.; Van Balkom, B.W.; Schiffelers, R.M.; Bouten, C.V.; Verhaar, M.C. Extracellular vesicles: Potential roles in regenerative medicine. Front. Immunol. 2014, 5, 608. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.G.; Salgado, A.J. Mesenchymal stem cells secretome: Current trends and future challenges. Neural. Regen. Res. 2019, 15, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, W.; Osugi, M.; Kawai, T.; Hibi, H. First-in-human study and clinical case reports of the alveolar bone regeneration with the secretome from human mesenchymal stem cells. Head Face Med. 2016, 12, 5. [Google Scholar] [CrossRef]
- Lin, H.; Chen, H.; Zhao, X.; Chen, Z.; Zhang, P.; Tian, Y.; Wang, Y.; Ding, T.; Wang, L.; Shen, Y. Advances in mesenchymal stem cell conditioned medium-mediated periodontal tissue regeneration. J. Transl. Med. 2021, 19, 456. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, W.; Osugi, M.; Kawai, T.; Ueda, M. Novel cell-free regeneration of bone using stem cell-derived growth factors. Int. J. Oral Maxillofac. Implant. 2013, 28, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned Media from Mesenchymal Stem Cells Enhanced Bone Regeneration in Rat Calvarial Bone Defects. Tissue Eng. Part A 2012, 18, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Inukai, T.; Katagiri, W.; Yoshimi, R.; Osugi, M.; Kawai, T.; Hibi, H.; Ueda, M. Novel application of stem cell-derived factors for periodontal regeneration. Biochem. Biophys. Res. Commun. 2013, 430, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Katagiri, W.; Osugi, M.; Sugimura, Y.; Hibi, H.; Ueda, M. Secretomes from bone marrow-derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy 2015, 17, 369–381. [Google Scholar] [CrossRef]
- Cornish, J.; Grey, A.; Callon, K.E.; Naot, D.; Hill, B.L.; Lin, C.Q.X.; Balchin, L.M.; Reid, I.R. Shared pathways of osteoblast mitogenesis induced by amylin, adrenomedullin, and IGF-1. Biochem. Biophys. Res. Commun. 2004, 318, 240–246. [Google Scholar] [CrossRef]
- Li, Y.; Yu, X.; Lin, S.; Li, X.; Zhang, S.; Song, Y.-H. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2007, 356, 780–784. [Google Scholar] [CrossRef]
- Han, X.; Amar, S. Role of insulin-like growth factor-1 signaling in dental fibroblast apoptosis. J. Periodontol. 2003, 74, 1176–1182. [Google Scholar] [CrossRef]
- Kaigler, D.; Krebsbach, P.H.; West, E.R.; Horger, K.; Huang, Y.C.; Mooney, D.J. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005, 19, 665–667. [Google Scholar] [CrossRef]
- Morishita, R.; Nakamura, S.; Hayashi, S.; Taniyama, Y.; Moriguchi, A.; Nagano, T.; Taiji, M.; Noguchi, H.; Takeshita, S.; Matsumoto, K.; et al. Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy. Hypertension 1999, 33, 1379–1384. [Google Scholar] [CrossRef]
- Bostrom, M.P.; Asnis, P. Transforming growth factor beta in fracture repair. Clin. Orthop. Relat. Res. 1998, 355S, S124–S131. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Symons, A.L.; Bartold, P.M. Expression of transforming growth factor-beta 1 (TGF-beta1) in the developing periodontium of rats. J. Dent. Res. 1998, 77, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Nishimura, M.; Sekiya, K.; Suehiro, F.; Kanawa, M.; Nikawa, H.; Hamada, T.; Kato, Y. Comprehensive Analysis of Chemotactic Factors for Bone Marrow Mesenchymal Stem Cells. Stem Cells Dev. 2007, 16, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, W.; Huang, J.; He, B.-C.; Zuo, G.-W.; Zhang, W.; Luo, Q.; Shi, Q.; Zhang, B.-Q.; Wagner, E.R.; et al. Insulin-like Growth Factor 2 (IGF-2) Potentiates BMP-9-Induced Osteogenic Differentiation and Bone Formation. J. Bone Miner. Res. 2010, 25, 2447–2459. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, W.L.; Zhan, X.; Zhang, C.; Hargreaves, K.M.; Jin, L.; Tong, E.H. Coculture of dental pulp stem cells with endothelial cells enhances osteo-/odontogenic and angiogenic potential in vitro. J. Endod. 2012, 38, 454–463. [Google Scholar] [CrossRef]
- Venugopal, C.; Shobha, K.; Rai, K.S.; Dhanushkodi, A. Neurogenic and cognitive enhancing effects of human dental pulp stem cells and its secretome in animal model of hippocampal neurodegeneration. Brain Res. Bull. 2022, 180, 46–58. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, V.; Rattan, V.; Jha, V.; Bhattacharyya, S. Secretome Cues Modulate the Neurogenic Potential of Bone Marrow and Dental Stem Cells. Mol. Neurobiol. 2017, 54, 4672–4682. [Google Scholar] [CrossRef]
- Huang, C.-C.; Narayanan, R.; Alapati, S.; Ravindran, S. Exosomes as Biomimetic Tools for Stem Cell Differentiation: Applications in Dental Pulp Tissue Regeneration. Biomaterials 2016, 111, 103–115. [Google Scholar] [CrossRef]
- Liu, H.; Li, D.; Zhang, Y.; Li, M. Inflammation, mesenchymal stem cells and bone regeneration. Histochem. Cell Biol. 2018, 149, 393–404. [Google Scholar] [CrossRef]
- Li, W.; Ren, G.; Huang, Y.; Su, J.; Han, Y.; Li, J.; Chen, X.; Cao, K.; Chen, Q.; Shou, P.; et al. Mesenchymal stem cells: A double-edged sword in regulating immune responses. Cell Death Differ. 2012, 19, 1505–1513. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, J.-y.; Zhou, G. Emerging functions and clinical applications of exosomes in human oral diseases. Cell Biosci. 2020, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Dong, C.; Yang, J.; Jin, Y.; Zheng, W.; Zhou, Q.; Liang, Y.; Bao, L.; Feng, G.; Ji, J.; et al. Exosomal microRNA-155-5p from PDLSCs regulated Th17/Treg balance by targeting sirtuin-1 in chronic periodontitis. J. Cell Physiol. 2019, 234, 20662–20674. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Dai, W.; Wang, H.; Xue, C.; Feng, J.; He, Y.; Wang, P.; Li, S.; Bai, D.; Shu, R. Periodontal ligament fibroblasts regulate osteoblasts by exosome secretion induced by inflammatory stimuli. Arch. Oral Biol. 2019, 105, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Maruyama, K.; Sakisaka, Y.; Suzuki, S.; Tada, H.; Suto, M.; Saito, M.; Yamada, S.; Nemoto, E. Cyclic Stretch Force Induces Periodontal Ligament Cells to Secrete Exosomes That Suppress IL-1β Production Through the Inhibition of the NF-κB Signaling Pathway in Macrophages. Front. Immunol. 2019, 10, 1310. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Ye, Y.; He, S.; Song, J. SHED-derived conditioned exosomes enhance the osteogenic differentiation of PDLSCs via Wnt and BMP signaling in vitro. Differentiation 2020, 111, 1–11. [Google Scholar] [CrossRef]
- Wakayama, H.; Hashimoto, N.; Matsushita, Y.; Matsubara, K.; Yamamoto, N.; Hasegawa, Y.; Ueda, M.; Yamamoto, A. Factors secreted from dental pulp stem cells show multifaceted benefits for treating acute lung injury in mice. Cytotherapy 2015, 17, 1119–1129. [Google Scholar] [CrossRef]
- Kou, X.; Xu, X.; Chen, C.; Sanmillan, M.L.; Cai, T.; Zhou, Y.; Giraudo, C.; Le, A.; Shi, S. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci. Transl. Med. 2018, 10, eaai8524. [Google Scholar] [CrossRef]
- Shi, H.Z.; Zeng, J.C.; Shi, S.H.; Giannakopoulos, H.; Zhang, Q.Z.; Le, A.D. Extracellular Vesicles of GMSCs Alleviate Aging-Related Cell Senescence. J. Dent. Res. 2021, 100, 283–292. [Google Scholar] [CrossRef]
- Shi, Q.; Qian, Z.; Liu, D.; Sun, J.; Wang, X.; Liu, H.; Xu, J.; Guo, X. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front. Physiol. 2017, 8, 904. [Google Scholar] [CrossRef]
- Koh, B.; Ab Rahman, F.H.; Matlan, N.A.; Rajan, M.; Musta’ain, A.Y.; Mohd Jeffry Lee, M.R.; Ramli, R.; Mohd Yunus, S.S.; Binti Hj Idrus, R.; Yazid, M.D. Potential role of dental pulp stem cells conditioned medium for odontoblastic differentiation. Biol. Res. 2022, 55, 11. [Google Scholar] [CrossRef]
- Sarra, G.; Machado, M.E.d.L.; Caballero-Flores, H.V.; Moreira, M.S.; Pedroni, A.C.F.; Marques, M.M. Effect of human dental pulp stem cell conditioned medium in the dentin-pulp complex regeneration: A pilot in vivo study. Tissue Cell 2021, 72, 101536. [Google Scholar] [CrossRef] [PubMed]
- Xian, X.; Gong, Q.; Li, C.; Guo, B.; Jiang, H. Exosomes with Highly Angiogenic Potential for Possible Use in Pulp Regeneration. J. Endod. 2018, 44, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Chen, H.; Gao, B. Potential Therapeutic Effects of Exosomes in Regenerative Endodontics. Arch. Oral Biol. 2020, 120, 104946. [Google Scholar] [CrossRef] [PubMed]
- Sicari, B.M.; Rubin, J.P.; Dearth, C.L.; Wolf, M.T.; Ambrosio, F.; Boninger, M.; Turner, N.J.; Weber, D.J.; Simpson, T.W.; Wyse, A.; et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci. Transl. Med. 2014, 6, 234ra258. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Zhang, W.; Schiele, N.R.; Khademhosseini, A.; Kuo, C.K.; Yelick, P.C. Developing a biomimetic tooth bud model. J. Tissue Eng. Regen. Med. 2017, 11, 3326–3336. [Google Scholar] [CrossRef]
- Karp, J.M.; Leng Teo, G.S. Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell 2009, 4, 206–216. [Google Scholar] [CrossRef]
- Galler, K.M.; Widbiller, M.; Schmalz, G. Cell-Free Approaches for Dental Pulp Tissue Engineering. J. Endod. 2020, 46, S143–S149. [Google Scholar] [CrossRef]
- Schmalz, G.; Widbiller, M.; Galler, K.M. Signaling Molecules and Pulp Regeneration. J. Endod. 2017, 43, S7–S11. [Google Scholar] [CrossRef]
- Duncan, H.F.; Kobayashi, Y.; Shimizu, E. Growth Factors and Cell Homing in Dental Tissue Regeneration. Curr. Oral Health Rep. 2018, 5, 276–285. [Google Scholar] [CrossRef]
- Widbiller, M.; Driesen, R.B.; Eidt, A.; Lambrichts, I.; Hiller, K.-A.; Buchalla, W.; Schmalz, G.; Galler, K.M. Cell Homing for Pulp Tissue Engineering with Endogenous Dentin Matrix Proteins. J. Endod. 2018, 44, 956–962. [Google Scholar] [CrossRef]
- Song, J.S.; Takimoto, K.; Jeon, M.; Vadakekalam, J.; Ruparel, N.B.; Diogenes, A. Decellularized Human Dental Pulp as a Scaffold for Regenerative Endodontics. J. Dent. Res. 2017, 96, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Alexander, A.; Vahdati, S.A.; Grandhi, A.; Baylink, D.; Shabahang, S. Effect of Residual Dental Pulp Tissue on Regeneration of Dentin-pulp Complex: An In Vivo Investigation. J. Endod. 2018, 44, 1796–1801. [Google Scholar] [CrossRef] [PubMed]
- Koh, B.; Sulaiman, N.; Ismadi, S.N.S.W.; Ramli, R.; Yunus, S.S.M.; Idrus, R.B.H.; Ariffin, S.H.Z.; Wahab, R.M.A.; Yazid, M.D. Mesenchymal stem cells: A comprehensive methods for odontoblastic induction. Biol. Proced. Online 2021, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Schulte, W. Implants and the periodontium. Int. Dent. J. 1995, 45, 16–26. [Google Scholar]
- Ivanovski, S.; Lee, R. Comparison of peri-implant and periodontal marginal soft tissues in health and disease. Periodontol. 2000 2018, 76, 116–130. [Google Scholar] [CrossRef]
- Zhan, C.; Huang, M.; Yang, X.; Hou, J. Dental nerves: A neglected mediator of pulpitis. Int. Endod. J. 2021, 54, 85–99. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, J.; Seidel, K.; Shi, S.; Klein, O.; Sharpe, P.; Chai, Y. Secretion of Shh by a Neurovascular Bundle Niche Supports Mesenchymal Stem Cell Homeostasis in the Adult Mouse Incisor. Cell Stem Cell 2018, 23, 147. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Tsiftsoglou, A.; Garefis, P.; Koidis, P.; Geurtsen, W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch. Oral Biol. 2011, 56, 709–721. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Polymeric Hydrogels as Technology Platform for Drug Delivery Applications. Gels 2017, 3, 25. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.-l.; Kan, C.-w. Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, C.; Huang, G.T.; Cheung, G.S.; Dissanayaka, W.L.; Zhu, W. Transplantation of dental pulp stem cells and platelet-rich plasma for pulp regeneration. J. Endod. 2012, 38, 1604–1609. [Google Scholar] [CrossRef]
- Han, Y.; Chen, Q.; Zhang, L.; Dissanayaka, W.L. Indispensable Role of HIF-1α Signaling in Post-implantation Survival and Angio-/Vasculogenic Properties of SHED. Front. Cell Dev. Biol. 2021, 9, 655073. [Google Scholar] [CrossRef] [PubMed]
- Rashid, I.; Pathak, A.K.; Kumar, R.; Srivastava, P.; Singh, M.; Murali, S.; Kushwaha, B. Genome-Wide Comparative Analysis of HIF Binding Sites in Cyprinus Carpio for In Silico Identification of Functional Hypoxia Response Elements. Front. Genet. 2019, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fang, L.; Guo, W.; Zhou, Y.; Yu, G.; Li, W.; Dong, K.; Liu, J.; Luo, Y.; Wang, B.; et al. Control of T(reg) cell homeostasis and immune equilibrium by Lkb1 in dendritic cells. Nat. Commun. 2018, 9, 5298. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, S.; Liu, Y.; Uyanne, J.; Shi, Y.; Shi, S.; Le, A.D. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J. Immunol. 2009, 183, 7787–7798. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, J.; Huang, F.; Wang, J.; Su, W.; Zhou, J.; Qi, Q.; Cao, F.; Sun, B.; Liu, Z.; et al. Human gingiva tissue-derived MSC ameliorates immune-mediated bone marrow failure of aplastic anemia via suppression of Th1 and Th17 cells and enhancement of CD4+Foxp3+ regulatory T cells differentiation. Am. J. Transl. Res. 2019, 11, 7627–7643, Erratum in Am. J. Transl. Res. 2020, 12, 1167. [Google Scholar]
- Luo, Y.; Wu, W.; Gu, J.; Zhang, X.; Dang, J.; Wang, J.; Zheng, Y.; Huang, F.; Yuan, J.; Xue, Y.; et al. Human gingival tissue-derived MSC suppress osteoclastogenesis and bone erosion via CD39-adenosine signal pathway in autoimmune arthritis. EBioMedicine 2019, 43, 620–631. [Google Scholar] [CrossRef]
- Bermudez, M.A.; Sendon-Lago, J.; Eiro, N.; Treviño, M.; Gonzalez, F.; Yebra-Pimentel, E.; Giraldez, M.J.; Macia, M.; Lamelas, M.L.; Saa, J.; et al. Corneal epithelial wound healing and bactericidal effect of conditioned medium from human uterine cervical stem cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 983–992. [Google Scholar] [CrossRef]
- Bermudez, M.A.; Sendon-Lago, J.; Seoane, S.; Eiro, N.; Gonzalez, F.; Saa, J.; Vizoso, F.; Perez-Fernandez, R. Anti-inflammatory effect of conditioned medium from human uterine cervical stem cells in uveitis. Exp. Eye Res. 2016, 149, 84–92. [Google Scholar] [CrossRef]
- Sendon-Lago, J.; Rio, L.G.; Eiro, N. Tailored Hydrogels as Delivery Platforms for Conditioned Medium from Mesenchymal Stem Cells in a Model of Acute Colitis in Mice. Pharmaceutics 2021, 13, 1127. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Eiró, N.; Sendon-Lago, J.; Seoane, S.; Bermúdez, M.A.; Lamelas, M.L.; Garcia-Caballero, T.; Schneider, J.; Perez-Fernandez, R.; Vizoso, F.J. Potential therapeutic effect of the secretome from human uterine cervical stem cells against both cancer and stromal cells compared with adipose tissue stem cells. Oncotarget 2014, 5, 10692–10708. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Francos, S.; Eiro, N. Mesenchymal Stem Cells as a Cornerstone in a Galaxy of Intercellular Signals: Basis for a New Era of Medicine. Int. J. Mol. Sci. 2021, 22, 3576. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, L.A.; Eiro, N.; Vaca, A.; Vizoso, F.J. Towards a New Concept of Regenerative Endodontics Based on Mesenchymal Stem Cell-Derived Secretomes Products. Bioengineering 2023, 10, 4. https://doi.org/10.3390/bioengineering10010004
Costa LA, Eiro N, Vaca A, Vizoso FJ. Towards a New Concept of Regenerative Endodontics Based on Mesenchymal Stem Cell-Derived Secretomes Products. Bioengineering. 2023; 10(1):4. https://doi.org/10.3390/bioengineering10010004
Chicago/Turabian StyleCosta, Luis A., Noemi Eiro, Andrea Vaca, and Francisco J. Vizoso. 2023. "Towards a New Concept of Regenerative Endodontics Based on Mesenchymal Stem Cell-Derived Secretomes Products" Bioengineering 10, no. 1: 4. https://doi.org/10.3390/bioengineering10010004
APA StyleCosta, L. A., Eiro, N., Vaca, A., & Vizoso, F. J. (2023). Towards a New Concept of Regenerative Endodontics Based on Mesenchymal Stem Cell-Derived Secretomes Products. Bioengineering, 10(1), 4. https://doi.org/10.3390/bioengineering10010004